Metabolomic Profiling of the White, Violet, and Red Flowers of Rhododendron schlippenbachii Maxim.
Abstract
:1. Introduction
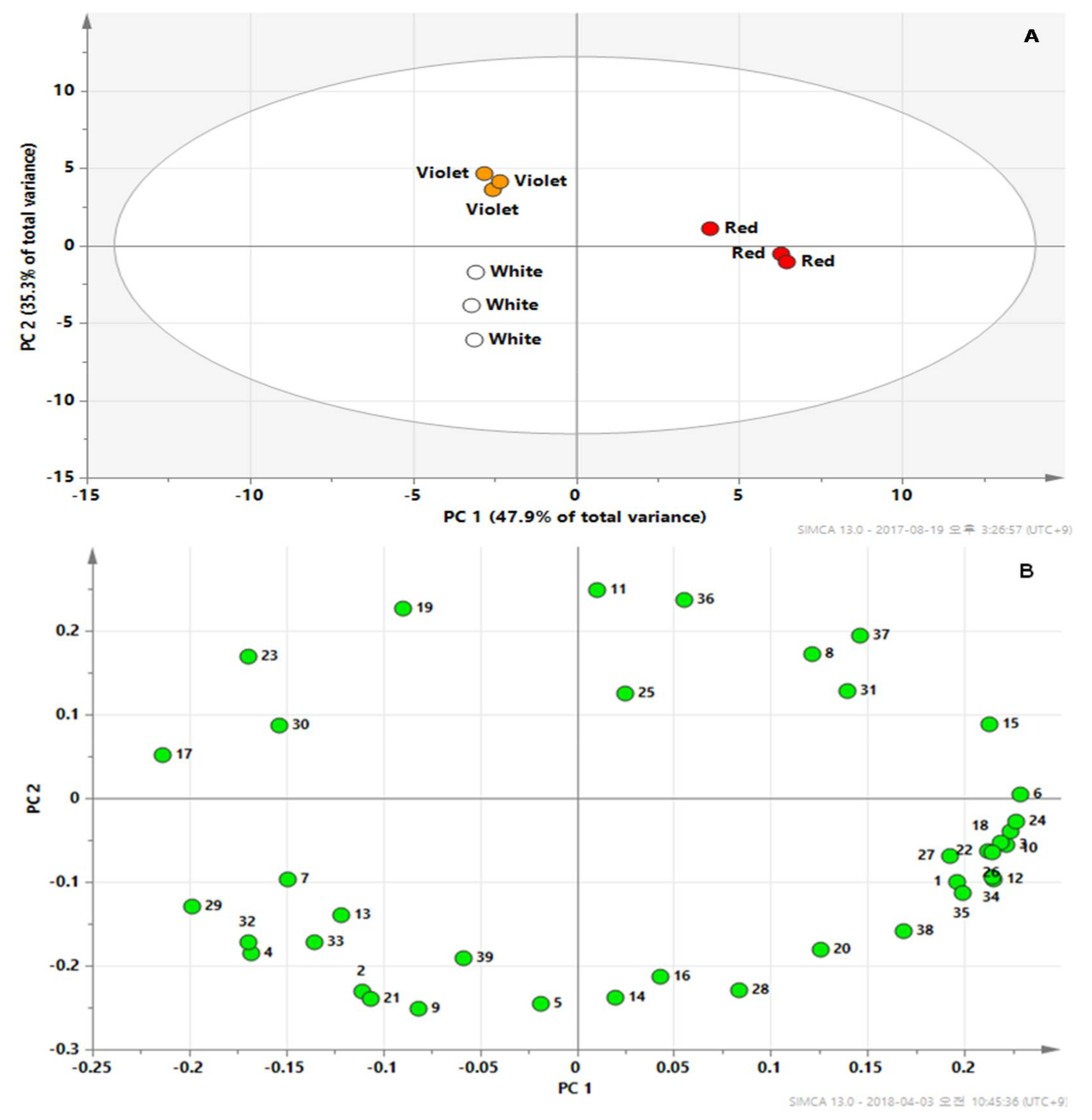
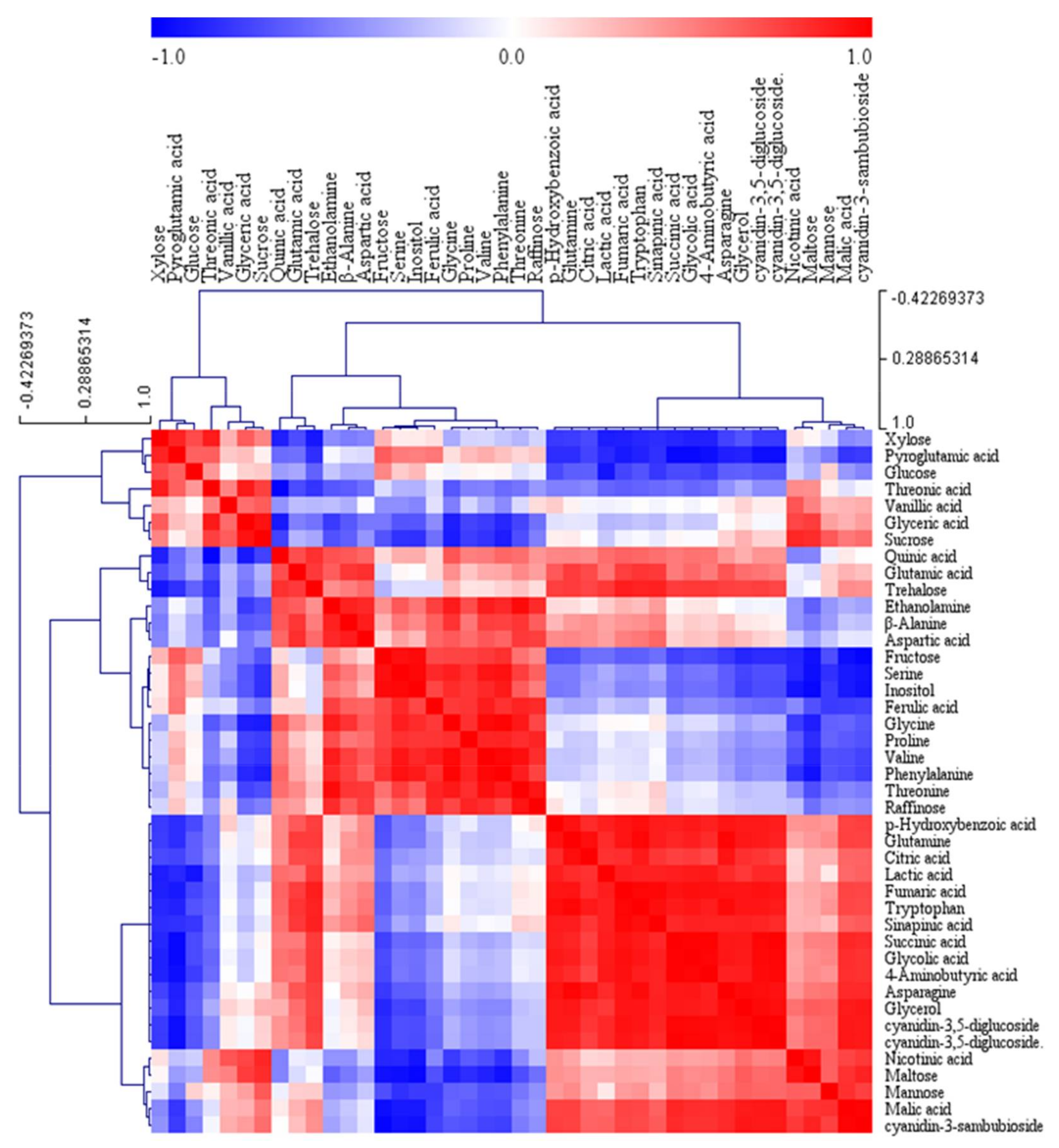
2. Results and Discussion
2.1. Anthocyanin Analysis
2.2. Metabolic Profiles Using GC-TOFMS Analysis
3. Materials and Methods
3.1. Plant Materials
3.2. Anthocyanin Extraction and HPLC Analysis
3.3. LC-MS/MS Analysis for the Quantification of Anthocyanin Contents
3.4. GC‒TOFMS Analysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carballeira, N.M.; Cartagena, M.; Tasdemir, D. Fatty acid composition of turkish Rhododendron species. J. Am. Oil Chem. Soc. 2008, 85, 605. [Google Scholar] [CrossRef]
- Popescu, R.; Kopp, B. The genus Rhododendron: An ethnopharmacological and toxicological review. J. Ethnopharmacol. 2013, 147, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, A.; Durmus, I.; Turedi, S.; Nuhoglu, I.; Ozturk, S. Mad honey poisoning-related asystole. Emerg. Med. J. 2007, 24, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, Ö.; Karakaş, F.P.; Pehlivan, D.; Nas, N. The antibacterial and antifungal effects of Rhododendron derived mad honey and extracts of four Rhododendron species. Turk. J. Biol. 2009, 33, 151–158. [Google Scholar]
- Kim, M.H.; Nugroho, A.; Choi, J.; Park, J.H.; Park, H.J. Rhododendrin, an analgesic/anti-inflammatory arylbutanoid glycoside, from the leaves of Rhododendron aureum. Arch. Pharm. Res. 2011, 34, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Bhandary, M.R.; Kawabata, J. Antidiabetic activity of Laligurans (Rhododendron arboreum Sm.) flower. J. Food Sci. Technol. Nepal 2009, 4, 61–63. [Google Scholar]
- Wang, C.M.; Hsu, Y.M.; Jhan, Y.L.; Tsai, S.J.; Lin, S.X.; Su, C.H.; Chou, C.H. Structure elucidation of procyanidins isolated from Rhododendron formosanum and their anti-oxidative and anti-bacterial activities. Molecules 2015, 20, 12787–12803. [Google Scholar] [CrossRef] [PubMed]
- Sancheti, S.; Um, B.H.; Seo, S.Y. 1,2,3,4,6-penta-O-galloyl-β-d-glucose: A cholinesterase inhibitor from Terminalia chebula. S. Afr. J. Bot. 2010, 76, 285–288. [Google Scholar] [CrossRef]
