Study of Photochemical Cytosine to Uracil Transition via Ultrafast Photo-Cross-Linking Using Vinylcarbazole Derivatives in Duplex DNA
Abstract
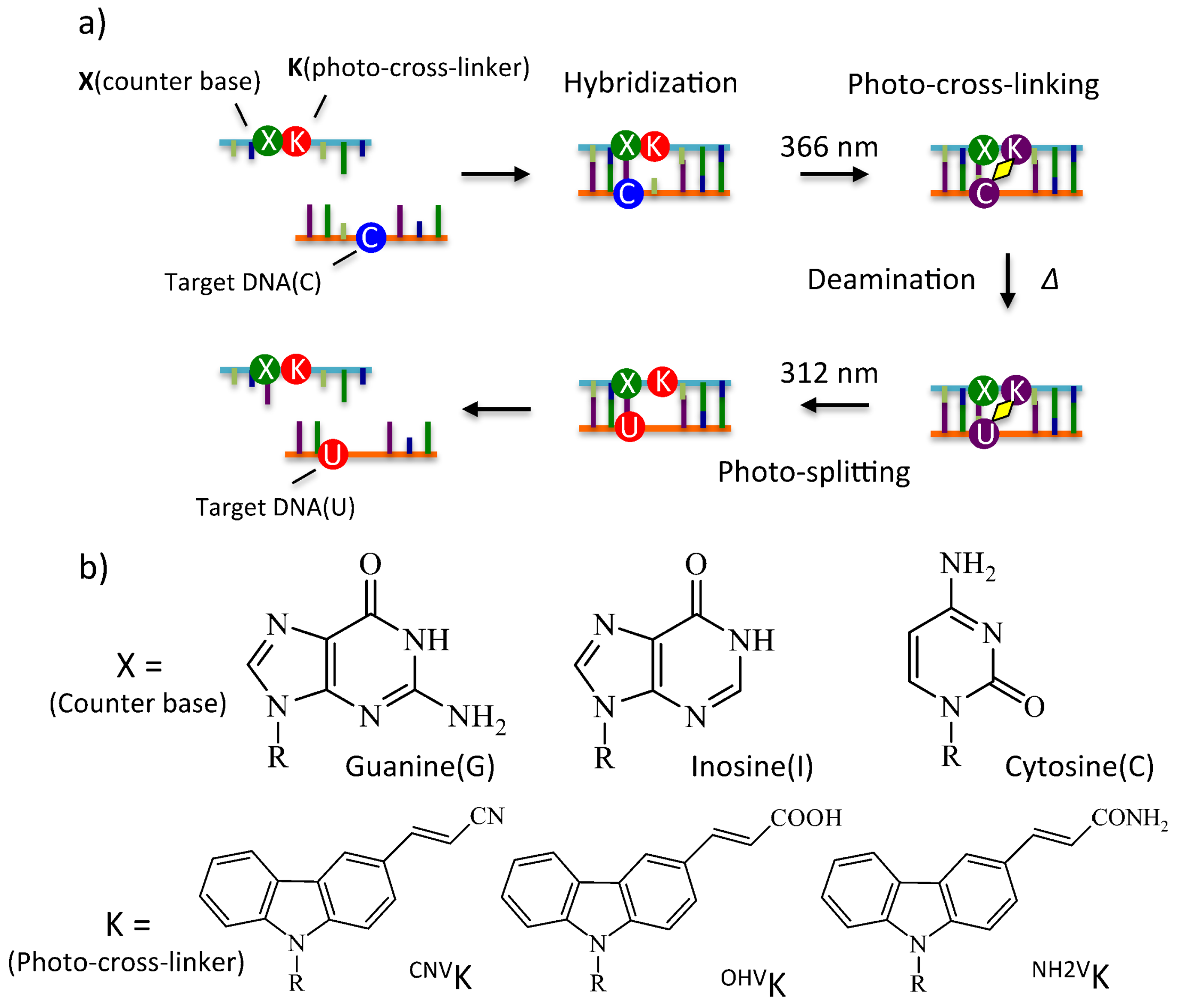
1. Introduction
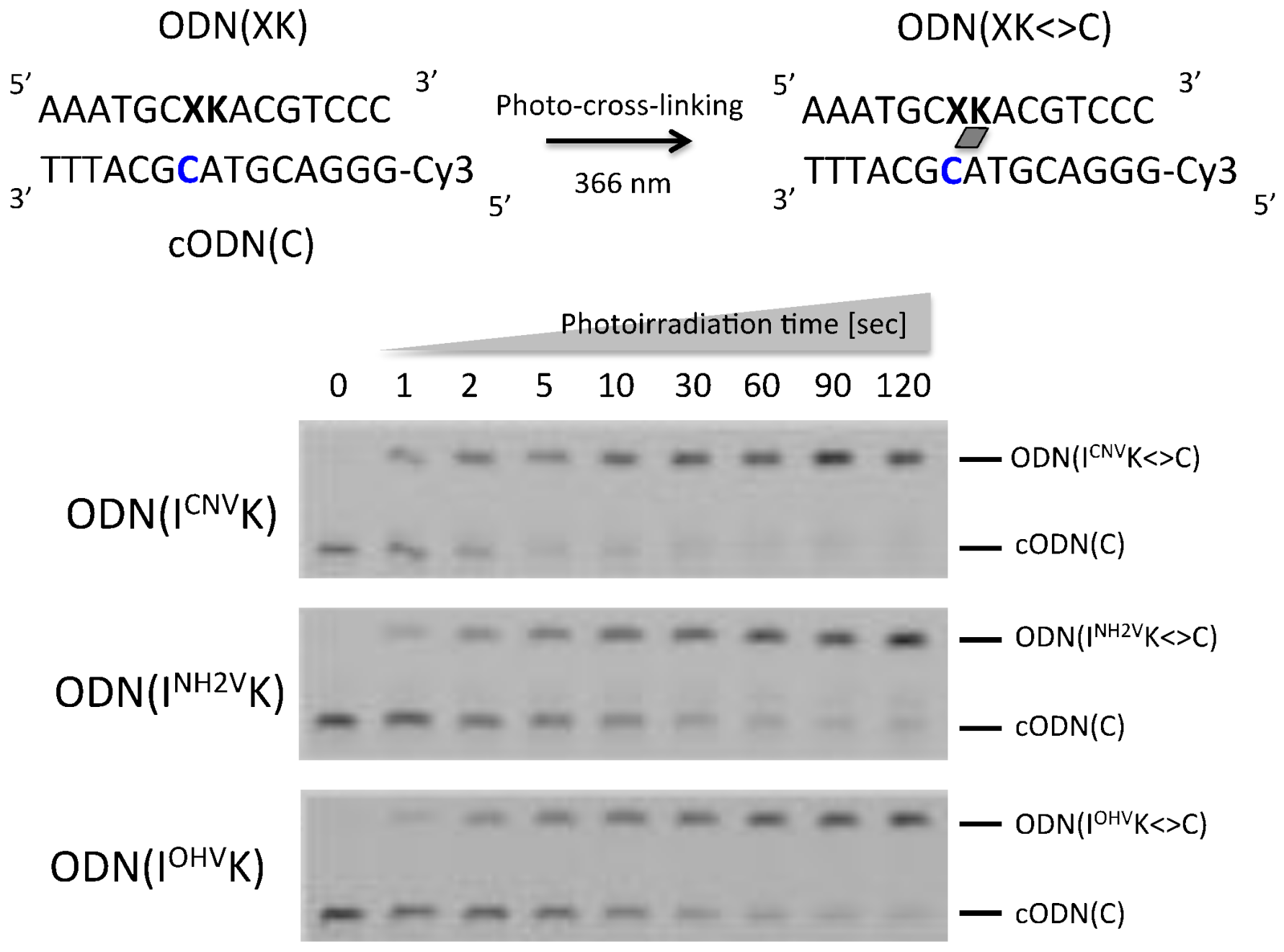
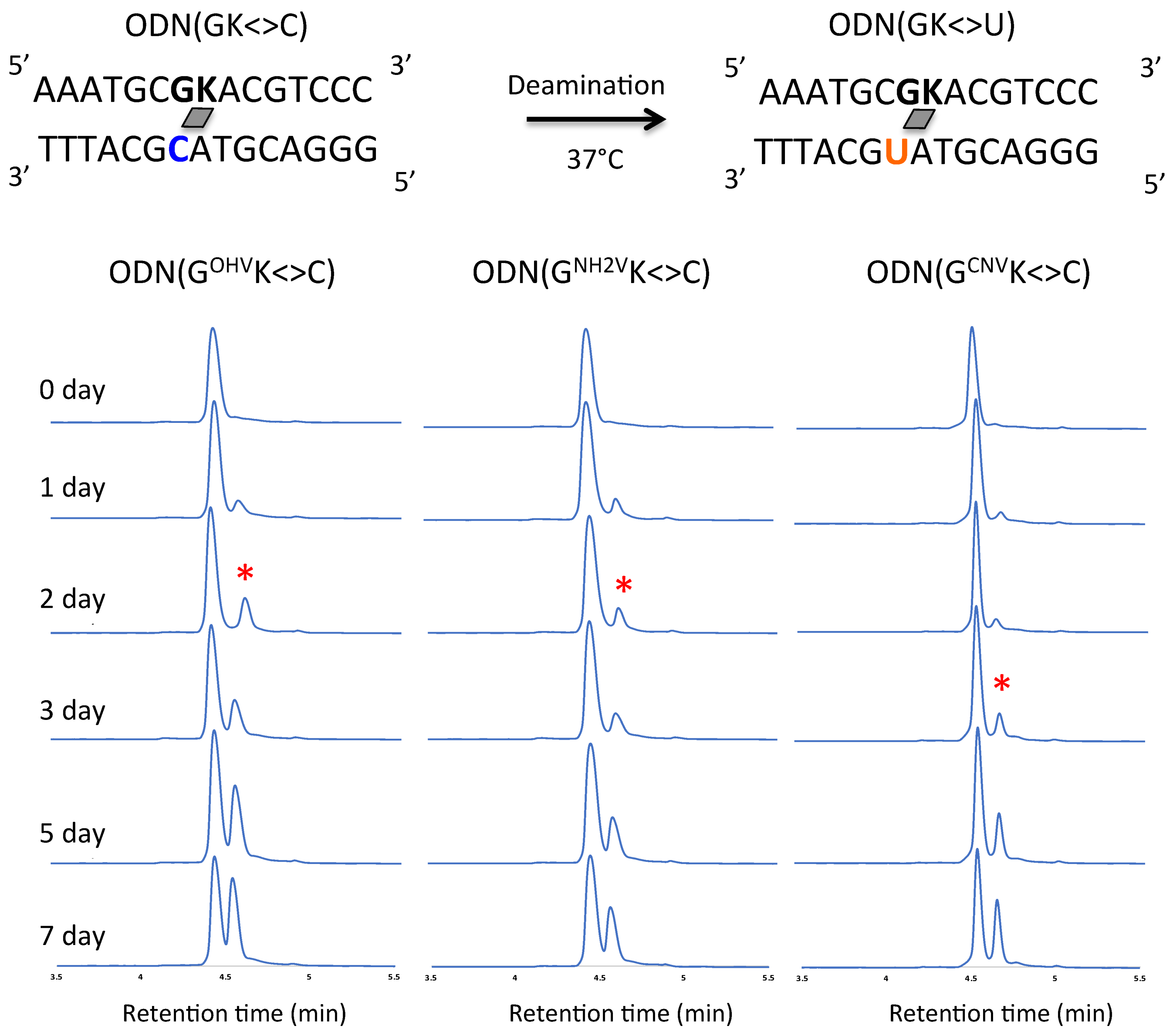
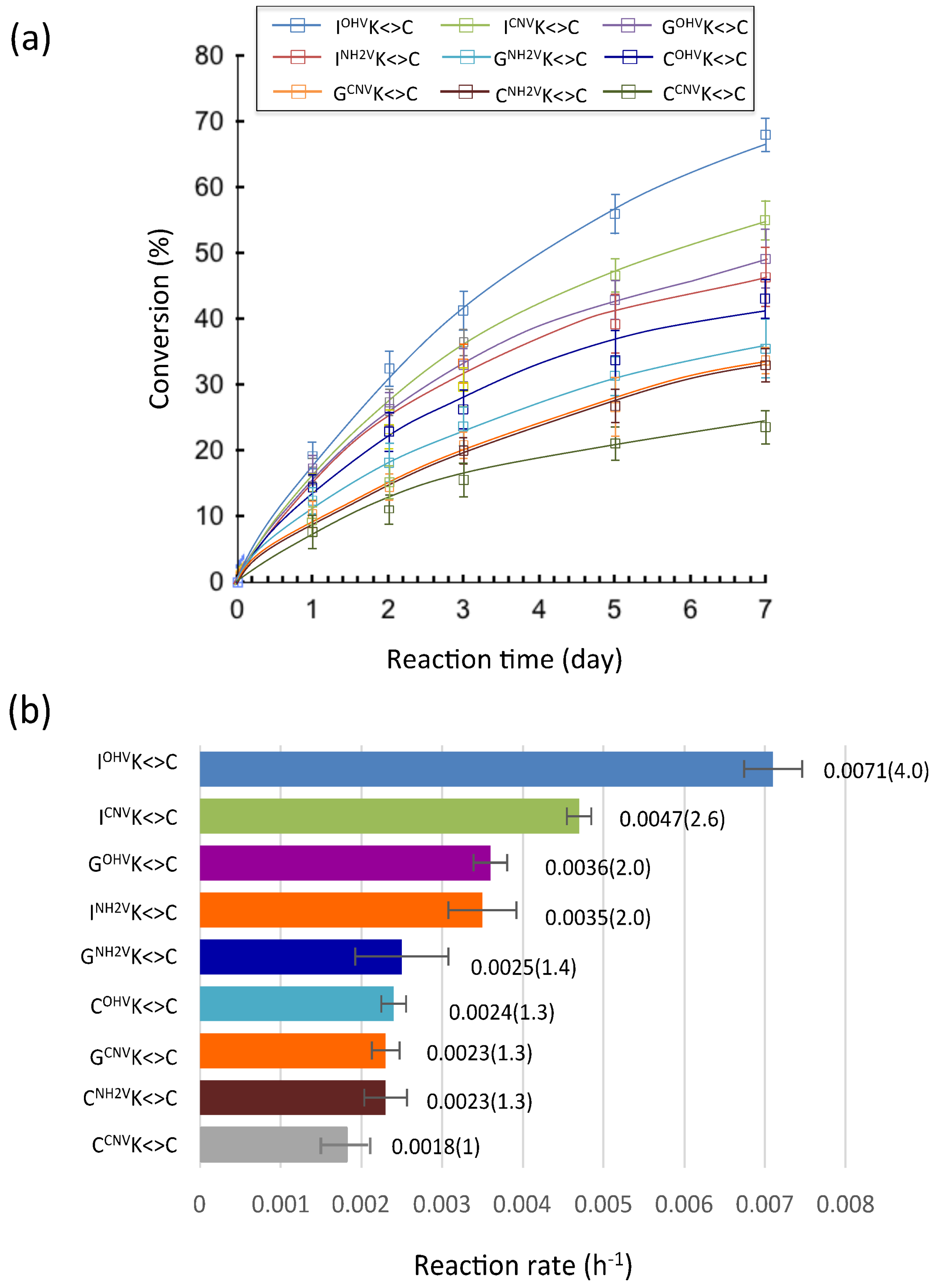
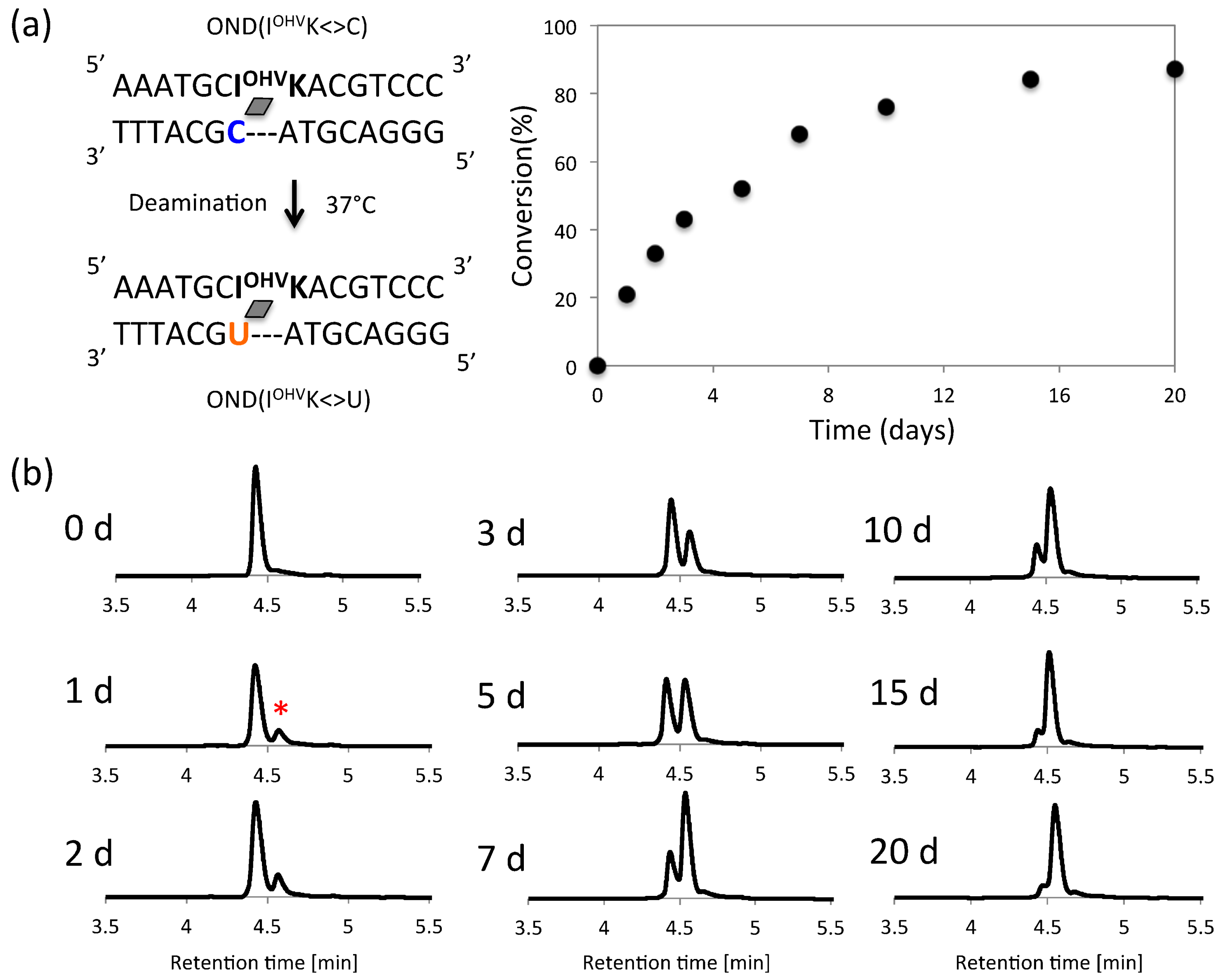
2. Results and Discussion
3. Materials and Methods
3.1. General
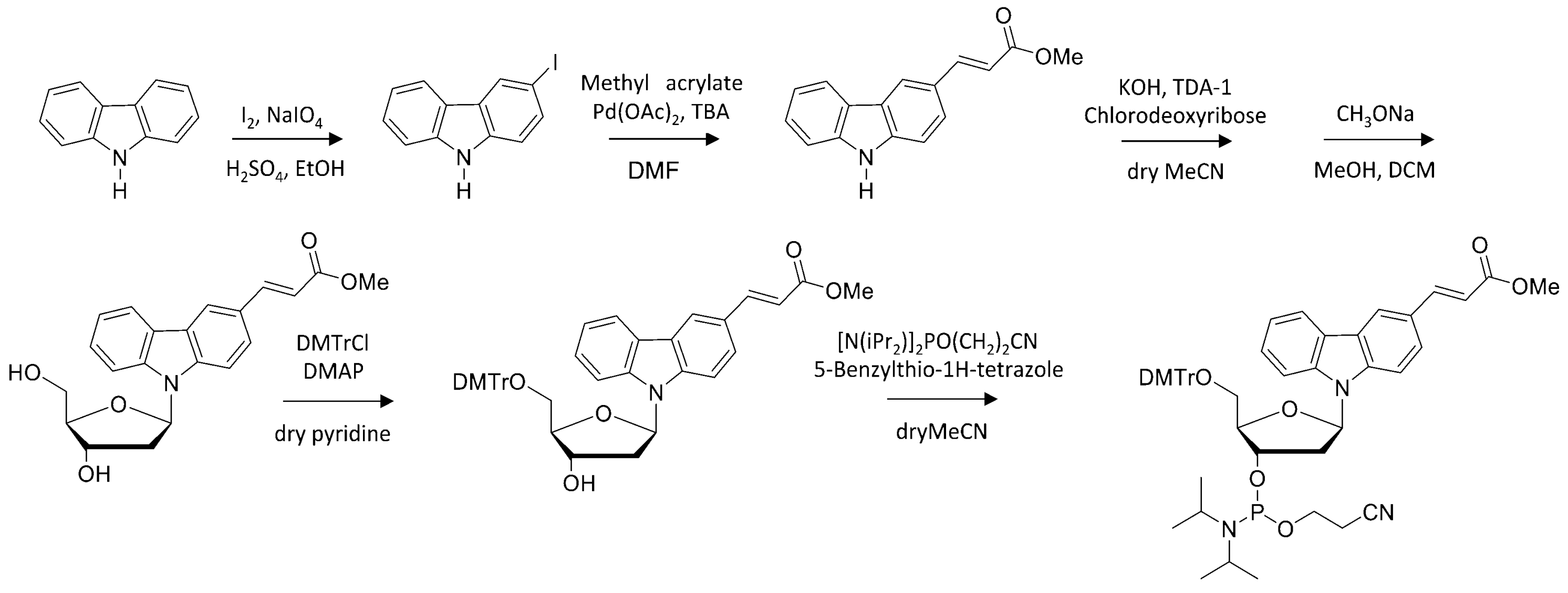
3.2. Synthesis and Preparation of Modified Oligonucleotides
3.3. Denaturing PAGE Analysis
3.4. Isolation of Photo-Cross-Linked dsDNA
3.5. Deamination
3.6. Photo-Splitting and UPLC Analysis
3.7. Partition Coefficient (LogP)
3.8. Enzymatic Digestion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cascalho, M. Advantages and disadvantages of cytidine deamination. J. Immunol. 2004, 172, 6513–6518. [Google Scholar] [CrossRef] [PubMed]
