Effects of Lignans from Schisandra chinensis Rattan Stems against Aβ1-42-Induced Memory Impairment in Rats and Neurotoxicity in Primary Neuronal Cells
Abstract
1. Introduction
2. Results
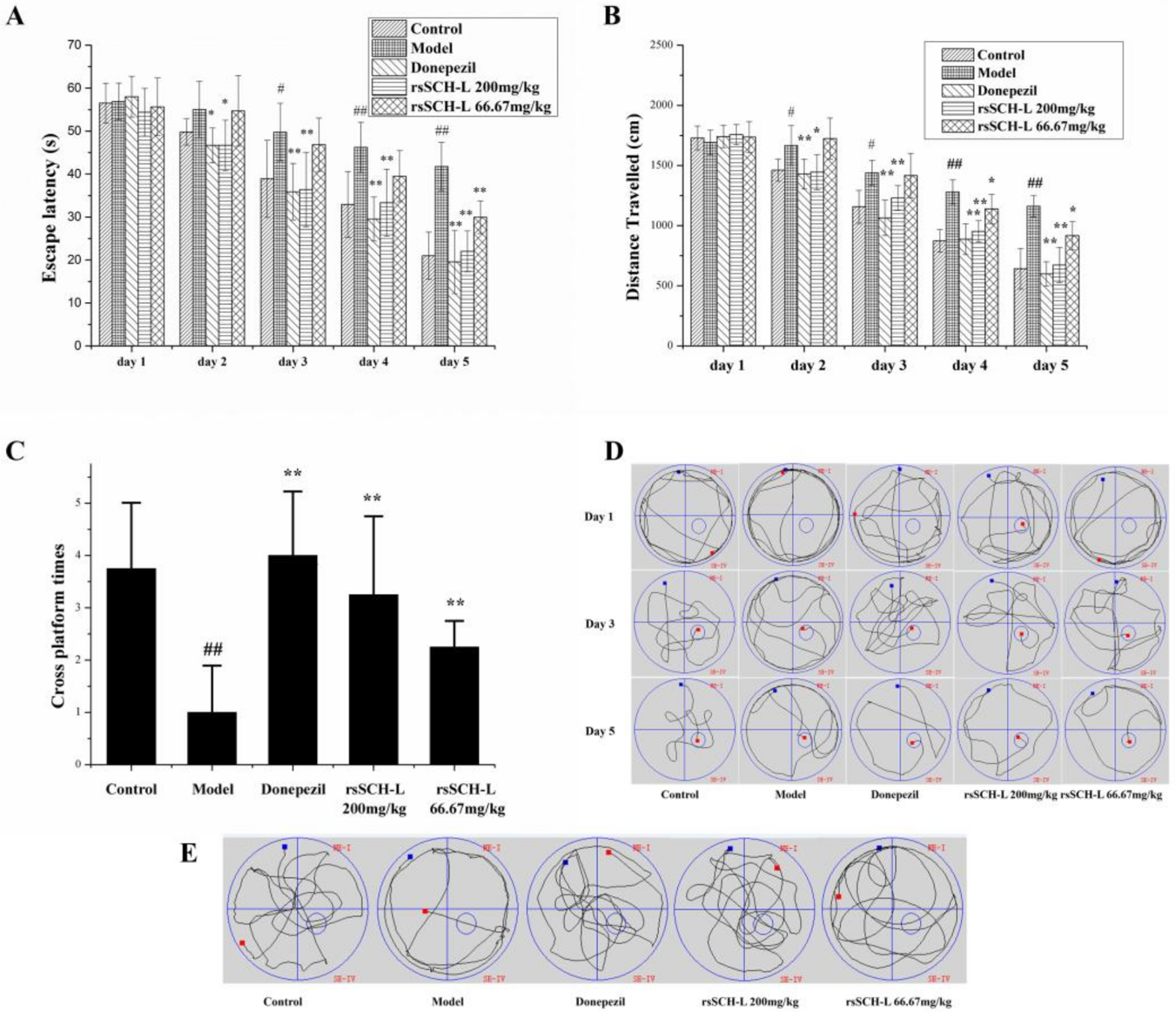
2.1. Effects of rsSCH-L on the Morris Water Maze Test in Aβ1-42-Induced AD Model Rat
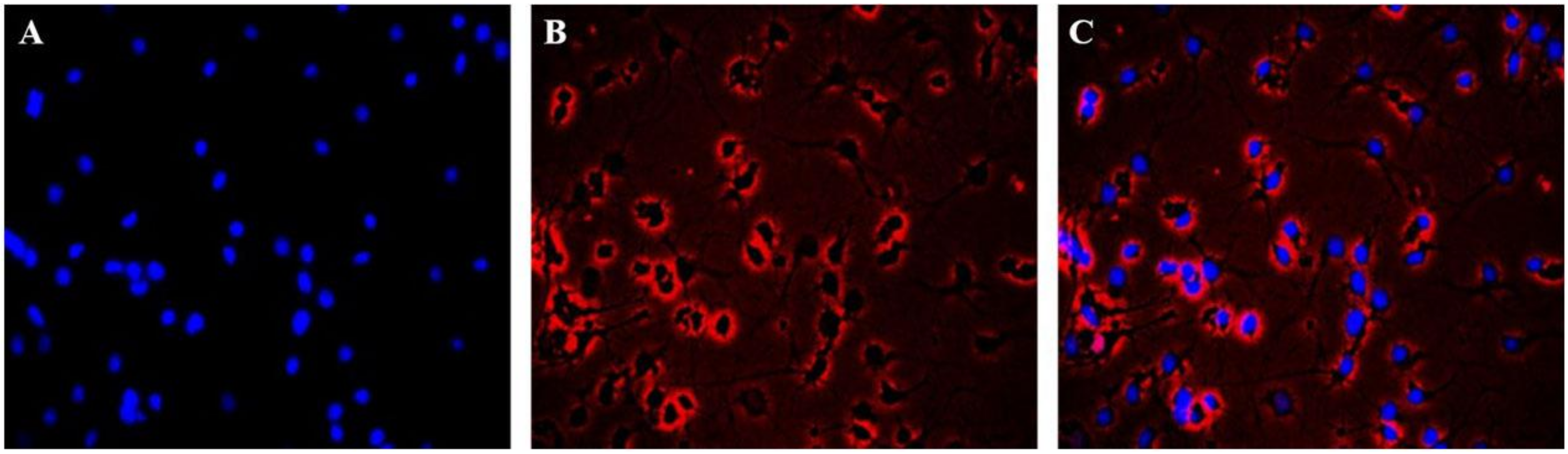
2.2. Immunofluorescence Identification of Neuronal Cells
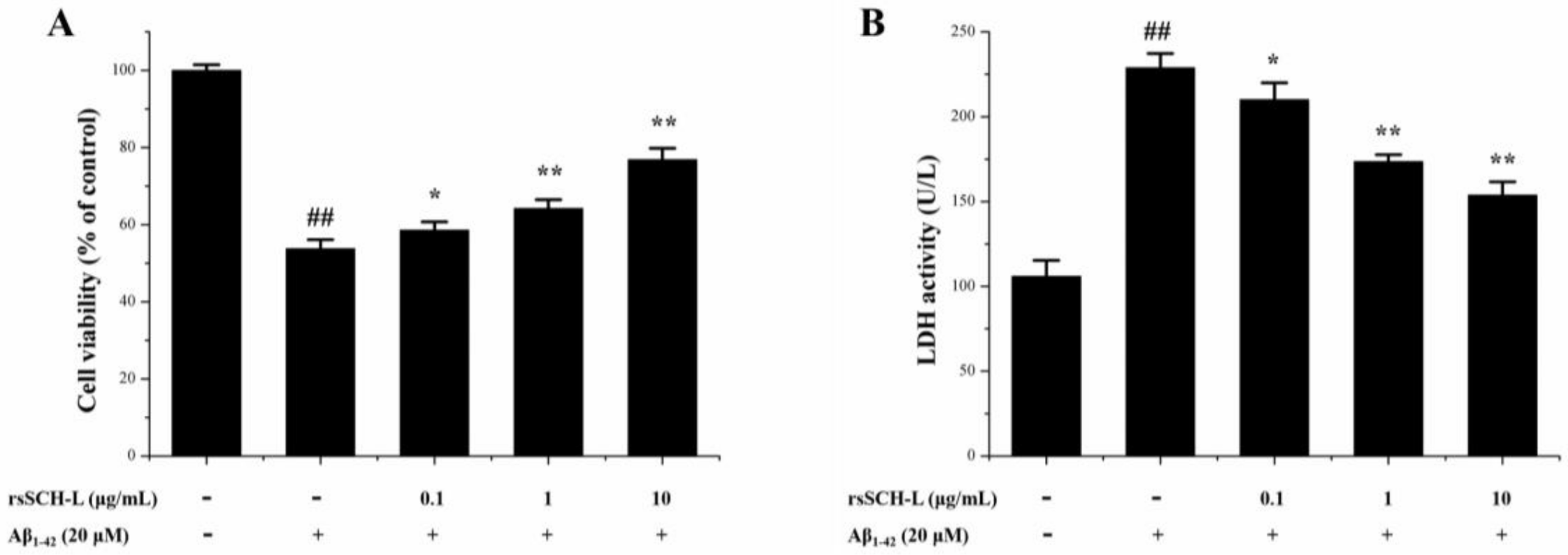
2.3. Effects of rsSCH-L on Cell Viability and LDH Release in Aβ1-42-Induced Neuronal Cells
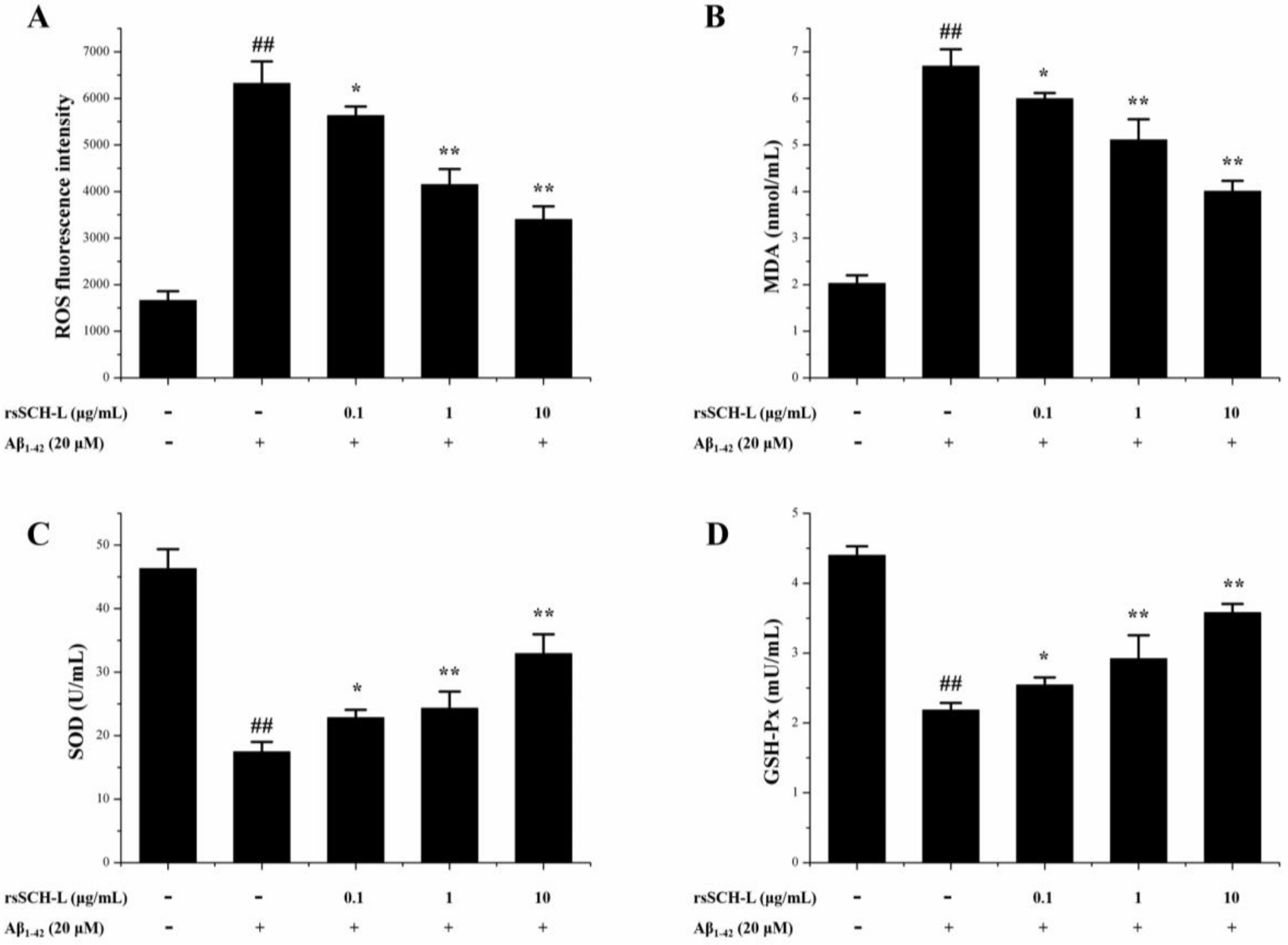
2.4. Effects of rsSCH-L on the Levels of ROS, MDA, and Activity of SOD and GSH-Px Release in Aβ1-42-Induced Neuronal Cells
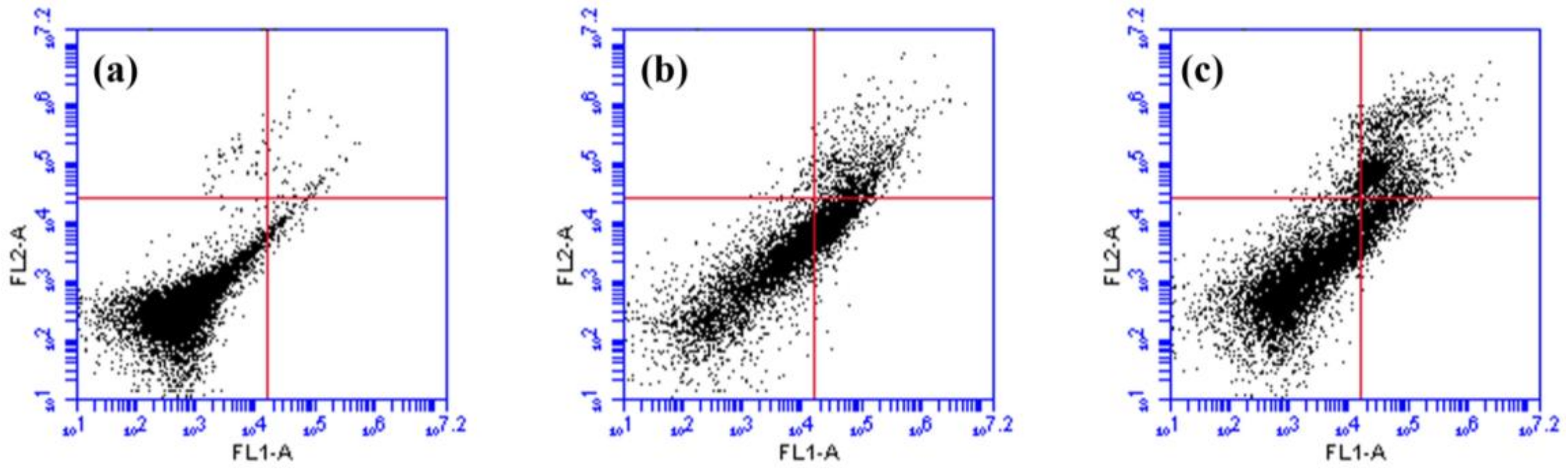
2.5. Effects of rsSCH-L on Apoptosis in Aβ1-42-Induced Neuronal Cells
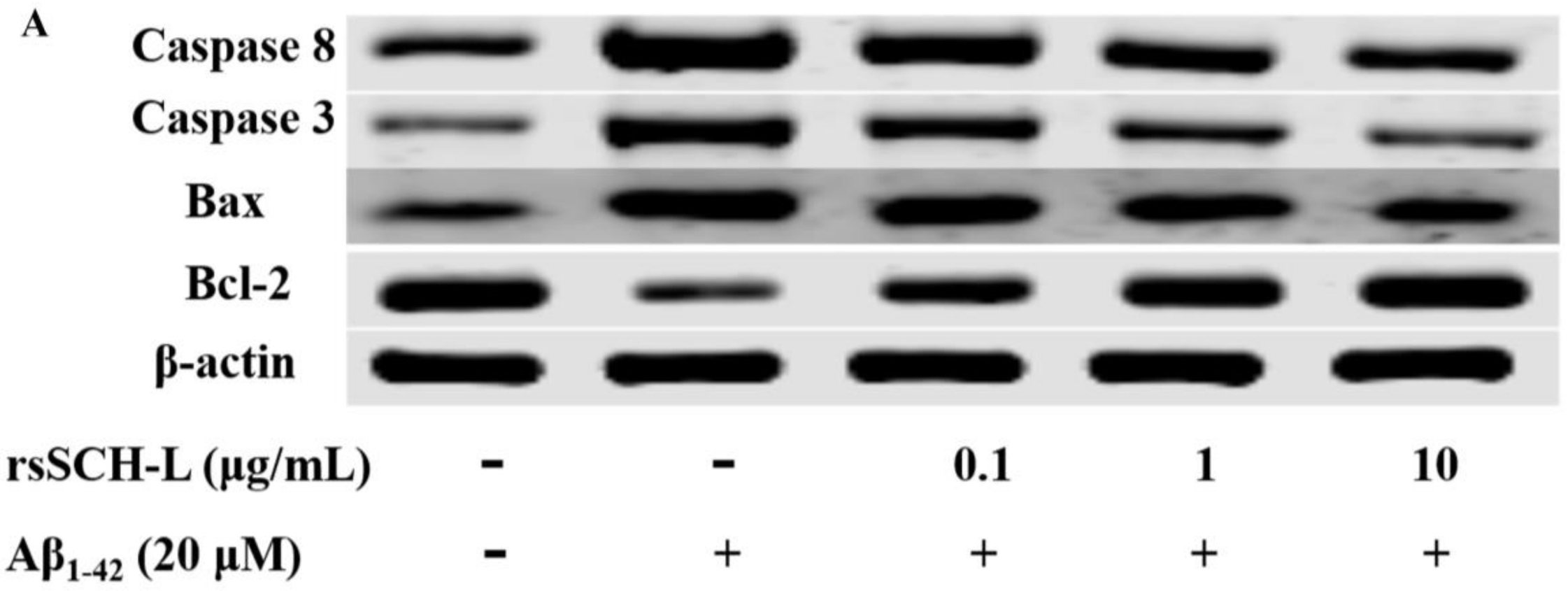
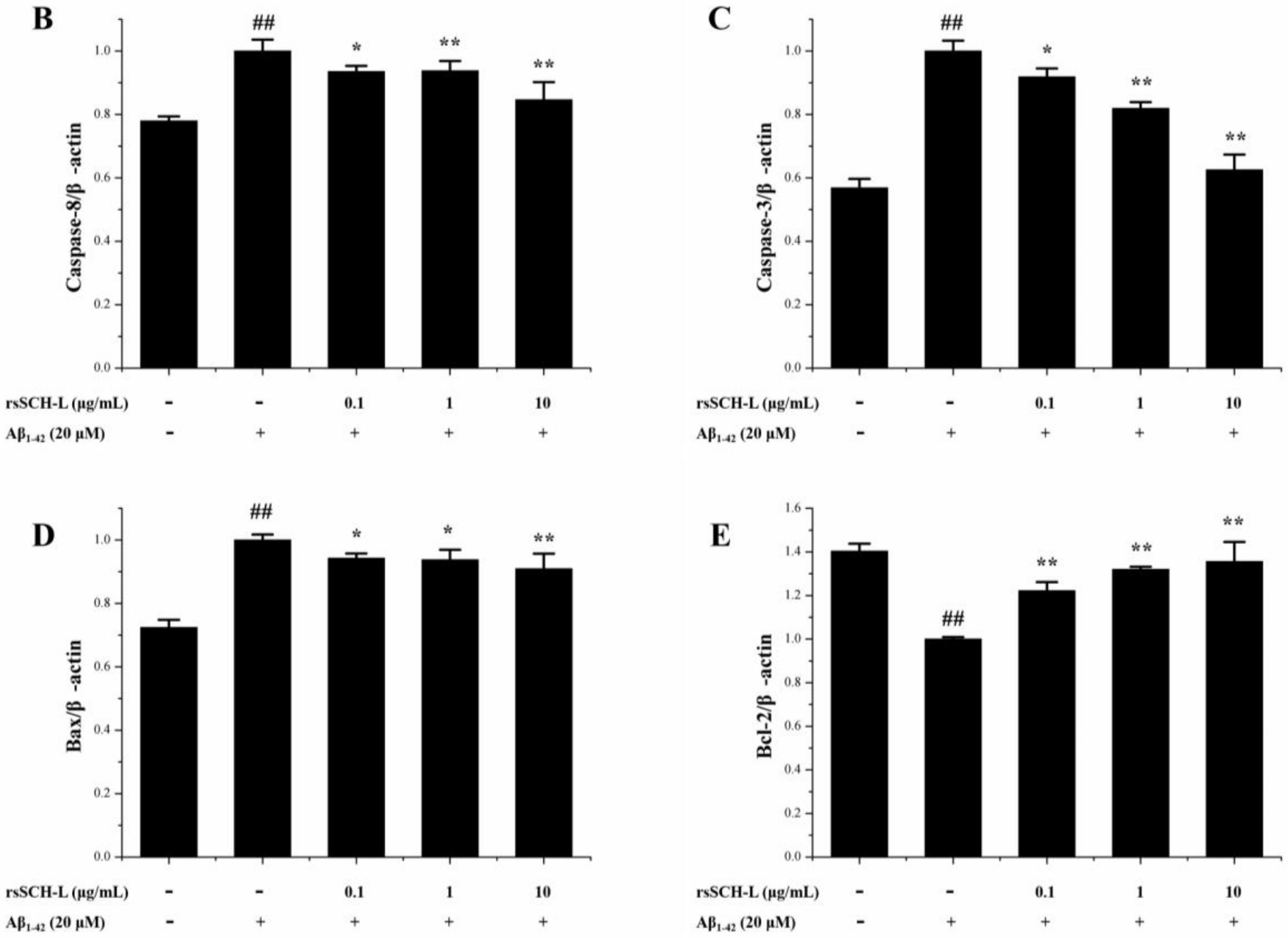
2.6. Effects of rsSCH-L Regulated Caspase-3, Caspase-8, Bax, and Bcl-2 Levels in Aβ1-42-Induced Neuronal Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of rsSCH-L
4.3. Animals
4.4. Morris Water Maze Test
4.5. Primary Neuronal Cells Culture
4.6. Immunofluorescence Identification
4.7. MTT Assay
4.8. LDH Assay
4.9. Assays of Oxidative Stress
4.10. Quantification of Apoptosis
4.11. Western Blot Assay
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Calderon-Garcidueñas, A.L.; Duyckaerts, C. Alzheimer disease. Handb. Clin. Neurol. 2017, 145, 325–337. [Google Scholar] [PubMed]
- Knuesel, I.; Nyffeler, M.; Mormède, C.; Muhia, M.; Meyer, U.; Pietropaolo, S.; Yee, B.K.; Pryce, C.R.; LaFerla, F.M.; Marighetto, A.; et al. Age-related accumulation of Reelin in amyloid-like deposits. Neurobiol. Aging 2009, 30, 697–716. [Google Scholar] [CrossRef] [PubMed]