- Rafiq, M.; Sancheti, S.S.; Kim, H.-R.; You, Y.H.; Seo, S.Y. Antihyperglycemic and antioxidant activities of Rhododendron schlippenbachii maxim. Bark and its various fractions. J. Med. Plant Res. 2013, 7, 713–719. [Google Scholar]
- Sancheti, S.; Sancheti, S.; Lee, S.H.; Lee, J.E.; Seo, S.Y. Screening of Korean medicinal plant extracts for α-glucosidase inhibitory activities. Iran. J. Pharm. Res. 2011, 10, 261. [Google Scholar] [PubMed]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Clifford, M.N. Anthocyanins-nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Dey, P.M.; Harborne, J.B. Plant Phenolics. In Methods in Plant Biochemistry, 1st ed.; Academic Press Ltd.: London, UK, 1989; Volume 1, pp. 1–608. [Google Scholar]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Galvano, F.; La Fauci, L.; Lazzarino, G.; Fogliano, V.; Ritieni, A.; Ciappellano, S.; Battistini, N.C.; Tavazzi, B.; Galvano, G. Cyanidins: Metabolism and biological properties. J. Nutr. Biochem. 2004, 15, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Min, S.W.; Ryu, S.N.; Kim, D.H. Anti-inflammatory effects of black rice, cyanidin-3-O-β-d-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int. Immunopharmacol. 2010, 10, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Watanabe, M.; Ohshima, K.; Norinobu, S.; Choi, S.-W.; Kawakishi, S.; Osawa, T. Antioxidative activity of the anthocyanin pigments cyaniding-3-O-β-d-glucoside and cyanidin. J. Agric. Food Chem. 1994, 42, 2407–2410. [Google Scholar] [CrossRef]
- Amorini, A.M.; Lazzarino, G.; Galvano, F.; Fazzina, G.; Tavazzi, B.; Galvano, G. Cyanidin-3-O-β-glucopyranoside protects myocardium and erythrocytes from oxygen radical-mediated damages. Free Radic. Res. 2003, 37, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Miyashita, K.; Nakanishi, T.; Sano, M.; Tamano, S.; Kadota, T.; Koda, T.; Nakamura, M.; Imaida, K.; Ito, N. Pronounced inhibition by a natural anthocyanin, purple corn color, of 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP)-associated colorectal carcinogenesis in male F344 rats pretreated with 1, 2-dimethylhydrazine. Cancer Lett. 2001, 171, 17–25. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-O-β-d-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Fimognari, C.; Berti, F.; Cantelli-Forti, G.; Hrelia, P. Effect of cyanidin 3-O-β-glucopyranoside on micronucleus induction in cultured human lymphocytes by four different mutagens. Environ. Mol. Mutagen. 2004, 43, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Villas-Boas, S.G.; Nielsen, J.; Smedsgaard, J.; Hansen, M.A.; Roessner-Tunali, U. Metabolome Analysis: An Introduction, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 1–319. [Google Scholar]
- Roessner, U.; Luedemann, A.; Brust, D.; Fiehn, O.; Linke, T.; Willmitzer, L.; Fernie, A.R. Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell 2001, 13, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liang, Y.; Dunn, W.B.; Shen, H.; Kell, D.B. Comparative evaluation of software for deconvolution of metabolomics data based on GC-TOF-MS. TrAC Trends Anal. Chem. 2008, 27, 215–227. [Google Scholar] [CrossRef]
- Sumner, L.W.; Mendes, P.; Dixon, R.A. Plant metabolomics: Large-scale phytochemistry in the functional genomics era. Phytochemistry 2003, 62, 817–836. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry–based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Kopka, J.; Fernie, A.; Weckwerth, W.; Gibon, Y.; Stitt, M. Metabolite profiling in plant biology: Platforms and destinations. Genome Biol. 2004, 5, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.S. Amino Acid Content in Rhododendron schlippenbachii Maxim. Flowers of Different Colors. Biosci. Biotechnol. Res. Asia 2016, 13, 1285–1289. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Einhorn, T.C.; Cheng, L. Comparison of phenolic metabolism and primary metabolism between green ‘Anjou’pear and its bud mutation, red ‘Anjou’. Physiol. Plant. 2014, 150, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Chen, H.-H. Orchid Biotechnology II, 1st ed.; World scientific Publishing: Singapore, 2011; pp. 1–355. [Google Scholar]