- Storici, F.; Lewis, L.K.; Resnick, M.A. In vivo site-directed mutagenesis using oligonucleotides. Nat. Biotechnol. 2001, 19, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Esvelt, K.M.; Wang, H.H. Genome-scale engineering for systems and synthetic biology. Mol. Syst. Biol. 2013, 9, 1–17. [Google Scholar] [CrossRef]
- Tan, W.S.; Carlson, D.F.; Walton, M.W.; Fahrenkrug, S.C.; Hackett, P.B. Precision editing of large animal genomes. Adv. Genet. 2012, 80, 37–97. [Google Scholar] [CrossRef] [PubMed]
- Puchta, H.; Fauser, F. Gene targeting in plants: 25 years later. Int. J. Dev. Biol. 2013, 57, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Sanger, J.D.; Joung, J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014, 32, 347–355. [Google Scholar] [CrossRef]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Bikard, D.; Cox, D.; Zhang, F.; Marraffini, L.A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Javier, N.D.; Michael, N.S. Altering the pathway of immunoglobulin hypermutation by inhibiting uracil-DNA glycosylase. Nature 2002, 419, 43–48. [Google Scholar] [CrossRef]
- White, M.K.; Kaminski, R.; Young, W.B.; Roehm, P.C.; Khalili, K. CRISPR Editing Technology in Biological and Biomedical Investigation. J. Cell. Biochem. 2017, 117, 3586–3594. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Kim, S.; Kim, J.M.; Kim, J.S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 2013, 31, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Tee, L.Y.; Wang, X.G.; Huang, Q.S.; Yang, S.H. Off-target Effects in CRISPR/Cas9-mediated Genome Engineering. Mol. Ther.-Nucleic Acids 2015, 4, e264. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.A.; Rahdar, M.; Porteus, M. Gene editing: Not just for translation anymore. Nat. Methods 2012, 9, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Grizot, S.; Arnould, S.; Duclert, A.; Epinat, J.C.; Chames, P.; Prieto, J.; Redondo, P.; Blanco, F.J.; Bravo, J.; et al. A combinatorial approach to create artificial homing endonucleases cleaving chosen sequences. Nucleic Acids Res. 2006, 34, e149. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kin, J. A guide to genome engineering with programmable nucleases. Nat. Rev. Genet. 2014, 15, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Frommer, M.; McDonald, L.E.; Millar, D.S.; Collis, C.M.; Watt, F.; Grigg, G.W.; Molloy, P.L.; Paul, C.L. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc. Natl. Acad. Sci. USA 1992, 89, 1827–1831. [Google Scholar] [CrossRef] [PubMed]
- Yamana, K.; Yoshikawa, A.; Nakano, H. Synthesis of a new photoisomerizable linker for connecting two oligonucleotide segments. Tetrahedron Lett. 1996, 37, 637–640. [Google Scholar] [CrossRef]
- Lee, B.L.; Blake, K.R.; Miller, P.S. Interaction of psoralen-derivatized oligodeoxyribonucleoside methylphosphonates with synthetic DNA containing a promoter for T7 RNA polymerase. Nucleic Acids Res. 1988, 16, 10681–10697. [Google Scholar] [CrossRef] [PubMed]
- Montes, C.V.; Memczak, H.; Gyssels, E.; Torres, T.; Madder, A.; Schneider, R.J. Photoinduced Cross-Linking of Short Furan-Modified DNA on Surfaces. Langmuir 2017, 33, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Kurz, M.; Gu, K.; Lohse, P.A. Psoralen photo-crosslinked mRNA-puromycin conjugates: A novel template for the rapid and facile preparation of mRNA-protein fusions. Nucleic Acids Res. 2000, 28, 83. [Google Scholar] [CrossRef]
- Liang, X.; Wakuda, R.; Fujioka, K.; Asanuma, H. Photoregulation of DNA transcription by using photoresponsive T7 promoters and clarification of its mechanism. FEBS J. 2010, 277, 1551–1561. [Google Scholar] [CrossRef]