- Burlá, C.; Rego, G.; Nunes, R. Alzheimer, dementia and the living will: A proposal. Med. Health Care Philos. 2014, 17, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Q.W. Clinical manifestation research of Alzheimer’ s disease. Chin. J. Clin. Ration. Drug Use 2014, 7, 127. [Google Scholar]
- Leszek, J.; Sochocka, M.; Gąsiorowski, K. The immunopathogenic role of reactive oxygen species in Alzheimer disease. Iran. J. Allerg. Asthma Immunol. 2012, 11, 203–216. [Google Scholar]
- Agostinho, P.; Cunha, R.A.; Oliveira, C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr. Pharm. Des. 2010, 16, 2766–2778. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lee, A.S.; Choi, I. Melandrii Herba extract attenuates H2O2-induced neurotoxicity in human neuroblastoma SH-SY5Y cells and Scopolamine-induced memory impairment in mice. Molecules 2017, 20, 1646. [Google Scholar] [CrossRef] [PubMed]
- Jiang, E.P.; Li, H.; Yu, C.R.; Yu, C.Y.; Jing, S.; Sun, H.X.; Wang, C.M.; Fan, X.T.; Chen, J.G.; Wang, S. Schisandrin B protects PC12 cells against oxidative stress of neurodegenerative diseases. Neuroreport 2015, 26, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Sánchez, F.F.; Gironès, X.; Ortega, A.; Alameda, F.; Lafuente, J.V. Oxidative stress in Alzheimer’s disease hippocampus: A topographical study. J. Neurol. Sci. 2010, 299, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, S.; Chen, X.; Yang, H.; Li, X.; Xu, Y.; Zhu, X. Orientin alleviates cognitive deficits and oxidative stress in Aβ1-42-induced mouse model of Alzheimer’s disease. Life Sci. 2015, 121, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, C.; Xu, M.; Li, X.; Bi, K.; Jia, Y. Total