- Mazza, G.; Miniati, E. Anthocyanins in Fruits, Vegetables, and Grains, 1st ed.; CRC Press: London, UK, 1993; pp. 1–362. [Google Scholar]
- Tsai, P.-J.; McIntosh, J.; Pearce, P.; Camden, B.; Jordan, B.R. Anthocyanin and antioxidant capacity in Roselle (Hibiscus sabdariffa L.) extract. Food Res. Int. 2002, 35, 351–356. [Google Scholar] [CrossRef]
- Wong, P.K.; Yusof, S.; Ghazali, H.; Che Man, Y. Physico-chemical characteristics of roselle (Hibiscus sabdariffa L.). Nutr. Food Sci. 2002, 32, 68–73. [Google Scholar] [CrossRef]
- Chen, F.; Sun, Y.; Zhao, G.; Liao, X.; Hu, X.; Wu, J.; Wang, Z. Optimization of ultrasound-assisted extraction of anthocyanins in red raspberries and identification of anthocyanins in extract using high-performance liquid chromatography-mass spectrometry. Ultrason. Sonochem. 2007, 14, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Goiffon, J.-P.; Mouly, P.P.; Gaydou, E.M. Anthocyanic pigment determination in red fruit juices, concentrated juices and syrups using liquid chromatography. Anal. Chim. Acta 1999, 382, 39–50. [Google Scholar] [CrossRef]
- Netzel, M.; Netzel, G.; Tian, Q.; Schwartz, S.; Konczak, I. Native Australian fruits—A novel source of antioxidants for food. Innov. Food Sci. Emerg. Technol. 2007, 8, 339–346. [Google Scholar] [CrossRef]
- Tanchev, S.; Timberlake, C. The anthocyanins of red cabbage (Brassica oleracea). Phytochemistry 1969, 8, 1825–1827. [Google Scholar] [CrossRef]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Twenty-five years of chemical ecology. Nat. Prod. Rep. 2001, 18, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, L.H.; Verpoorte, R. Secondary metabolism in tobacco. Plant Cell Tissue Org. Cult. 2002, 68, 105–125. [Google Scholar] [CrossRef]
- Yeoman, M.; Yeoman, C. Manipulating secondary metabolism in cultured plant cells. New Phytol. 1996, 134, 553–569. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Kim, N.S.; Eun, P.Y.; Kim, S.-J.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.-Y.; Kim, J.K.; Park, S.U. Metabolic profiling of pale green and purple kohlrabi (Brassica oleracea var. gongylodes). Appl. Biol. Chem. 2017, 60, 249–257. [Google Scholar] [CrossRef]
