Effects of Basal Defoliation on Wine Aromas: A Meta-Analysis
Abstract
1. Introduction
2. Results and Discussion
2.1. The Effects of Basal Defoliation on Varietal Aromas
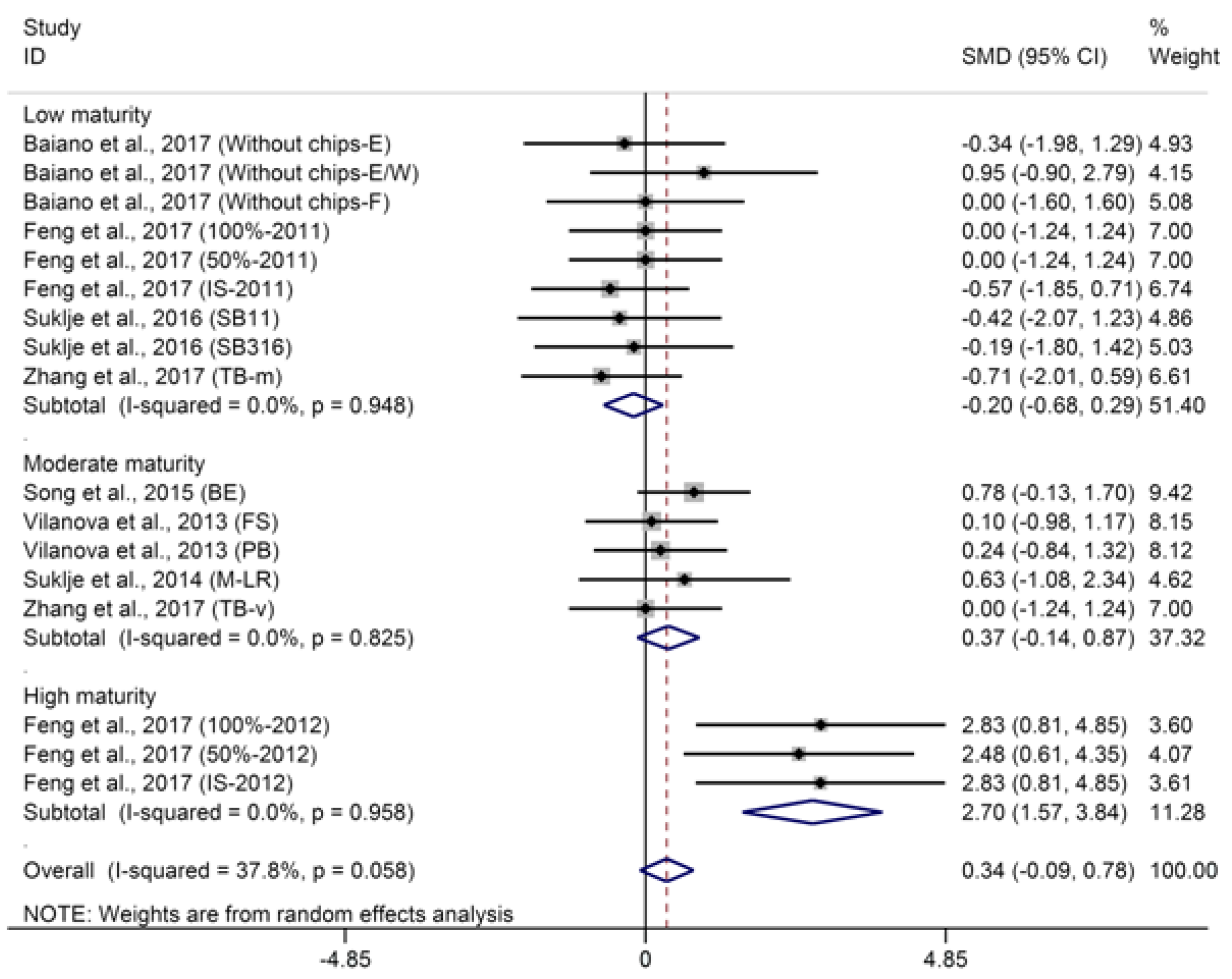
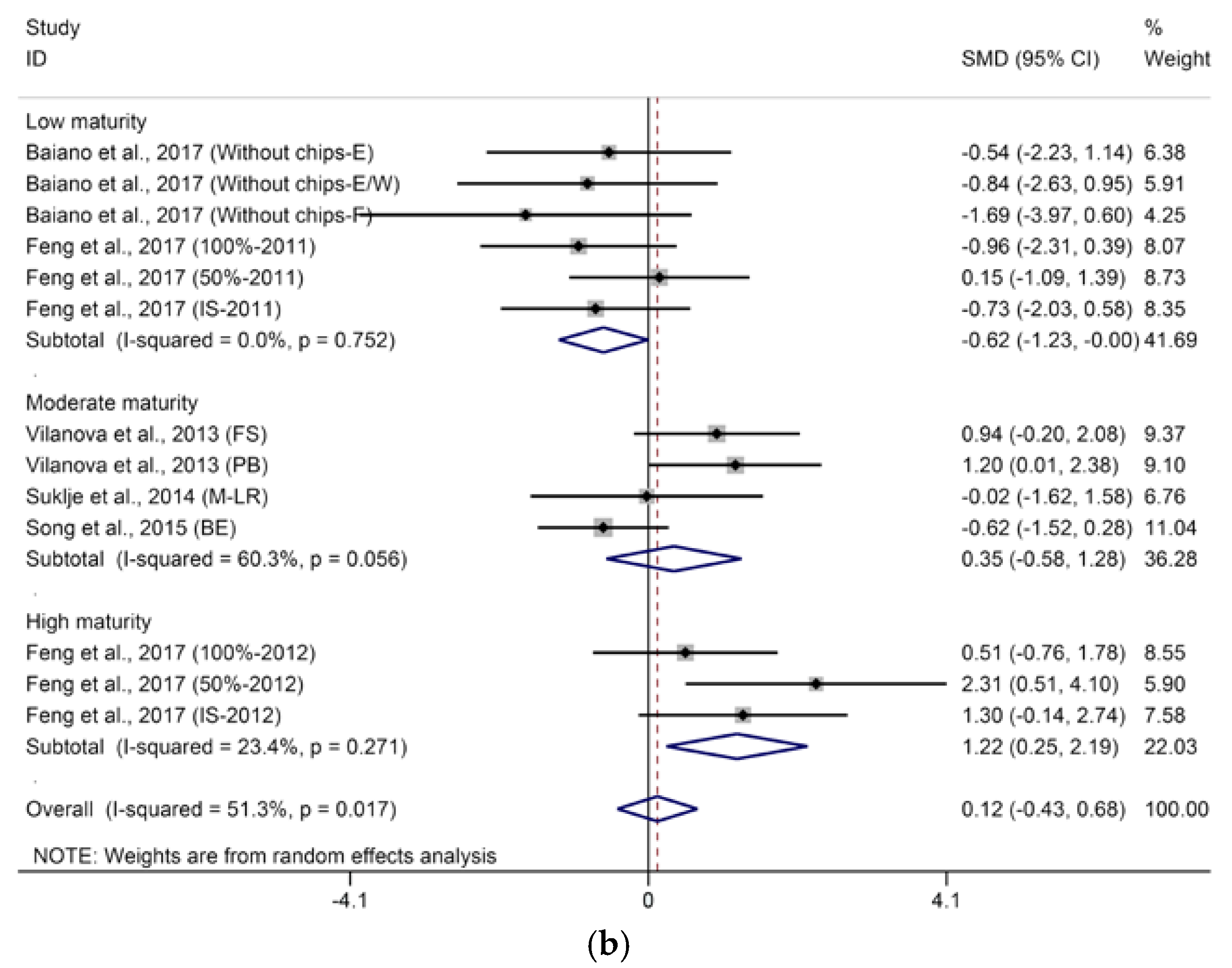