- Liu, J.; Geng, Y.; Pound, E.; Gyawall, S.; Ashton, J.R.; Hickey, J.; Woolley, A.T.; Harb, J.N. Metallization of branched DNA origami for nanoelectronic circuit fabrication. ACS Nano 2011, 5, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Iwase, R.; Namba, M.; Yamaoka, T.; Murakami, A. Gene regulation by decoy approach (I): Synthesis and properties of photo-crosslinked oligonucleotides. Nucleic Acids Symp. Ser. 1997, 37, 203–204. [Google Scholar]
- Fujimoto, K.; Konishi-Hiratsuka, K.; Sakamoto, T.; Yoshimura, Y. Site-specific photochemical RNA editing. Chem. Commun. 2010, 46, 7545–7547. [Google Scholar] [CrossRef]
- Fujimoto, K.; Konishi-Hiratsuka, K.; Sakamoto, T.; Yoshimura, Y. Site-Specific Cytosine to Uracil Transition by Using Reversible DNA Photo-crosslinking. ChemBioChem 2010, 11, 1661–1664. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Ooe, M.; Fujimoto, K. Critical Effect of Base Pairing of Target Pyrimidine on the Interstrand Photo-Cross-Linking of DNA via 3-Cyanovinylcarbazole Nucleoside. Bioconj. Chem. 2015, 26, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Ooe, M.; Sakamoto, T.; Fujimoto, K. Effect of nucleobase change on cytosine deamination through DNA photo-cross-linking reaction via 3-cyanovinylcarbazole nucleoside. Mol. BioSyst. 2017, 13, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Yasuharu, T.; Nakamura, S.; Fujimoto, K. Effect of substitution of photo-cross-linker in photochemical cytosine to uracil transition in DNA. Bioorg. Med. Chem. Lett. 2017, 27, 3905–3908. [Google Scholar] [CrossRef] [PubMed]
- OECD Guidline for Testing of Chemicals. Available online: http://www.oecd.org/chemicalsafety/risk-assessment/1948177.pdf (accessed on 15 December 2017).