- Zulak, K.G.; Weljie, A.M.; Vogel, H.J.; Facchini, P.J. Quantitative 1 H NMR metabolomics reveals extensive metabolic reprogramming of primary and secondary metabolism in elicitor-treated opium poppy cell cultures. BMC Plant Biol. 2008, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Li, X.X.; Chu, Y.N.; Zhang, M.X.; Wen, Y.Q.; Duan, C.Q.; Pan, Q.H. Three types of ultraviolet irradiation differentially promote expression of shikimate pathway genes and production of anthocyanins in grape berries. Plant Physiol. Biochem. 2012, 57, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Muhlemann, J.K.; Maeda, H.; Chang, C.-Y.; San Miguel, P.; Baxter, I.; Cooper, B.; Perera, M.A.; Nikolau, B.J.; Vitek, O.; Morgan, J.A. Developmental changes in the metabolic network of snapdragon flowers. PLoS ONE 2012, 7, e40381. [Google Scholar] [CrossRef] [PubMed]
- Lancien, M.; Gadal, P.; Hodges, M. Enzyme redundancy and the importance of 2-oxoglutarate in higher plant ammonium assimilation. Plant Physiol. 2000, 123, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Scheible, W.R.; Krapp, A.; Stitt, M. Reciprocal diurnal changes of phosphoenolpyruvate carboxylase expression and cytosolic pyruvate kinase, citrate synthase and NADP-isocitrate dehydrogenase expression regulate organic acid metabolism during nitrate assimilation in tobacco leaves. Plant Cell Environ. 2000, 23, 1155–1167. [Google Scholar] [CrossRef]
- Park, C.H.; Baskar, T.B.; Park, S.-Y.; Kim, S.-J.; Valan Arasu, M.; Al-Dhabi, N.A.; Kim, J.K.; Park, S.U. Metabolic profiling and antioxidant assay of metabolites from three radish cultivars (Raphanus sativus). Molecules 2016, 21, 157. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Chae, S.C.; Park, S.-Y.; Kim, J.K.; Kim, Y.J.; Chung, S.O.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. Anthocyanin and carotenoid contents in different cultivars of chrysanthemum (Dendranthema grandiflorum Ramat.) flower. Molecules 2015, 20, 11090–11102. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Uddin, M.R.; Park, W.T.; Kim, Y.B.; Seo, J.M.; Kim, S.-J.; Nou, I.-S.; Lee, J.; Kim, H.; Park, S.U. Accumulation of anthocyanin and related genes expression during the development of cabbage seedlings. Process Biochem. 2014, 49, 1084–1091. [Google Scholar] [CrossRef]
