Oxadiazole-Based Highly Efficient Bipolar Fluorescent Emitters for Organic Light-Emitting Diodes
Abstract
1. Introduction
2. Results and Discussion
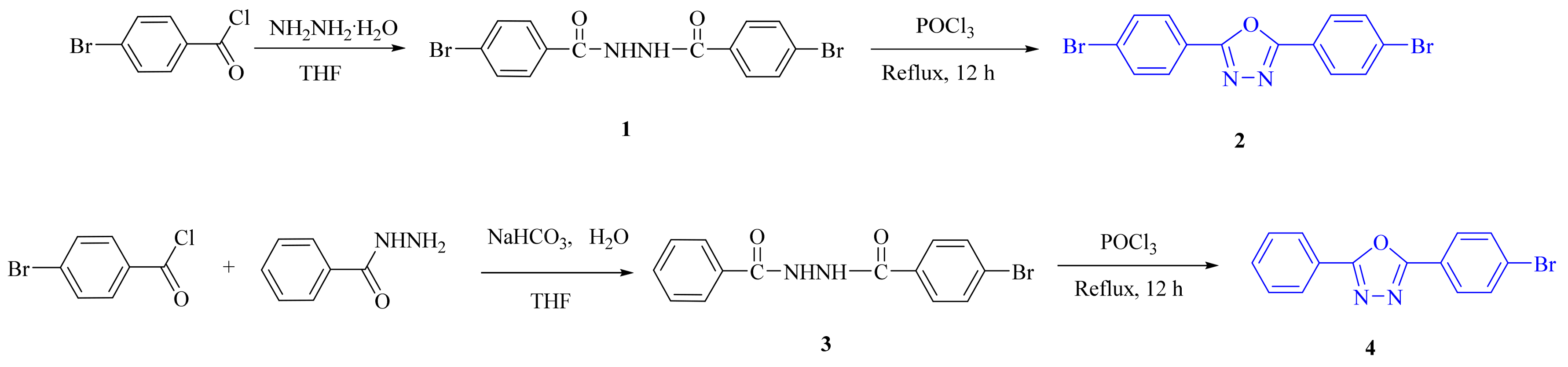
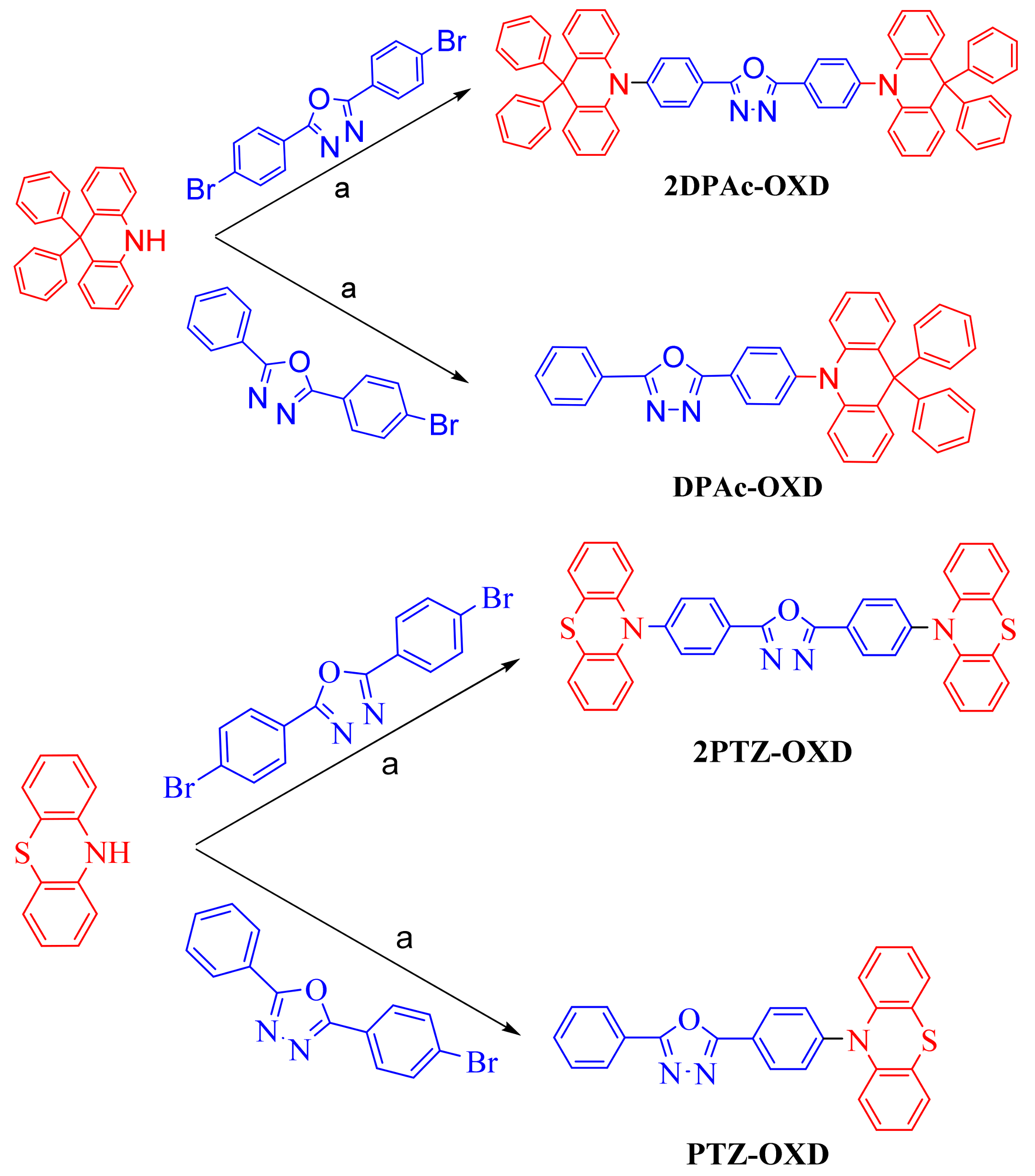
2.1. Synthesis
2.2. Thermal Properties
2.3. Photophysical and Electrochemical Properties
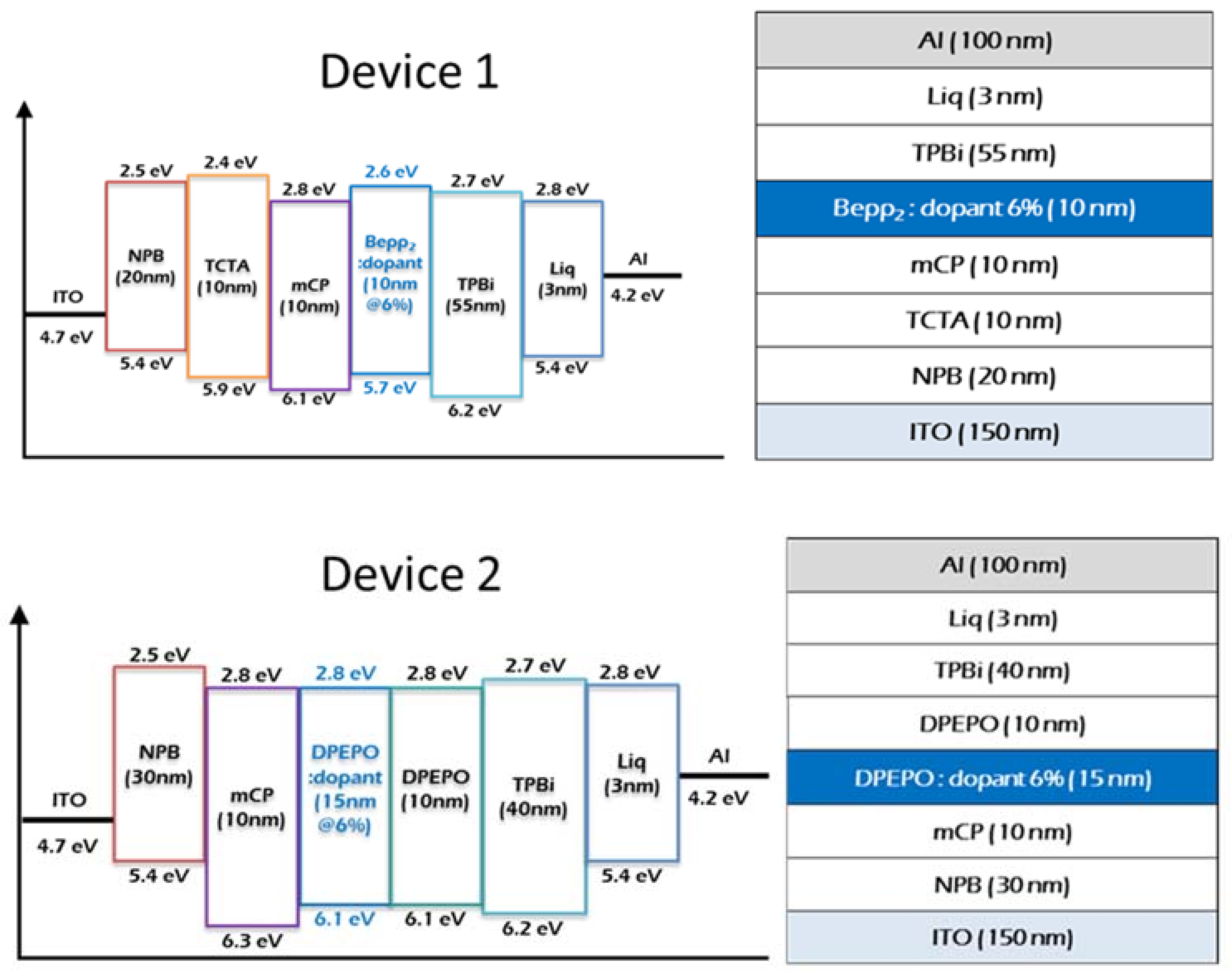
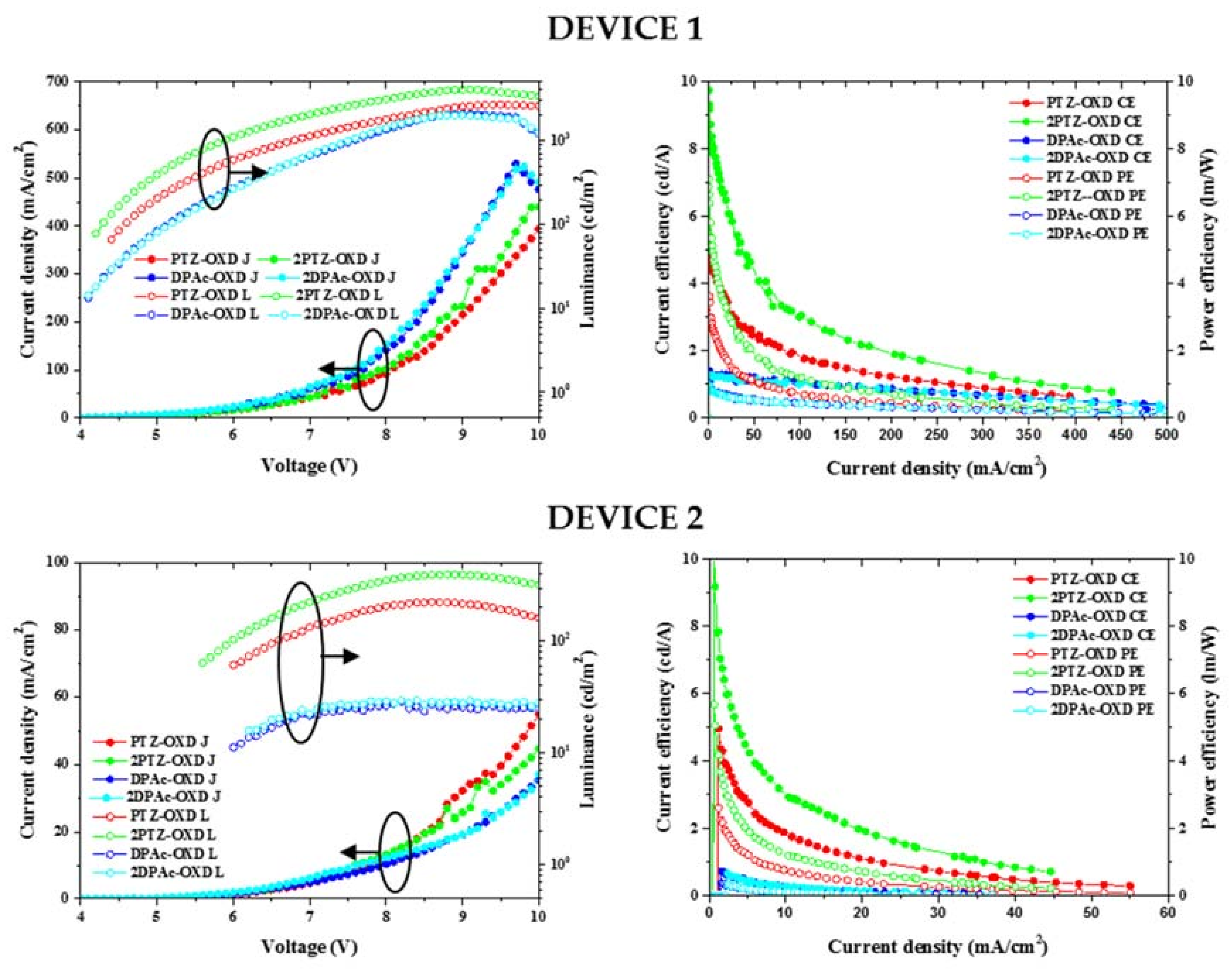
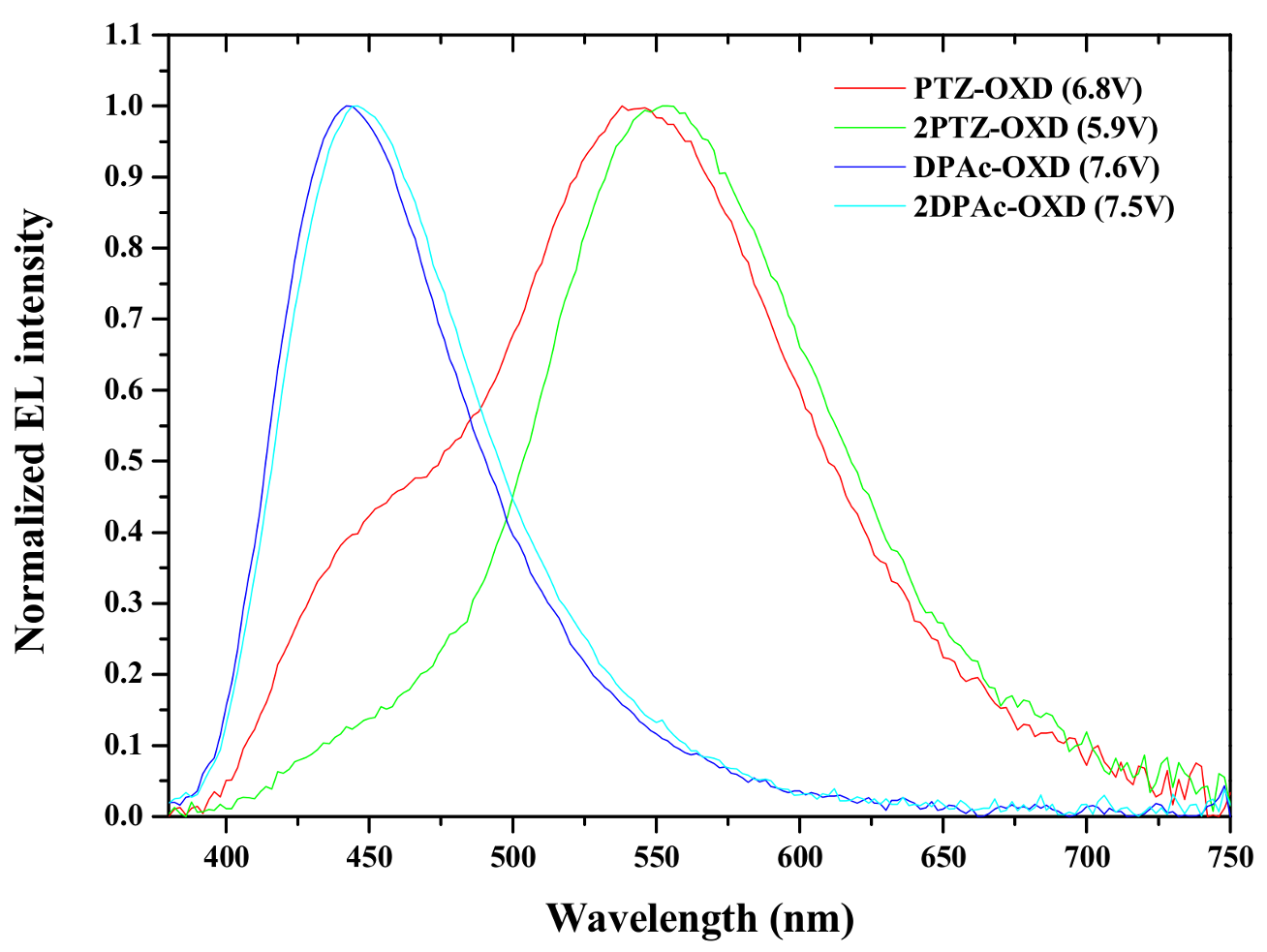
2.4. Device Characteristics
3. Materials and Methods
3.1. General Procedures
3.2. Synthetic Procedures
3.2.1. 4-Bromo-N′-(4-bromobenzoyl)benzohydrazide (1)
3.2.2. 2,5-Bis(4-bromophenyl)-1,3,4-oxadiazole (2)
3.2.3. 2-(4-Bromophenyl)-5-phenyl-1,3,4-oxadiazole (4)
3.2.4. 2,5-Bis(4-(9,9-diphenyl-9,10-dihydroacridine)phenyl)-1,3,4-oxadiazole (2DPAc-OXD)
3.2.5. 2-(4-(9,9-Diphenyl-9,10-dihydroacridine)phenyl)-1,3,4-oxadiazole (DPAc-OXD)
3.2.6. 2,5-Bis(4-(10H-phenothiazin-10-yl)-1,3,4-oxadizole (2PTZ-OXD)
3.2.7. 2-(4-(10H-phenothiazin-10-yl)-1,3,4-oxadiazole(PTZ-OXD)
3.3. OLED Fabrication and Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- D’Andrade, B.W.; Forrest, S.R. White organic light-emitting devices for solid-state lighting. Adv. Mater. 2004, 16, 1585–1595. [Google Scholar] [CrossRef]
- Reddy, S.S.; Sree, V.G.; Cho, W.; Jin, S.-H. Achieving pure deep-blue electroluminescence with CIE y ≤ 0.06 via a rational design approach for highly efficient non-doped solution-processed organic light-emitting diodes. Chem. Asian J. 2016, 11, 3275–3282. [Google Scholar] [CrossRef] [PubMed]
