Synthesis and Anticandidal Activity of New Imidazole-Chalcones
Abstract
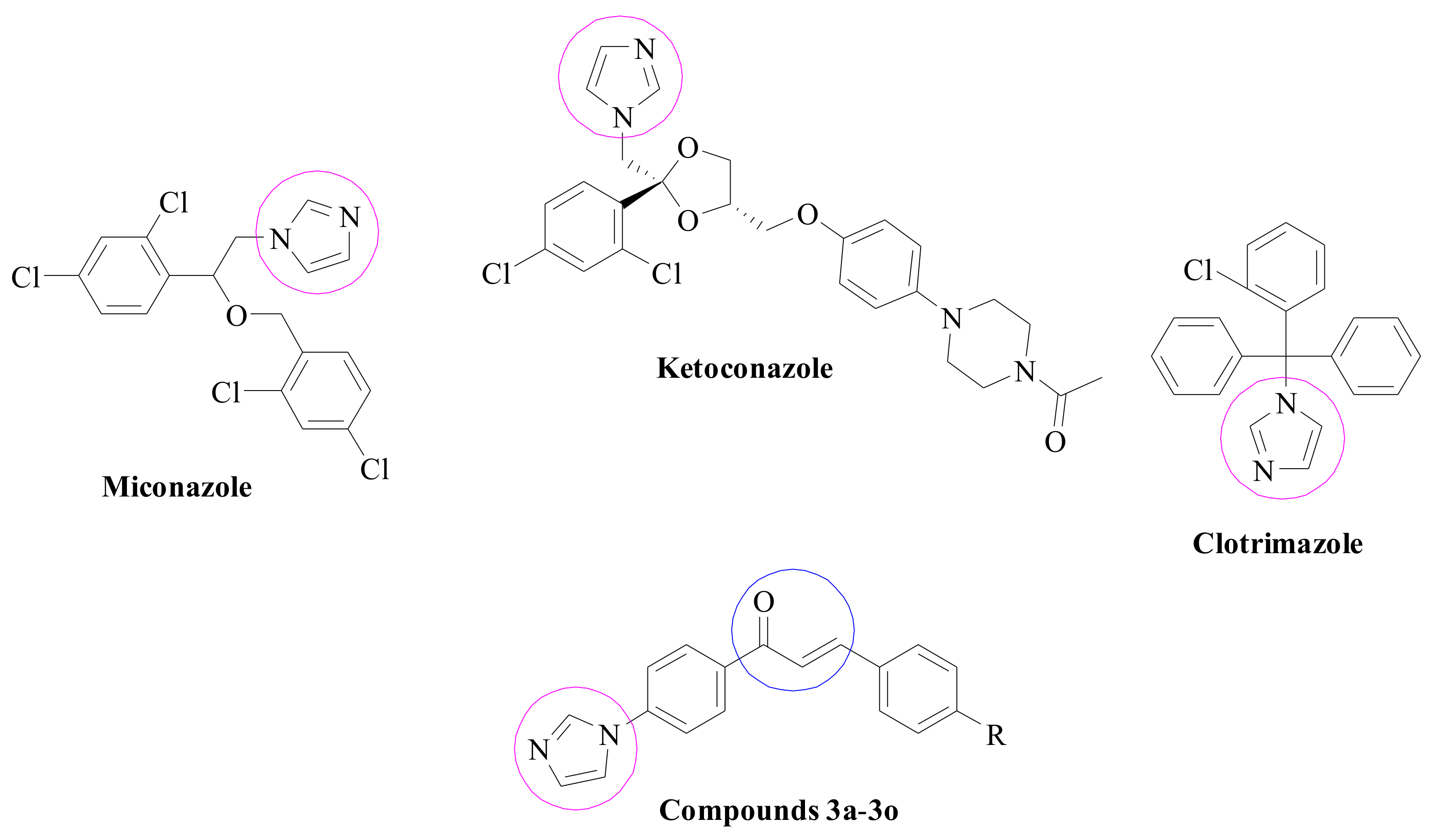
1. Introduction
2. Results and Discussion
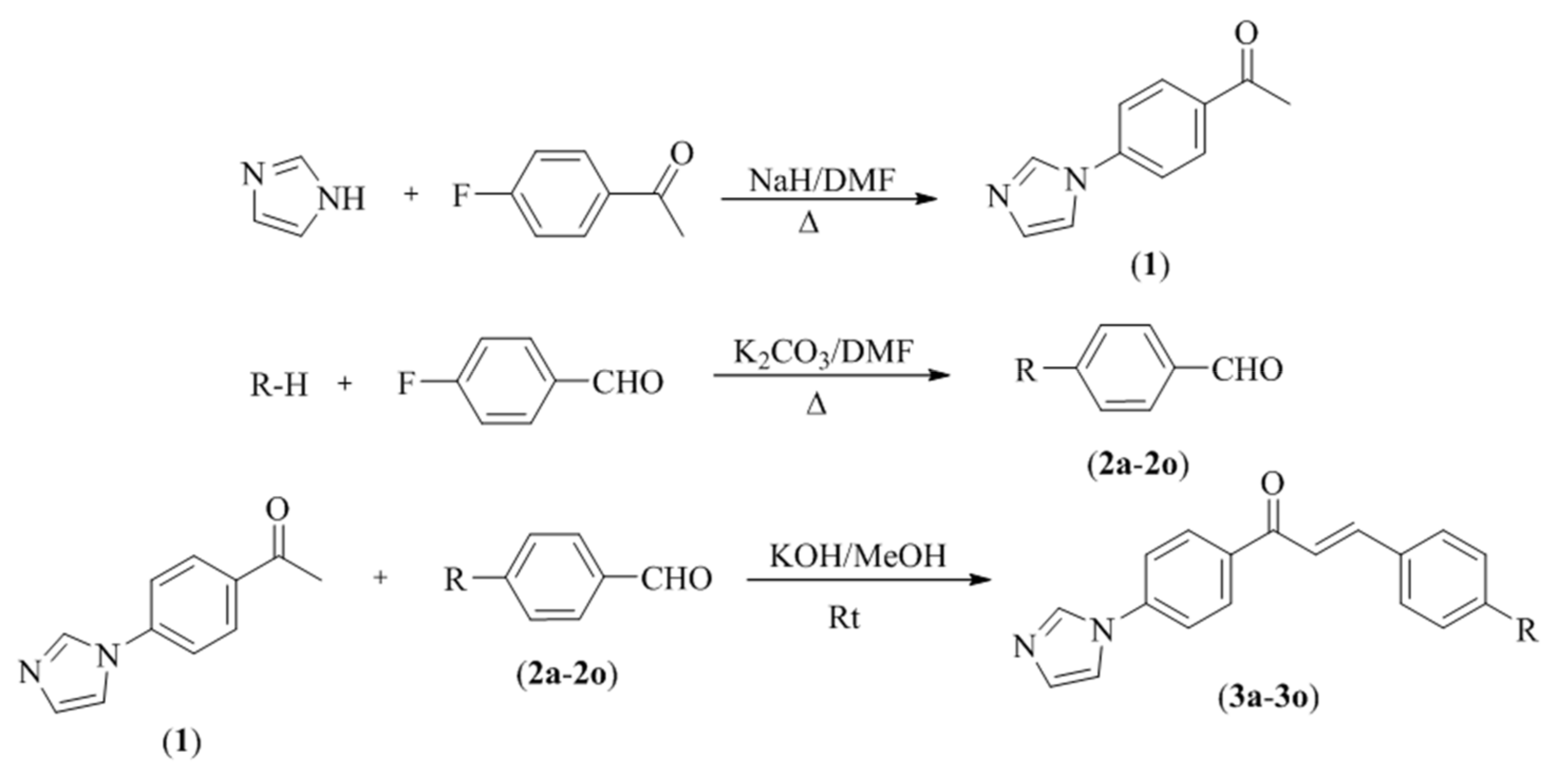
2.1. Chemistry
2.2. Antifungal Activity
2.3. Quantification of the Ergosterol Level
2.4. Cytotoxicity Test
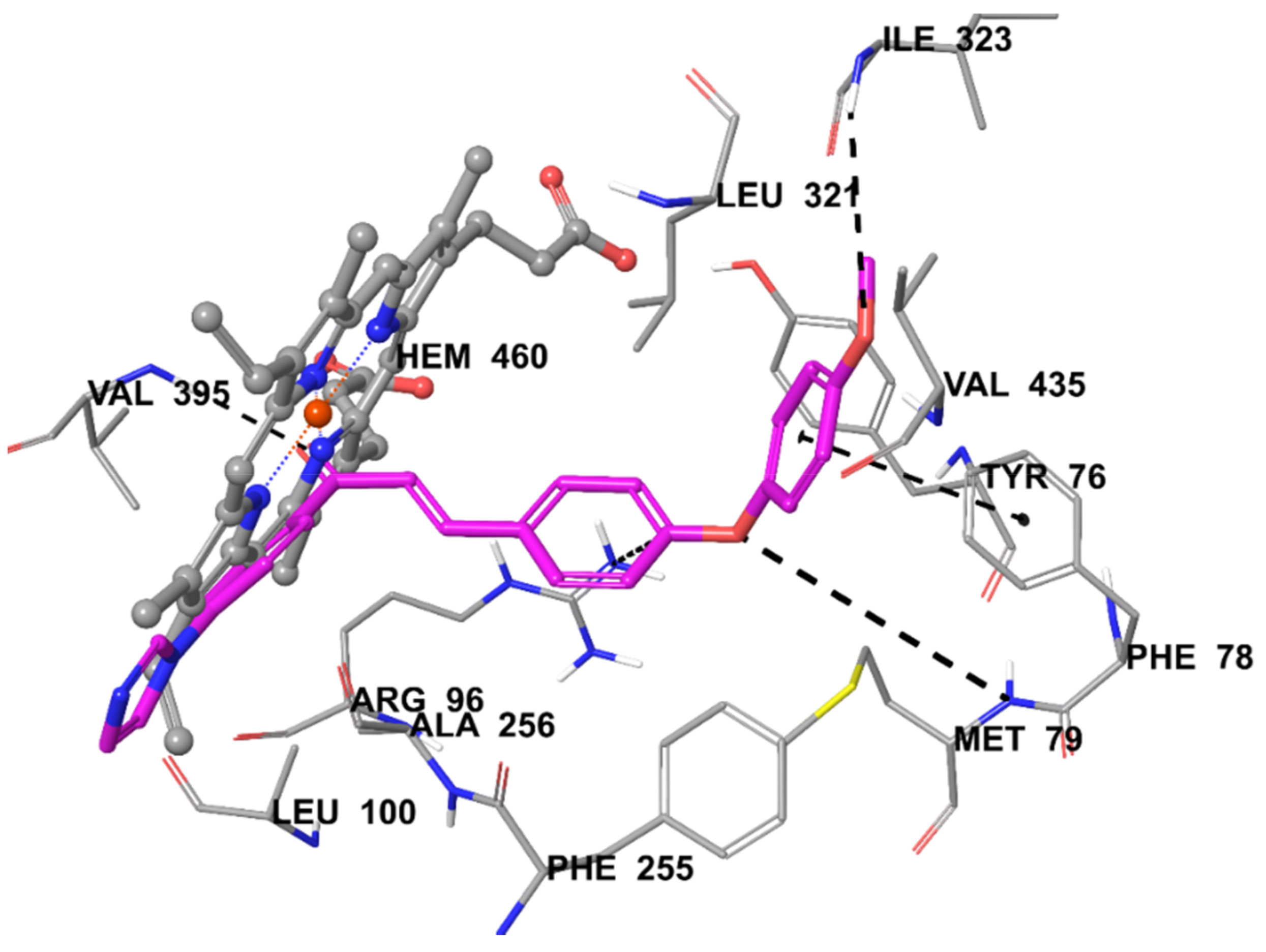
2.5. Molecular Docking Studies
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of 4′-(Imidazol-1-yl)acetophenone (1)
3.1.2. Synthesis of Four Substituted Benzaldehydes (2a–2o)
3.1.3. General Procedure for the Synthesis of Target Compounds (3a–3o)
3.2. Antifungal Activity
3.3. Quantification of Ergosterol Level
3.4. Cytotoxicity Test
3.5. Molecular Docking Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, H.; Wub, J.; Zhang, W.; Zhao, L.; Zhang, Y.H.; Shen, C.W. Design, Synthesis and Biological Evaluation of Novel Non-Azole Derivatives as Potential Antifungal Agents. Chin. Chem. Lett. 2015, 26, 1161–1164. [Google Scholar] [CrossRef]
- Canuto, M.M.; Rodero, F.G. Antifungal Drug Resistance to Azoles and Polyenes. Infect. Dis. 2002, 2, 550–563. [Google Scholar] [CrossRef]
- Jiang, Z.; Gu, J.; Wang, C.; Wang, S.; Liu, N.; Jiang, Y.; Dong, G.; Wang, Y.; Liu, Y.; Yao, J.; et al. Design, synthesis and antifungal activity of novel triazole derivatives containing substituted 1,2,3-triazole-piperdine side chains. Eur. J. Med. Chem. 2014, 82, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Cast’on-Osorio, J.J.; Rivero, A.; Torre-Cisneros, J. Epidemiology of Invasive Fungal Infection. Int. J. Antimicrob. Agents 2008, 32, 103–109. [Google Scholar] [CrossRef]