lignans of Schisandra chinensis ameliorates Aβ1-42-induced neurodegeneration with cognitive impairment in mice and primary mouse neuronal cells. PLoS ONE 2016, 11, e0152772. [Google Scholar] [CrossRef] [PubMed]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 2014, 42, S125–S152. [Google Scholar] [PubMed]
- Su, Y.; Wang, Q.; Wang, C.; Chan, K.; Sun, Y.; Kuang, H.X. The treatment of Alzheimer’s disease using Chinese medicinal plants: From disease models to potential clinical applications. J. Ethnopharmacol. 2014, 152, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Qi, Y.D.; Xu, L.J.; Peng, Y.; Zhang, B.G.; Xiao, P.G. Investigation of Traditional Pharmacology of Schisandraceae in China. China J. Chin. Mater. Med. 2012, 37, 1353–1359. [Google Scholar]
- Ma, Y.X.; Huang, Y.X.; Zhou, H.C.; Wang, Y.L.; Li, J. Schisandra chinensis modern pharmacological and clinical research progress. Inf. Tradit. Chin. Med. 2014, 31, 125–126. [Google Scholar]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochwmistry Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. An overview of neuroprotective and cognitive enhancement properties of lignans from Schisandra chinensis. Biomed. Pharmacother. 2018, 97, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Song, J.X.; Lin, X.; Wong, R.N.; Sze, S.C.; Tong, Y.; Shaw, P.C.; Zhang, Y.B. Protective effects of dibenzocyclooctadiene lignans from Schisandra chinensis against beta-amyloid and homocysteine neurotoxicity in PC12 cells. Phytother. Res. 2015, 25, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.J.; Lee, H.K.; Lee, K.Y.; Jeon, B.J.; Kim, D.H.; Park, J.H.; Song, J.H.; Huh, J.; Lee, J.H.; Sung, S.H. The effects of lignan-riched extract of Shisandra chinensis on amyloid-β-induced cognitive impairment and neurotoxicity in the cortex and hippocampus of mouse. J. Ethnopharmacol. 