- Shirota, Y.; Kinoshita, M.; Noda, T.; Okumoto, K.; Ohara, T. A novel class of emitting amorphous molecular materials as bipolar radical formants: 2-{4-[bis(4-methylphenyl)amino]phenyl}-5-(dimesitylboryl)thiophene and 2-{4-[bis(9,9-dimethylfluorenyl)amino]phenyl}-5-(dimesitylboryl)thiophene. J. Am. Chem. Soc. 2000, 122, 11021–11022. [Google Scholar] [CrossRef]
- Scott, J.C.; Karg, S.; Carter, S.A. Bipolar charge and current distributions in organic light-emitting diodes. J. Appl. Phys. 1997, 82, 1454–1460. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Fujiyama, T.; Tanaka, H.; Yokoyama, M. Material design of organic thin films for bipolar charge transport. Chem. Mater. 1990, 2, 341–342. [Google Scholar] [CrossRef]
- Woo, S.-J.; Kim, Y.; Kim, M.-J.; Baek, J.Y.; Kwon, S.K.; Kim, Y.H.; Kim, J.J. Strategies for the molecular design of donor–acceptor-type fluorescent emitters for efficient deep blue organic light emitting diodes. Chem. Mater. 2018, 30, 857–863. [Google Scholar] [CrossRef]
- Reddy, S.S.; Sree, V.G.; Gunasekar, K.; Cho, W.; Gal, Y.S.; Song, M.; Kang, J.W.; Jin, S.H. Highly efficient bipolar deep-blue fluorescent emitters for solution-processed non-doped organic light-emitting diodes based on 9,9-dimethyl-9,10-dihydroacridine/phenanthroimadazole derivatives. Adv. Opt. Mater. 2016, 4, 1236–1246. [Google Scholar] [CrossRef]
- Thirion, D.; Rault-Berthelot, J.; Vignau, L.; Poriel, C. Synthesis and properties of a blue bipolar indenofluorene emitter based on a D-π-A design. Org. Lett. 2011, 13, 4418–4421. [Google Scholar] [CrossRef] [PubMed]
- Abdurahman, A.; Obolda, A.; Peng, Q.; Li, F. Efficient deep blue fluorescent oleds with ultra-low efficiency roll-off based on 4h-1,2,4-triazole cored D-A and D-A-D type emitters. Dyes Pigm. 2018, 153, 10–17. [Google Scholar] [CrossRef]