- Borges, N.M.; Kenny, P.W.; Montanari, C.A.; Prokopozyk, I.M.; Ribeiro, J.F.; Rocha, J.R.; Sartori, G.R. The influence of hydrogen bonding on partition coefficients. J. Comput.-Aided Mol. Des. 2017, 31, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Sklenak, S.; Yao, L.; Cukier, R.I.; Yan, H. Catalytic mechanism of yeast cytosine deaminase: An ONIOM computational study. J. Am. Chem. Soc. 2004, 126, 14879–14889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Yan, H.; Cao, Z. Combined QM(DFT)/MM molecular dynamics simulations of the deamination of cytosine by yeast cytosine deaminase (yCD). J. Comput. Chem. 2016, 37, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Fujimoto, K. Ultrafast reversible photo-cross-linking reaction: Toward in situ DNA manipulation. Org. Lett. 2008, 10, 3227–3230. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Entry | Sequence of ODNs (5′ to 3′) |
|---|---|
| ODN(GCNVK) | AAATGCGCNVKACGTCCC |
| ODN(GNH2VK) | AAATGCGNH2VKACGTCCC |
| ODN(GOHVK) | AAATGCGOHVKACGTCCC |
| ODN(CCNVK) | AAATGCCCNVKACGTCCC |
| ODN(CNH2VK) | AAATGCCNH2VKACGTCCC |
| ODN(COHVK) | AAATGCCOHVKACGTCCC |
| ODN(ICNVK) | AAATGCICNVKACGTCCC |
| ODN(INH2VK) | AAATGCINH2VKACGTCCC |
| ODN(IOHVK) | AAATGCIOHVKACGTCCC |
| Entry | Retention Time (min) | LogP | |
|---|---|---|---|
| ODN(IOHVK<>C) | 13.8 | 0.52 | |
| ODN(GOHVK<>C) | 14.2 | 0.54 | |
| ODN(COHVK<>C) | 14.7 | 0.57 | |
| ODN(INH2VK<>C) | 15.4 | 0.61 | |
| ODN(GNH2VK<>C) | 16.0 | 0.64 | |
| ODN(ICNVK<>C) | 16.3 | 0.66 | |
| ODN(GCNVK<>C) | 16.6 | 0.68 | |
| ODN(CCNVK<>C) | 17.3 | 0.72 | |
| ODN(CNH2VK<>C) | 17.8 | 0.75 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sethi, S.; Nakamura, S.; Fujimoto, K. Study of Photochemical Cytosine to Uracil Transition via Ultrafast Photo-Cross-Linking Using Vinylcarbazole Derivatives in Duplex DNA. Molecules 2018, 23, 828. https://doi.org/10.3390/molecules23040828
Sethi S, Nakamura S, Fujimoto K. Study of Photochemical Cytosine to Uracil Transition via Ultrafast Photo-Cross-Linking Using Vinylcarbazole Derivatives in Duplex DNA. Molecules. 2018; 23(4):828. https://doi.org/10.3390/molecules23040828
Chicago/Turabian StyleSethi, Siddhant, Shigetaka Nakamura, and Kenzo Fujimoto. 2018. "Study of Photochemical Cytosine to Uracil Transition via Ultrafast Photo-Cross-Linking Using Vinylcarbazole Derivatives in Duplex DNA" Molecules 23, no. 4: 828. https://doi.org/10.3390/molecules23040828
APA StyleSethi, S., Nakamura, S., & Fujimoto, K. (2018). Study of Photochemical Cytosine to Uracil Transition via Ultrafast Photo-Cross-Linking Using Vinylcarbazole Derivatives in Duplex DNA. Molecules, 23(4), 828. https://doi.org/10.3390/molecules23040828