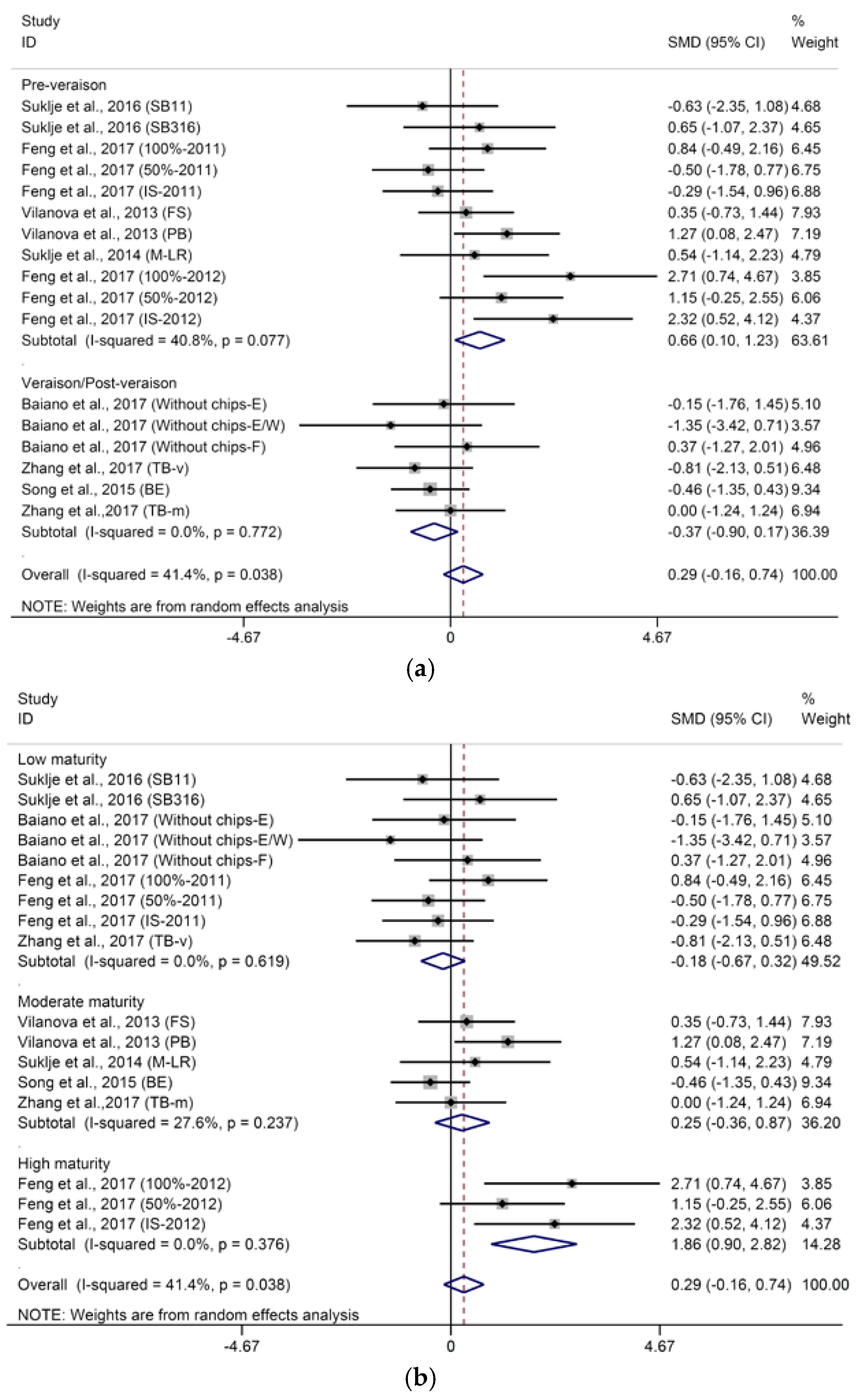
2.1.1. C13-Norisoprenoids
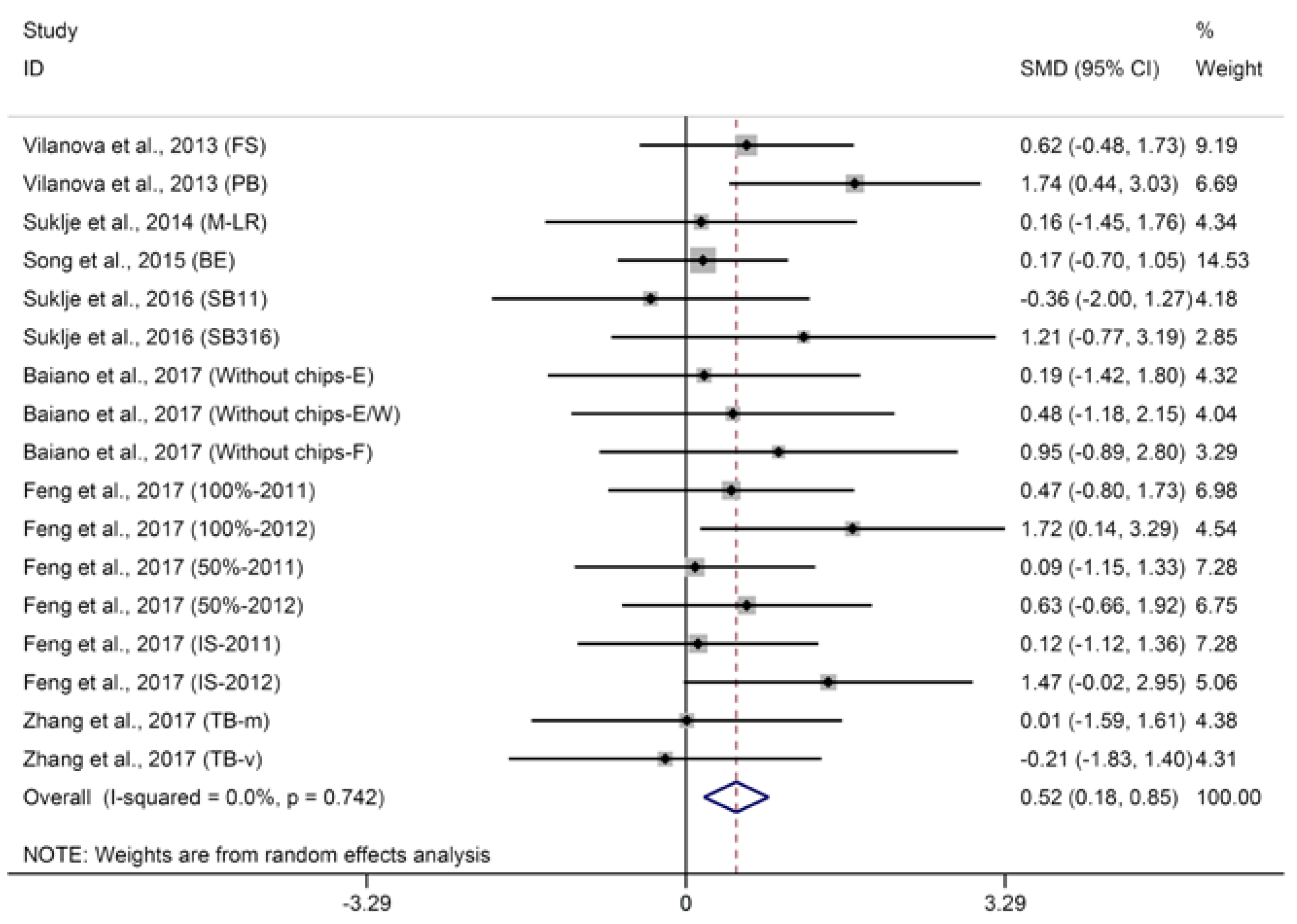
2.1.2. Terpenes