- Liu, X.K.; Zheng, C.J.; Lo, M.F.; Xiao, J.; Chen, Z.; Liu, C.L.; Lee, C.S.; Fung, M.K.; Zhang, X.H. Novel blue fluorophor with high triplet energy level for high performance single-emitting-layer fluorescence and phosphorescence hybrid white organic light-emitting diodes. Chem. Mater. 2013, 25, 4454–4459. [Google Scholar] [CrossRef]
- Lin, S.L.; Chan, L.H.; Lee, R.H.; Yen, M.Y.; Kuo, W.J.; Chen, C.T.; Jeng, R.J. Highly efficient carbazole-π-dimesitylborane bipolar fluorophores for nondoped blue organic light-emitting diodes. Adv. Mater. 2008, 20, 3947–3952. [Google Scholar] [CrossRef]
- Fisher, A.L.; Linton, K.E.; Kamtekar, K.T.; Pearson, C.; Bryce, M.R.; Petty, M.C. Efficient deep-blue electroluminescence from an ambipolar fluorescent emitter in a single-active-layer device. Chem. Mater. 2011, 23, 1640–1642. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, M.K.; Kim, S.H.; Hong, J.I. Efficient deep-blue emitters based on triphenylamine-linked benzimidazole derivatives for nondoped fluorescent organic light-emitting diodes. Org. Electron. 2013, 14, 2497–2504. [Google Scholar] [CrossRef]
- Ramaiah, D.; Thurakkal, S.; Sanju, K.S.; Soman, A.; Unni, N.; Joseph, J. Design and synthesis of solution processable green fluorescent D-π-A dyads for oled applications. New J. Chem. 2018, 42, 5456–5464. [Google Scholar]