2013, 146, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Guo, J.T.; Li, Z.Y.; Wang, C.F.; Wang, Z.B.; Wang, Q.H.; Kuang, H.X. New Thymoquinol glycosides and neuroprotective Dibenzocyclooctane lignans from the rattan stems of Schisandra chinensis. Chem. Biodivers. 2016, 13, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Tan, J.Y.; Liu, Y.; Liu, B.; Jin, S.; Guo, H.W.; Kuang, H.X. A UPLC-TOF/MS-based metabolomics study of rattan stems of Schisandra chinensis effects on Alzheimer’s disease rats model. Biomed. Chromatogr. 2017, 32. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Zheng, G.Y.; Guo, J.T.; Liu, Y.; Kuang, H.X. Chemical constituents from the stems of Schisandra chinensis. Inf. Tradit. Chin. Med. 2016, 33, 7–10. [Google Scholar]
- Li, Y.; Sun, B.H.; Huang, J.; Gao, H.Y.; Wu, L.J. Isolation and identification of chemical constituents from the stems of Schisandra chinensis. J. Shenyang Pharm. Univ. 2009, 26, 438–440. [Google Scholar]
- Zhu, L.; Li, B.; Liu, X.; Huang, G.; Meng, X. Purification of six lignans from the stems of Schisandra chinensis by using high-speed counter-current chromatography combined with preparative high-performance liquid chromatography. Food Chem. 2015, 186, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Mu, F.Y.; Jin, M.H.; Liu, R.J. Analysis of content of the Schisandra chimensis Baill fruit, rattan and fruit handles. J. Med. Sci. Yanbian Univ. 2005, 28, 28–30. [Google Scholar]