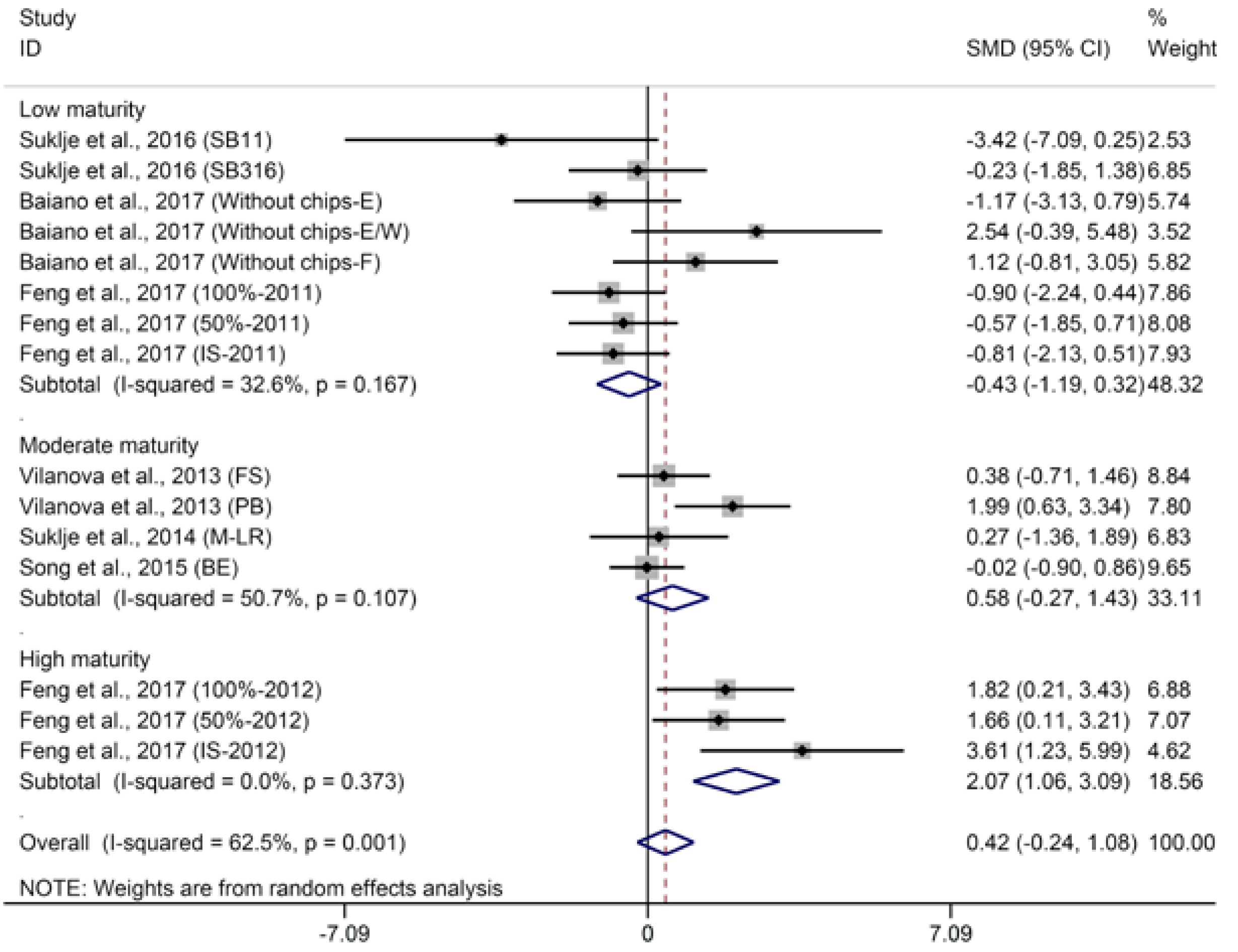
2.1.3. Methoxypyrazines
2.2. Effects of Basal Defoliation on Aromas Related to Fermentation