- Zhang, G.; Auer-Berger, M.; Gehrig, D.; Blom, P.; Baumgarten, M.; Schollmeyer, D.; List-Kratochvil, E.; Müllen, K. Blue light emitting polyphenylene dendrimers with bipolar charge transport moieties. Molecules 2016, 21, 1400. [Google Scholar] [CrossRef] [PubMed]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kuwabara, H.; Potscavage, W.J.; Huang, S.; Hatae, Y.; Shibata, T.; Adachi, C. Anthraquinone-based intramolecular charge-transfer compounds: Computational molecular design, thermally activated delayed fluorescence, and highly efficient red electroluminescence. J. Am. Chem. Soc. 2014, 136, 18070–18081. [Google Scholar] [CrossRef] [PubMed]
- Yook, K.S.; Lee, J.Y.; Yook, K.S. Bipolar host materials for organic light-emitting diodes. Chem. Rec. 2016, 16, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Kulkarni, A.P.; Jenekhe, S.A. Phenoxazine-based emissive donor−acceptor materials for efficient organic light-emitting diodes. Chem. Mater. 2005, 17, 5225–5227. [Google Scholar] [CrossRef]
- Goes, M.; Verhoeven, J.W.; Hofstraat, H.; Brunner, K. Oled and pled devices employing electrogenerated, intramolecular charge-transfer fluorescence. ChemPhysChem 2003, 4, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.P.; Kong, X.; Jenekhe, S.A. High-performance organic light-emitting diodes based on intramolecular charge-transfer emission from donor–acceptor molecules: Significance of electron-donor strength and molecular geometry. Adv. Funct. Mater. 2006, 16, 1057–1066. [Google Scholar] [CrossRef]
- Zhong, H.; Lai, H.; Fang, Q. New conjugated triazine based molecular materials for application in optoelectronic devices: Design, synthesis, and properties. J. Phys. Chem. C 2011, 115, 2423–2427. [Google Scholar] [CrossRef]