- Mocan, A.; Schafberg, M.; Crisan, G.; Rohn, S. Determination of lignans and phenolic components of Schisandra chinensis (Turcz.) Baill. using HPLC-ESI-ToF-MS and HPLC-online TEAC: Contribution of individual components to overall antioxidant activity and comparison with traditional antioxidant assays. J. Funct. Foods 2016, 24, 579–594. [Google Scholar] [CrossRef]
- Yang, B.Y.; Chen, Z.L.; Liu, Y.; Kuang, H.X. Separation and analysis of chemical components in Schisandra chinensis rattan stems. Chin. Tradit. Pat. Med. 2017, 39, 2334–2340. [Google Scholar]
- Liu, J.X.; Hou, W.; Dou, F.M.; Jin, Y.P.; Wang, Y.S.; Wang, Y.P. Chemical constituents of n-butanol fraction from stems of Schisandra chinensis. Chin. Tradit. Herb. Drugs 2015, 46, 1878–1882. [Google Scholar]
- Li, Y.Z.; Ren, S.; Yan, X.T.; Li, H.P.; Li, W.; Zheng, B.; Wang, Z.; Liu, Y.Y. Improvement of Cisplatin-induced renal dysfunction by Schisandra chinensis stems via anti-inflammation and anti-apoptosis effects. J. Ethnopharmacol. 2018, 217, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, X.; Liu, F.; Wang, J.; Feng, J. A review of polysaccharides from Schisandra chinensis and Schisandra sphenanthera: Properties, functions and applications. Carbohydr. Polym. 2018, 184, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Su, G.; Wang, S.; Zhang, Q.; Zhang, J.; Zheng, L.; Sun, B.; Zhao, M. Neuroprotective effects of acetylcholinesterase inhibitory peptides from Anchovy (Coilia mystus) against glutamate-induced toxicity in PC12 cells. J. Agric. Food Chem. 2017, 65, 11192–11201. [Google Scholar] [CrossRef] [PubMed]