- Kim, M.S.; Baek, S.-H.; Park, S.U.; Im, K.-H.; Kim, J.K. Targeted metabolite profiling to evaluate unintended metabolic changes of genetic modification in resveratrol-enriched rice (Oryza sativa L.). Appl. Biol. Chem. 2017, 60, 205–214. [Google Scholar] [CrossRef]
Sample Availability: Samples of compounds are not available from the authors. |

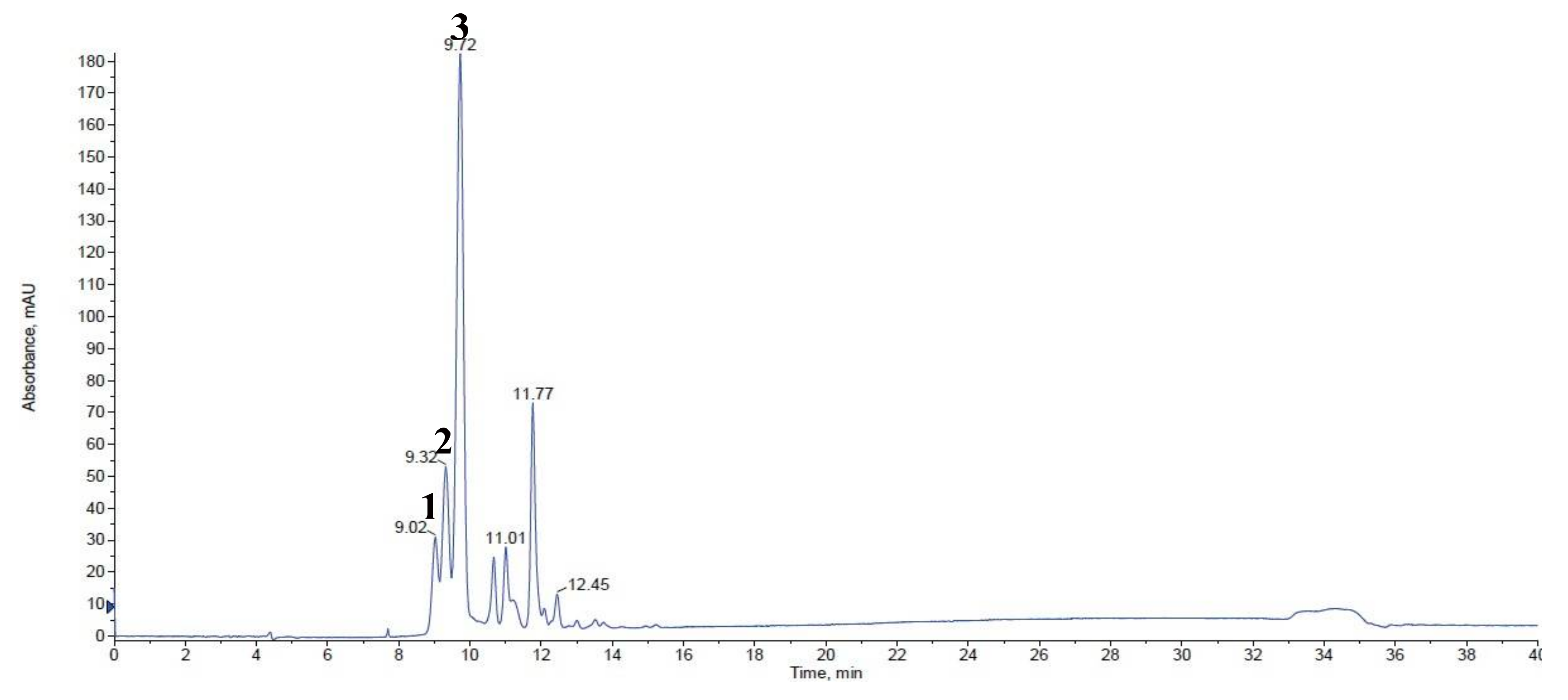
| No. 1 | Retention Time | Trivial Name | [M + H]+ (m/z) | MS/MS (m/z) | White | Violet | Red |
|---|---|---|---|---|---|---|---|
| 1 | 9.02 | cyanidin-3,5-diglucoside | 611 | 449/287 | ND 2 | ND | 0.11 ± 0.01 |
| 2 | 9.32 | cyanidin-3,5-diglucoside | 611 | 449/287 | ND | ND | 0.21 ± 0.00 |
| 3 | 9.72 | cyanidin-3-sambubioside | 581 | 287 | ND | 0.30 ± 0.01 b | 0.70 ± 0.01 a |
| Total | ND | 0.30 ± 0.01 b | 1.02 ± 0.02 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.H.; Yeo, H.J.; Kim, N.S.; Park, Y.E.; Park, S.-Y.; Kim, J.K.; Park, S.U. Metabolomic Profiling of the White, Violet, and Red Flowers of Rhododendron schlippenbachii Maxim. Molecules 2018, 23, 827. https://doi.org/10.3390/molecules23040827
Park CH, Yeo HJ, Kim NS, Park YE, Park S-Y, Kim JK, Park SU. Metabolomic Profiling of the White, Violet, and Red Flowers of Rhododendron schlippenbachii Maxim. Molecules. 2018; 23(4):827. https://doi.org/10.3390/molecules23040827
Chicago/Turabian StylePark, Chang Ha, Hyeon Ji Yeo, Nam Su Kim, Ye Eun Park, Soo-Yun Park, Jae Kwang Kim, and Sang Un Park. 2018. "Metabolomic Profiling of the White, Violet, and Red Flowers of Rhododendron schlippenbachii Maxim." Molecules 23, no. 4: 827. https://doi.org/10.3390/molecules23040827
APA StylePark, C. H., Yeo, H. J., Kim, N. S., Park, Y. E., Park, S.-Y., Kim, J. K., & Park, S. U. (2018). Metabolomic Profiling of the White, Violet, and Red Flowers of Rhododendron schlippenbachii Maxim. Molecules, 23(4), 827. https://doi.org/10.3390/molecules23040827