- Marchetti, O.; Bille, J.; Fluckiger, U.; Eggimann, P.; Ruef, C.; Garbino, J.; Calandra, T.; Glauser, M.P.; Tauber, M.G.; Pittet, D. Fungal Infection Network of Switzerland (FUNGINOS), Epidemiology of Candidemia in Swiss Tertiary Care Hospitals: Secular Trends, 1991–2000. Clin. Infect. Dis. 2003, 38, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Viviani, M.A.; Arathoon, E.; Chiou, C.; Ghannous, M.; Groll, A.H.; Odds, F.C. New targets and delivery systems for antifungal therapy. Med. Mycol. 2000, 38, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B. Antifungal therapy in oropharyngeal mycotic infections. Oral Surg. Oral Med. Oral Pathol. 1990, 69, 32–41. [Google Scholar] [CrossRef]
- Guida, R.A. Candidiasis of the oropharynx and esophagus. Ear Nose Throat J. 1988, 67, 832–840. [Google Scholar] [PubMed]
- Warrilow, A.G.; Parker, J.E.; Kelly, D.E.; Kelly, S.L. Azole Affinity of Sterol 14α-Demethylase (CYP51) Enzymes from Candida albicans and Homo sapiens. Antimicrob. Agents Chemother. 2013, 57, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Lupetti, A.; Danesi, R.; Campa, M.; Del Tacca, M.; Kelly, S. Molecular basis of resistance to azole antifungals. Mol. Med. 2002, 8, 77–81. [Google Scholar] [CrossRef]
- Moraca, F.; De Vita, D.; Pandolfi, F.; Di Santo, R.; Costi, R.; Cirilli, R.; D’Auria, F.D.; Panella, S.; Palamara, A.T.; Simonetti, G.; et al. Synthesis, biological evaluation and structureeactivity correlation study of a series of imidazol-based compounds as Candida albicans inhibitors. Eur. J. Med. Chem. 2014, 83, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Downer-Riley, N.K.; Jackson, Y.A. Recent