- Linton, K.E.; Fisher, A.L.; Pearson, C.; Fox, M.A.; Palsson, L.O.; Bryce, M.R.; Petty, M.C. Colour tuning of blue electroluminescence using bipolar carbazole-oxadiazole molecules in single-active-layer organic light emitting devices (oleds). J. Mater. Chem. 2012, 22, 11816–11825. [Google Scholar] [CrossRef]
- Duan, L.; Qiao, J.; Sun, Y.; Qiu, Y. Strategies to design bipolar small molecules for oleds: Donor-acceptor structure and non-donor-acceptor structure. Adv. Mater. 2011, 23, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, A.Y.; Li, B.-X.; Huang, J.; Zhao, L.; Wang, B.-Z.; Li, J.-W.; Zhu, X.-H.; Peng, J.; Cao, Y.; et al. Asymmetrically 4,7-disubstituted benzothiadiazoles as efficient non-doped solution-processable green fluorescent emitters. Org. Lett. 2009, 11, 5318–5321. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chen, Z.; Fung, M.K.; Zheng, C.; Ou, X.; Zhang, X.; Yuan, Y.; Lee, C.S. Carbazole/sulfone hybrid D-π-A-structured bipolar fluorophores for high-efficiency blue-violet electroluminescence. Chem. Mater. 2013, 25, 2630–2637. [Google Scholar] [CrossRef]
- Zhang, M.; Xue, S.; Dong, W.; Wang, Q.; Fei, T.; Gu, C.; Ma, Y. Highly-efficient solution-processed oleds based on new bipolar emitters. ChemComm 2010, 46, 3923–3925. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, H.; Inbasekaran, M.; Woo, E.P. Blue-green organic light-emitting diodes based on fluorene-oxadiazole compounds. Appl. Phys. Lett. 1998, 73, 3055–3057. [Google Scholar] [CrossRef]
- Lian, M.; Yu, Y.; Zhao, J.; Huang, Z.; Yang, X.; Zhou, G.; Wu, Z.; Wang, D. Novel phosphorescent polymers containing both ambipolar segments and functionalized IrIII phosphorescent moieties: Synthesis, photophysical, redox, and electrophosphorescence investigation. J. Mater. Chem. C 2014, 2, 9523–9535. [Google Scholar] [CrossRef]