- Prasanthi, J.R.; Dasari, B.; Marwarha, G.; Larson, T.; Chen, X.; Geiger, J.D.; Ghribi, O. Caffeine protects against oxidative stress and Alzheimer’s disease-like pathology in rabbit hippocampus induced by cholesterol-enriched diet. Free Radic. Biol. Med. 2010, 49, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Vergallo, A.; Aguilar, L.F.; Benda, N.; Broich, K.; Cuello, A.C.; Cummings, J.; Dubois, B.; Federoff, H.J.; Fiandaca, M.; et al. Precision pharmacology for Alzheimer’s disease. Pharmacol. Res. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Batarseh, Y.S.; Mohamed, L.A.; Al Rihani, S.B.; Mousa, Y.M.; Siddique, A.B.; El Sayed, K.A.; Kaddoumi, A. Oleocanthal ameliorates amyloid-β oligomers’ toxicity on astrocytes and neuronal cells: In vitro studies. Neuroscience 2017, 352, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.W.; Zhang, T.; Fu, H.; Liu, G.X.; Wang, X.M. Schisandrin B exerts anti-neuroinflammatory activity by inhibiting the Toll-like receptor 4-dependent MyD88/IKK/NF-κB signaling pathway in lipopolysaccharide-induced microglia. Eur. J. Pharmacol. 2012, 692, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, T.; Li, W.; Gao, N.; Zhang, T. Resveratrol attenuates oxidative damage through activating mitophagy in an in vitro model of Alzheimer’s disease. Toxicol. Lett. 2018, 282, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Hirai, K.; Hsiao, K.; Pappolla, M.A.; Harris, P.L.; Siedlak, S.L.; Tabaton, M.; Perry, G. Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. J. Neurochem. 1998, 70, 2212–2215. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, H.; Li, Z.; Yang, Z. Triptolide attenuated injury via inhibiting oxidative stress in Amyloid-Beta25-35-treated differentiated PC12 cells. Life Sci. 2016, 145, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Farajdokht, F.; Amani, M.; Mirzaei, B.F.; Alihemmati, A.; Mohaddes, G.; Babri, S. Troxerutin protects hippocampal neurons against amyloid