Advances in the Synthesis of 1,3-Azoles. Curr. Top. Med. Chem. 2016, 16, 3617–3626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Z.; Gan, L.L.; Wang, H.; Zhou, C.H. New Progress in Azole Compounds as Antimicrobial Agents. Mini Rev. Med. Chem. 2017, 17, 122–166. [Google Scholar] [CrossRef] [PubMed]
- Kaplancikli, Z.A.; Turan-Zitouni, G.; Özdemir, A.; Revial, G. Synthesis and anticandidal activity of some imidazopyridine derivatives. J. Enzym. Inhib. Med. Chem. 2008, 23, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Reis, D.; Recio Despaigne, A.A.; Da Silva, J.G.; Silva, N.F.; Vilela, C.F.; Mendes, I.C.; Takahashi, J.A.; Beraldo, H. Structural Studies and Investigation on the Activity of Imidazole-Derived Thiosemicarbazones and Hydrazones against Crop-Related Fungi. Molecules 2013, 18, 12645–12662. [Google Scholar] [CrossRef] [PubMed]
- Niimi, M.; Firth, N.A.; Cannon, R.D. Antifungal drug resistance of oral fungi. Odontology 2010, 98, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Groll, A.H.; Chiou, C.; Walsh, T.J. Newer systemic antifungal agents. Drugs 2004, 64, 1997–2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ramamoorthy, Y.; Kılıcarslan, T.; Nolte, H.; Tyndale, R.F.; Sellers, E.M. Inhıbıtıon of Cytochromes P450 by Antıfungal Imıdazole Derıvatıves Drug Metabolısm And Dısposıtıon. DMD 2002, 30, 314–318. [Google Scholar] [CrossRef]
- Lopez, S.N.; Castelli, M.V.; Zacchino, S.A.; Domınguez, J.N.; Lobo, G.; Charris-Charris, J.; Corte, J.G.C.; Ribas, J.C.; Devia, C.; Rodrı´guezd, A.M.; et al. In Vitro Antifungal Evaluation and Structure-Activity Relationships of a New Series of Chalcone Derivatives and Synthetic Analogues, with Inhibitory Properties against Polymers of the Fungal Cell Wall. Bioorg. Med. Chem. 2001, 9, 1999–2013. [Google Scholar] [CrossRef]