- Wróblowska, M.; Kudelko, A.; Kuźnik, N.; Łaba, K.; Łapkowski, M. Synthesis of extended 1,3,4-oxadiazole and 1,3,4-thiadiazole derivatives in the Suzuki cross-coupling reactions. J. Heterocycl. Chem. 2017, 54, 1550–1557. [Google Scholar] [CrossRef]
- Kwon, W.; Ahn, B.; Kim, D.M.; Ko, Y.-G.; Hahm, S.G.; Kim, Y.; Kim, H.; Ree, M. Morphology-dependent electrical memory characteristics of a well-defined brush polymer bearing oxadiazole-based mesogens. J. Phys. Chem. C 2011, 115, 19355–19363. [Google Scholar] [CrossRef]
- Mi, B.; Gao, Z.; Liao, Z.; Huang, W.; Chen, C.H. Molecular hosts for triplet emitters in organic light-emitting diodes and the corresponding working principle. Sci. China Chem. 2010, 53, 1679–1694. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, Z.; Liu, Y.; Dai, Y.; Chen, J.; Ma, D. A hybrid white organic light-emitting diode with stable color and reduced efficiency roll-off by using a bipolar charge carrier switch. Org. Electron. 2012, 13, 1049–1055. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, D.; Wei, Y.; Xu, H. Extremely condensing triplet states of DPEPO-type hosts through constitutional isomerization for high-efficiency deep-blue thermally activated delayed fluorescence diodes. Chem. Sci. 2016, 7, 2870–2882. [Google Scholar] [CrossRef]