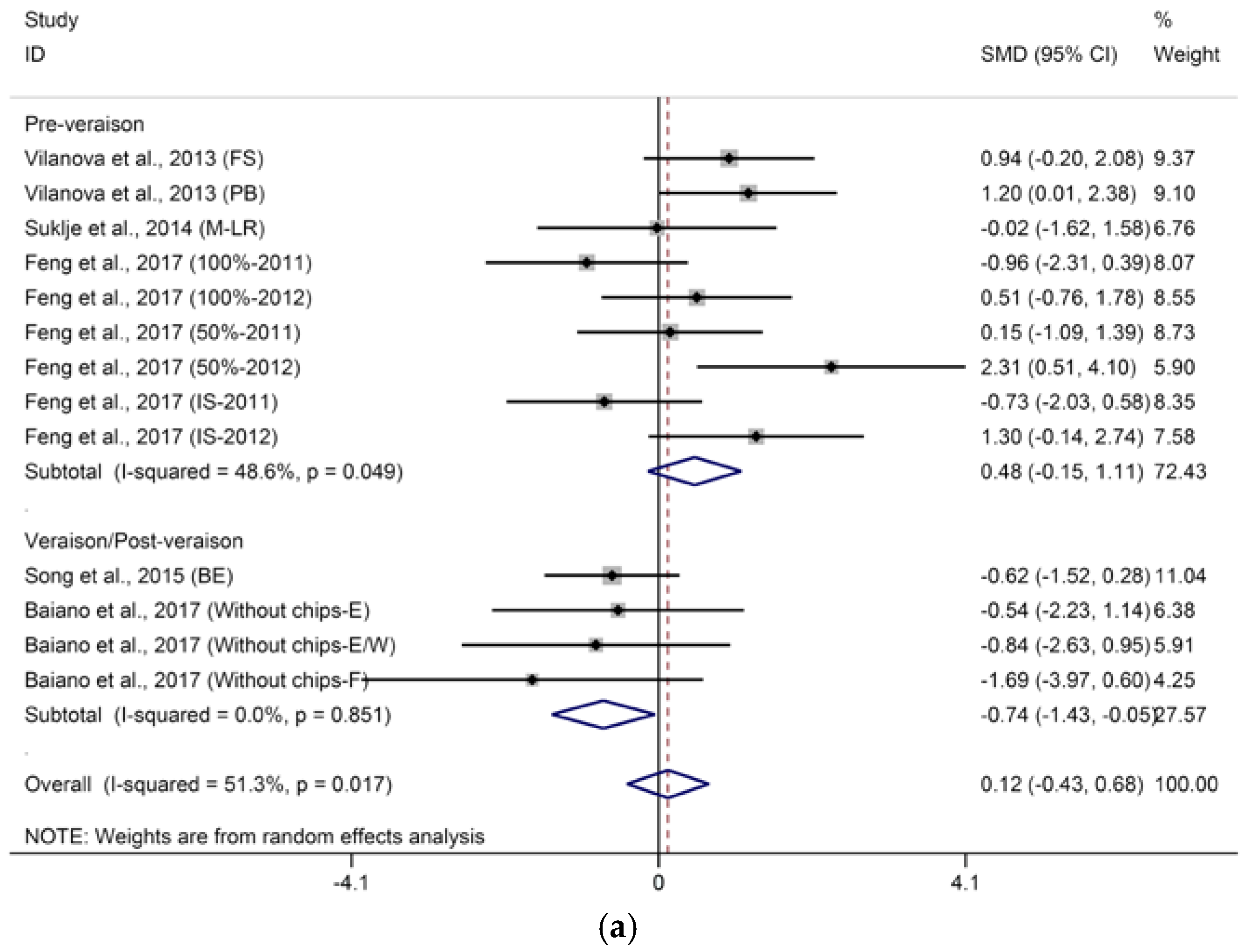
2.2.1. C6-Compounds
2.2.2. Higher Alcohols and Their Derived Acetates
2.2.3. Fatty Acids and Their Derived Ethyl Esters
3. Materials and Methods
3.1. Data Selection
- There were ≥2 repetitions.