- Batovska, D.; Parushev, S.; Slavova, A.; Bankova, V.; Tsvetkova, I.; Ninova, M.; Najdenski, H. Study on the substituents’ effects of a series of synthetic chalcones against the yeast Candida albicans. Eur. J. Med. Chem. 2007, 42, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, Z.; Kedzia, B.; Schroeder, G. Synthesis, physicochemical properties and antimicrobial evaluation of new (E)-chalcones. Eur. J. Med. Chem. 2008, 43, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Boecka, P.; Leala, P.C.; Yunesa, R.A.; Filhob, V.C.; Lopez, S.; Sortinoc, M.; Escalantec, A.; Furlanc, R.L.E.; Zacchinoc, S. Antifungal Activity and Studies on Mode of Action of Novel Xanthoxyline-Derived Chalcones. Arch. Pharm. Chem. Life Sci. 2005, 338, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lahtchev, K.L.; Batovska, D.I.; Parushev, S.P.; Ubiyvovk, V.M.; Sibirny, A.A. Antifungal activity of chalcones: A mechanistic study using various yeast strains. Eur. J. Med. Chem. 2008, 43, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Borra, R.C.; Lotufo, M.A.; Gagioti, S.M.; Barros Fde, M.; Andrade, P.M. A simple method to measure cell viability in proliferation and cytotoxicity assays. Braz. Oral Res. 2009, 23, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiter assay plate: Simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Ozkay, Y.; Tunalı, Y.; Karaca, H.; Işıkdağ, I. Antimicrobial activity