beta-induced oxidative stress and apoptosis. EXCLI J. 2017, 16, 1081–1089. [Google Scholar] [PubMed]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Liao, Z.; Guo, L.; Xu, X.; Wu, B.; Xu, M.; Zhao, X.; Bi, K.; Jia, Y. Schisandrin C ameliorates learning and memory deficits by Aβ1-42 -induced oxidative stress and neurotoxicity in mice. Phytother. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Liu, J.; Chen, R.; Tang, Y.; Chen, H.; Gu, L.; Li, M.; Cao, S.; Qin, D.; et al. Inhibitory effect of Lychee Seed Saponins on apoptosis induced by Aβ25-35 through regulation of the apoptotic and NF-κB pathways in PC12 cells. Nutrients 2017, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J.; Lin, C.Y.; Mong, M.C.; Ho, M.W.; Yin, M.C. s-Ethyl cysteine and s-propyl cysteine alleviate beta-amyloid induced cytotoxicity in nerve growth factor differentiated PC12 cells. J. Agric. Food Chem. 2010, 58, 7104–7108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.X.; Yu, Y.; Du, J.R. Protective effects of Z-ligustilide against cytotoxicity induced by Abeta25-35 in neuron cells. J. Sichuan Univ. 2012, 43, 34–37. [Google Scholar]
- Koh, E.J.; Seo, Y.J.; Choi, J.; Lee, H.Y.; Kang, D.H.; Kim, K.J.; Lee, B.Y. Spirulina maxima extract prevents neurotoxicity via promoting activation of BDNF/CREB signaling pathways in neuronal cells and mice. Molecules 2017, 22, 1363. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.D.; Yang, X.L.; Chen, T.; Ji, J.; Lan, L.; Hu, R.; Ji, H. Treatment with Akebia Saponin D ameliorates Aβ1-42-induced memory impairment and neurotoxicity in rats. Molecules 2016, 21, 323. [Google Scholar] [CrossRef]