- Braveenth, R.; Bae, I.-J.; Han, J.-H.; Qiong, W.; Seon, G.; Raagulan, K.; Yang, K.; Park, Y.H.; Kim, M.; Chai, K.Y. Utilizing a Spiro Core with Acridine- and Phenothiazine-Based New Hole Transporting Materials for Highly Efficient Green Phosphorescent Organic Light-Emitting Diodes. Molecules 2018, 23, 713. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Zhao, S.; Luo, Y.; Aziz, H. Causes of efficiency roll-off in phosphorescent organic light emitting devices: Triplet-triplet annihilation versus triplet-polaron quenching. Appl. Phys. Lett. 2010, 97, 268. [Google Scholar] [CrossRef]
- Zheng, T.; Choy, W.C.; Ho, C.L.; Wong, W.Y. Improving efficiency roll-off in organic light emitting devices with a fluorescence-interlayer-phosphorescence emission architecture. Appl. Phys. Lett. 2009, 95, 264. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
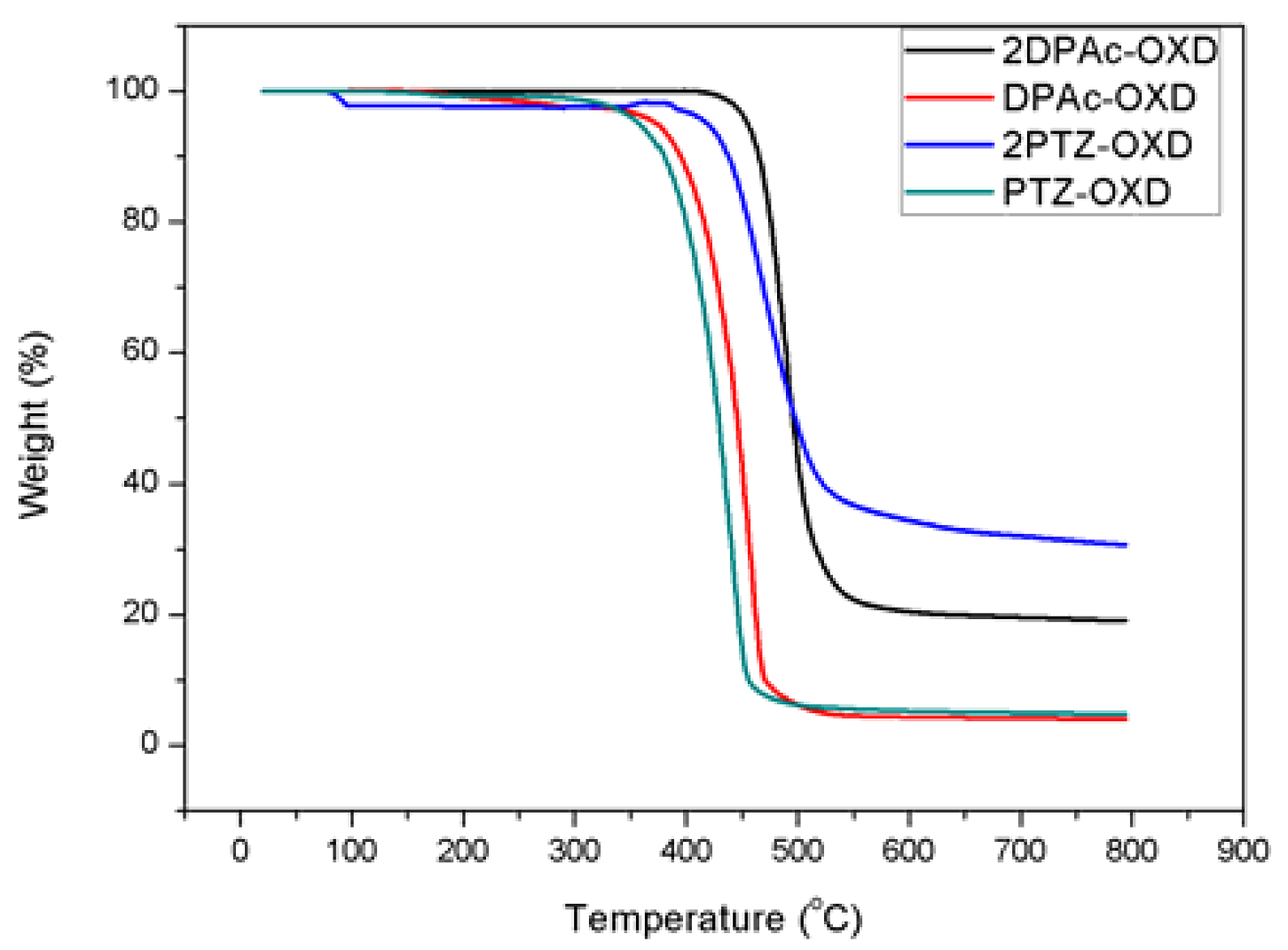
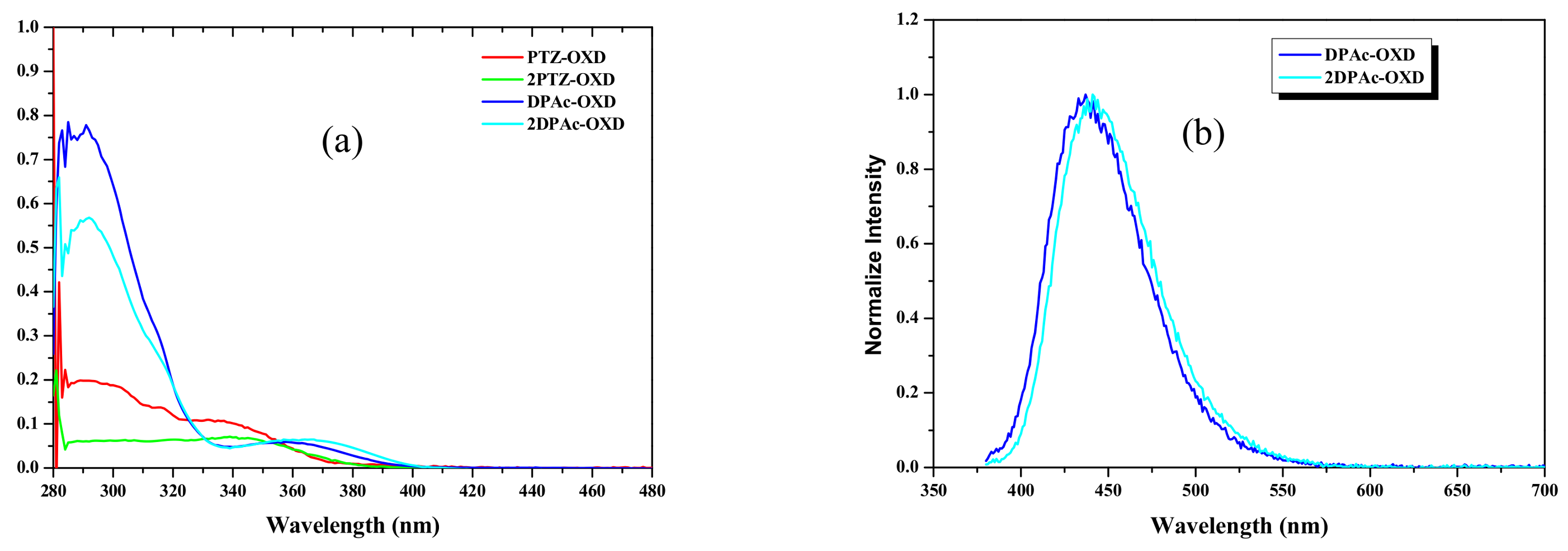
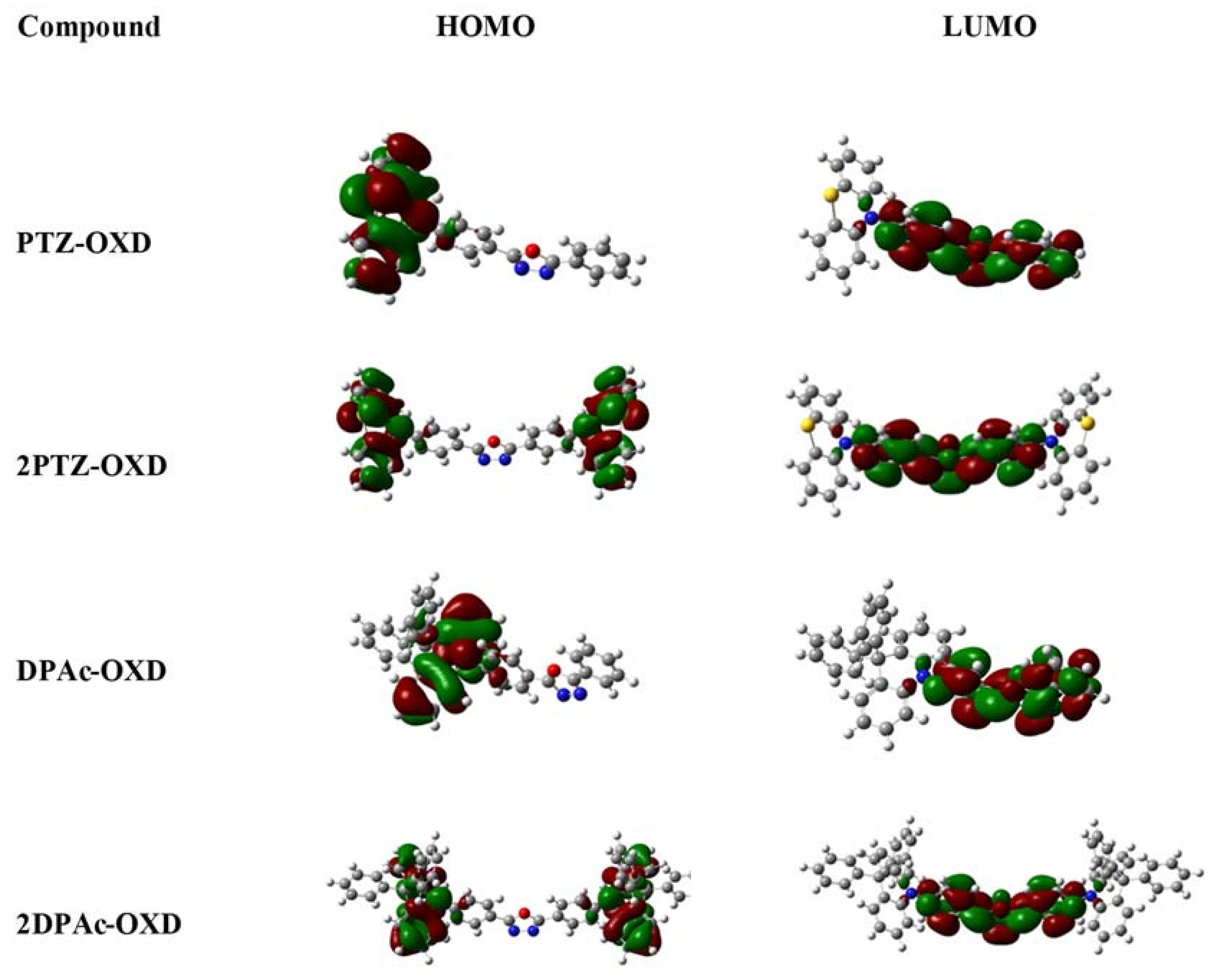
| Dopant | Td a (°C) | UV-vis b (nm) | PL max c (nm) | HOMO d (eV) | LUMO e (eV) | Eg f (eV) | ET g (eV) |
|---|---|---|---|---|---|---|---|
| PTZ-OXD 2PTZ-OXD DPAc-OXD 2DPAc-OXD | 358 419 375 455 | 376 384 396 402 | 500 512 435 442 | −5.48 −5.48 −5.69 −5.68 | −2.18 −2.25 −2.56 −2.60 | 3.30 3.23 3.13 3.08 | 2.48 2.42 2.85 2.81 |
| Calculation Values | PTZ-OXD | 2PTZ-OXD | DPAc-OXD | 2DPAc-OXD |
|---|---|---|---|---|
| S1 (eV) a T1 (eV) b ΔEST (eV) c D-A rotation(°) HOMO (eV) LUMO (eV) | 3.71 3.36 0.35 77.05 −5.975 −2.185 | 3.63 3.34 0.29 78.91 −5.485 −2.255 | 3.83 3.37 0.46 81.26 −5.695 −2.565 | 3.78 3.34 0.44 81.70 −5.685 −2.605 |
| Device properties | PTZ-OXD | 2PTZ-OXD | DPAc-OXD | 2DPAc-OXD |
|---|---|---|---|---|
| Turn on voltage (V) | 4.4 a 6.0 b | 4.2 a 5.6 b | 4.1 a 6.0 b | 4.1 a 6.2 b |
| Driving voltage (V) | 6.8 a | 5.9 a | 7.6 a | 7.5 a |