of a new series of benzimidazole derivatives. Arch. Pharm. Res. 2011, 34, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Bard, M.; Lees, N.D.; Turi, T.; Craft, D.; Cofrin, L.; Barbuch, R.; Koegel, C.; Loper, J.C. Sterol synthesis and viability of erg11 (cytochrome P450 lanosterol demethylase) mutations in Saccharomyces cerevisiae and Candida albicans. Lipids 1993, 28, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Gooday, G.W. Cell membrane. In The Growing Fungus; Chapman & Hall: London, UK, 1995; pp. 62–64. [Google Scholar]
- Gollapudy, R.; Ajmani, S.; Kulkarni, S.A. Modeling and interactions of Aspergillus fumigatus lanosterol 14-α demethylase ‘A’ with azole antifungals. Bioorg. Med. Chem. 2004, 12, 2937–2950. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.A.; Sagartz, J.E.; Morris, D.L. The application of discovery toxicology and pathology towards the design of safer pharmaceutical lead candidates. Nat. Rev. Drug Discov. 2007, 6, 636–649. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity ISO-10993-5, 3rd ed.; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- González-Chávez, R.; Martínez, R.; Torre-Bouscoulet, M.E.; Gallo, M.; González-Chávez, M.M. De novo design of non-coordinating indolones as potential inhibitors for lanosterol 14-α-demethylase (CYP51). Chem. Pharm. Bull. 2014, 62, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Podust, L.M.; Poulos, T.L.; Waterman, M.R. Crystal structure of cytochrome P450 14α-sterol demethylase (CYP51) from Mycobacterium tuberculosis in complex with azole inhibitors. Proc. Natl. Acad. Sci. USA 2001, 98, 3068–3073. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Definitive Document EDef 7.1: Method for the Determination of Broth Dilution MICs of Antifungal Agents for Fermentative Yeasts. Clin. Microbiol. Infect. 2008, 14, 398. [Google Scholar]