- Hu, D.; Li, C.; Han, N.; Miao, L.; Wang, D.; Liu, Z.; Wang, H.; Yin, J. Deoxyschizandrin isolated from the fruits of Schisandra chinensis ameliorates Aβ1-42-induced memory impairment in mice. Planta Med. 2012, 78, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Parmar, H.S.; Houdek, Z.; Pesta, M.; Vaclava, C.; Dvorak, P.; Hatina, J. Protective effect of Aspirin against oligomeric Aβ42 induced mitochondrial alterations and neurotoxicity in differentiated EC P19 neuronal cells. Curr. Alzheimer Res. 2017, 14, 810–819. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.-Y.; Han, W.; Han, H.; Liu, Y.; Guan, W.; Li, X.-M.; Kuang, H.-X. Effects of Lignans from Schisandra chinensis Rattan Stems against Aβ1-42-Induced Memory Impairment in Rats and Neurotoxicity in Primary Neuronal Cells. Molecules 2018, 23, 870. https://doi.org/10.3390/molecules23040870
Yang B-Y, Han W, Han H, Liu Y, Guan W, Li X-M, Kuang H-X. Effects of Lignans from Schisandra chinensis Rattan Stems against Aβ1-42-Induced Memory Impairment in Rats and Neurotoxicity in Primary Neuronal Cells. Molecules. 2018; 23(4):870. https://doi.org/10.3390/molecules23040870
Chicago/Turabian StyleYang, Bing-You, Wei Han, Hua Han, Yan Liu, Wei Guan, Xiao-Mao Li, and Hai-Xue Kuang. 2018. "Effects of Lignans from Schisandra chinensis Rattan Stems against Aβ1-42-Induced Memory Impairment in Rats and Neurotoxicity in Primary Neuronal Cells" Molecules 23, no. 4: 870. https://doi.org/10.3390/molecules23040870
APA StyleYang, B.-Y., Han, W., Han, H., Liu, Y., Guan, W., Li, X.-M., & Kuang, H.-X. (2018). Effects of Lignans from Schisandra chinensis Rattan Stems against Aβ1-42-Induced Memory Impairment in Rats and Neurotoxicity in Primary Neuronal Cells. Molecules, 23(4), 870. https://doi.org/10.3390/molecules23040870