| Current (mA) | 0.05 a 0.05 b | 0.03 a 0.03 b | 0.04 a 0.06 b | 0.05 a 0.10 b |
| Current efficiency (cd/A) | 5.03 a 4.97 b | 9.20 a 10.10 b | 1.30 a 0.72 b | 1.28 a 0.66 b |
| Power efficiency (Lm/W) | 3.59 a 2.60 b | 6.88 a 5.67 b | 0.99 a 0.38 b | 0.98 a 0.33 b |
| EQE (%) | 1.94 a | 3.38 a | 1.84 a | 1.81 a |
| 2.26 b | 3.99 b | 1.08 b | 0.88 b | |
| Luminance (at 1000 cd/m2) | 1008 | 1020 | 1029 | 992 |
| CIE (x,y) | (0.38, 0.50) a (0.43, 0.14) b | (0.40, 0.53) a (0.31, 0.49) b | (0.16, 0.12) a (0.18, 0.12) b | (0.17, 0.14) a (0.18, 0.15) b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Braveenth, R.; Zhang, H.Q.; Bae, I.-J.; Kim, M.; Chai, K.Y. Oxadiazole-Based Highly Efficient Bipolar Fluorescent Emitters for Organic Light-Emitting Diodes. Molecules 2018, 23, 843. https://doi.org/10.3390/molecules23040843
Wu Q, Braveenth R, Zhang HQ, Bae I-J, Kim M, Chai KY. Oxadiazole-Based Highly Efficient Bipolar Fluorescent Emitters for Organic Light-Emitting Diodes. Molecules. 2018; 23(4):843. https://doi.org/10.3390/molecules23040843
Chicago/Turabian StyleWu, Qiong, Ramanaskanda Braveenth, Heng Qiang Zhang, Il-Ji Bae, Miyoung Kim, and Kyu Yun Chai. 2018. "Oxadiazole-Based Highly Efficient Bipolar Fluorescent Emitters for Organic Light-Emitting Diodes" Molecules 23, no. 4: 843. https://doi.org/10.3390/molecules23040843
APA StyleWu, Q., Braveenth, R., Zhang, H. Q., Bae, I.-J., Kim, M., & Chai, K. Y. (2018). Oxadiazole-Based Highly Efficient Bipolar Fluorescent Emitters for Organic Light-Emitting Diodes. Molecules, 23(4), 843. https://doi.org/10.3390/molecules23040843