- Breivik, O.N.; Owades, J.L. Spectrophotometric semi-microdetermination of ergosterol in yeast. Agric. Food Chem. 1957, 5, 360–363. [Google Scholar] [CrossRef]
- Karaca Gençer, H.; Acar Çevik, U.; Levent, S.; Sağlık, B.N.; Korkut, B.; Özkay, Y.; Ilgın, S.; Öztürk, Y. New Benzimidazole-1,2,4-Triazole Hybrid Compounds: Synthesis, Anticandidal Activity and Cytotoxicity Evaluation. Molecules 2017, 22, 507. [Google Scholar] [CrossRef] [PubMed]
- Sağlık, B.N.; Ilgın, S.; Özkay, Y. Synthesis of new donepezil analogues and investigation of their effects on cholinesterase enzymes. Eur. J. Med. Chem. 2016, 124, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Demir Özkay, Ü.; Can, Ö.D.; Sağlık, B.N.; Acar Çevik, U.; Levent, S.; Özkay, Y.; Ilgın, S.; Atlı, Ö. Design, synthesis, and AChE inhibitory activity of new benzothiazole-piperazines. Bioorg. Med. Chem. Lett. 2016, 26, 5387–5394. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Gheewala, N.; Suthar, A.; Shah, A. In-vitro cytotoxicity activity of Solanum nigrum extract against Hela cell line and Vero cell line. Int. J. Pharm. Pharm. Sci. 2009, 1, 38–46. [Google Scholar]
- Maestro, version 10.6; Schrödinger, LLC: New York, NY, USA, 2016.
- Schrödinger, version 2016-2; LLC: New York, NY, USA, 2016.
- LigPrep, version 3.8; Schrödinger, LLC: New York, NY, USA, 2016.
- Glide, version 7.1; Schrödinger, LLC: New York, NY, USA, 2016.
Sample Availability: Samples of the compounds 3a–3o are available from the authors. |
| Compounds | R |
|---|---|
| 3a | 4-methylphenoxy |
| 3b | 4-methylphenylthio |
| 3c | 4-methoxyphenoxy |
| 3d | 4-methoxyphenylthio |
| 3e | pyrrolidinyl |
| 3f | morpholinyl |
| 3g | piperidinyl |
| 3h | 3-methylpiperidinyl |
| 3i | 4-methylpiperidinyl |
| 3j | 3,5-dimethylpiperidinyl |
| 3k | 4-benzylpiperidinyl |
| 3l | 4-methylpiperazinyl |
| 3m | 4-ethylpiperazinyl |
| 3n | 4-(2-dimethylaminoethyl)piperazinyl |
| 3o | 4-(3-dimethylaminopropyl)piperazinyl |
| Comp. | C. albicans | C. glabrata | C. krusei | C. parapsilosis |
|---|---|---|---|---|
| 3a | 3.125 | 3.125 | 0.78 | 0.78 |
| 3b | 3.125 | 3.125 | 0.78 | 0.78 |
| 3c | 1.56 | 0.78 | 0.78 | 0.78 |
| 3d | 1.56 | 0.78 | 1.56 | 3.125 |
| 3e | 50 | 12.50 | 12.50 | 12.50 |
| 3f | 50 | 12.50 | 12.50 | 12.50 |
| 3g | 50 | 12.50 | 25 | 12.50 |
| 3h | 50 | 12.50 | 25 | 12.50 |
| 3i | 50 | 12.50 | 12.50 | 12.50 |
| 3j | 50 | 12.50 | 25 | 25 |
| 3k | 50 | 25 | 25 | 12.50 |
| 3l | 50 | 12.50 | 25 | 12.50 |
| 3m | 50 | 12.50 | 12.50 | 25 |
| 3n | 50 | 12.50 | 12.50 | 12.50 |
| 3o | 50 | 12.50 | 12.50 | 12.50 |
| Ketoconazole | 0.78 | 1.56 | 1.56 | 1.56 |
| Fluconazole | 0.78 | 1.56 | 1.56 | 0.78 |
| Compound | IC50 (µg/mL) | Inhibition of Ergosterol Biosynthesis (%) | ||
|---|---|---|---|---|
| 0.78 µg/mL | 1.56 µg/mL | 3.12 µg/mL | ||
| 3a | >500 | 66.19 ± 2.23 | 79.45 ± 3.16 | 86.47 ± 4.77 |
| 3b | >500 | 68.59 ± 1.98 | 81.62 ± 4.07 | 83.49 ± 3.18 |
| 3c | 436.04 ± 1.03 | 71.14 ± 4.9 | 78.16 ± 2.70 | 84.28 ± 4.65 |
| 3d | 387.64 ± 20.20 | 52.47 ± 1.83 | 67.14 ± 2.70 | 74.14 ± 2.21 |
| Ketoconazole | - | 60.99 ± 2.94 | 73.12 ± 4.16 | 84.56 ± 3.01 |
| Fluconazole | - | 61.74 ± 1.70 | 70.12 ± 3.22 | 82.13 ± 4.45 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osmaniye, D.; Kaya Cavusoglu, B.; Saglik, B.N.; Levent, S.; Acar Cevik, U.; Atli, O.; Ozkay, Y.; Kaplancikli, Z.A. Synthesis and Anticandidal Activity of New Imidazole-Chalcones. Molecules 2018, 23, 831. https://doi.org/10.3390/molecules23040831
Osmaniye D, Kaya Cavusoglu B, Saglik BN, Levent S, Acar Cevik U, Atli O, Ozkay Y, Kaplancikli ZA. Synthesis and Anticandidal Activity of New Imidazole-Chalcones. Molecules. 2018; 23(4):831. https://doi.org/10.3390/molecules23040831
Chicago/Turabian StyleOsmaniye, Derya, Betul Kaya Cavusoglu, Begum Nurpelin Saglik, Serkan Levent, Ulviye Acar Cevik, Ozlem Atli, Yusuf Ozkay, and Zafer Asim Kaplancikli. 2018. "Synthesis and Anticandidal Activity of New Imidazole-Chalcones" Molecules 23, no. 4: 831. https://doi.org/10.3390/molecules23040831
APA StyleOsmaniye, D., Kaya Cavusoglu, B., Saglik, B. N., Levent, S., Acar Cevik, U., Atli, O., Ozkay, Y., & Kaplancikli, Z. A. (2018). Synthesis and Anticandidal Activity of New Imidazole-Chalcones. Molecules, 23(4), 831. https://doi.org/10.3390/molecules23040831