- Control and treatment measurements were reported.
- Basal defoliation was applied rather than other techniques to improve cluster exposure such as shoot thinning.
3.2. Database
3.3. Data Analysis
- MDf: 1 = “total soluble solids showed no significant difference between control and treated berries”; 2 = “total soluble solids in treated berries were significantly higher than that in control berries”.
- BM: 1 (low maturity) = “°Brix < 23”; 2 (moderate maturity) = “23 < °Brix < 25”; 3 (high maturity) = “°Brix > 25”.
- DT: 1 = “pre-veraison”; 2 = “veraison/post-veraison”.
- DS: 1 = “all leaves removed from the base of shoot to the node just above the apical cluster”; 2 = “not all leaves removed from the base of shoot to the node just above the apical cluster”.
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alessandrini, M.; Battista, F.; Panighel, A.; Flamini, R.; Tomasi, D. Effect of pre-bloom leaf removal on grape aroma composition and wine sensory profile of Semillon cultivar. J. Sci. Food Agric. 2018, 98, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, X.; Needs, S.; Liu, D.; Fuentes, S.; Howell, K. The influence of apical and basal defoliation on the canopy structure and biochemical composition of Vitis vinifera cv. Shiraz grapes and wine. Front. Chem. 2017, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Mosetti, D.; Herrera, J.C.; Sabbatini, P.; Green, A.; Alberti, G.; Peterlunger, E.; Lisjak, K.; Castellarin, S.D. Impact of leaf removal after berry set on fruit composition and bunch rot in ‘Sauvignon blanc’. Vitis 2016, 55, 57–64. [Google Scholar] [CrossRef]
- Young, P.R.; Eyeghe-Bickong, H.A.; du Plessis, K.; Alexandersson, E.; Jacobson, D.A.; Coetzee, Z.; Deloire, A.; Vivier, M.A. Grapevine plasticity in response to an altered microclimate: Sauvignon Blanc modulates specific metabolites in response to increased berry exposure. Plant Physiol. 2016, 170, 1235–1254. [Google Scholar] [CrossRef] [PubMed]
- Šuklje, K.; Antalick, G.; Coetzee, Z.; Schmidtke, L.M.; Baša Česnik, H.; Brandt, J.; du Toit, W.J.; Lisjak, K.; Deloire, A. Effect of leaf removal and ultraviolet radiation on the composition and sensory perception of Vitis vinifera L. cv. Sauvignon Blanc wine. Aust. J. Grape Wine Res. 2014, 20, 223–233. [Google Scholar] [CrossRef]
- Kuhn, N.; Guan, L.; Dai, Z.W.; Wu, B.-H.; Lauvergeat, V.; Gomès, E.; Li, S.-H.; Godoy, F.; Arce-Johnson, P.; Delrot, S. Berry ripening: Recently heard through the grapevine. J. Exp. Bot. 2014, 65, 4543–4559. [Google Scholar] [CrossRef] [PubMed]
- Poni, S.; Casalini, L.; Bernizzoni, F.; Civardi, S.; Intrieri, C. Effects of early defoliation on shoot photosynthesis, yield components, and grape composition. Am. J. Enol. Vitic. 2006, 57, 397–407. [Google Scholar]
- Diago, M.P.; Ayestaran, B.; Guadalupe, Z.; Poni, S.; Tardaguila, J. Impact of prebloom and fruit set basal leaf removal on the flavonol and anthocyanin composition of Tempranillo grapes. Am. J. Enol. Vitic. 2012, 63, 367–376. [Google Scholar] [CrossRef]
- Frinoi, T.; Zhuang, S.; Palliotti, A.; Sivilotti, P.; Falchi, R.; Sabbatini, P. Leaf removal and cluster thinning efficiencies are highly modulated by environmental conditions in cool climate viticulture. Am. J. Enol. Vitic. 2017. [Google Scholar] [CrossRef]
- Keller, M. Managing grapevine to optimize fruit development in a challenging environment: A climate change primer for viticulturists. Aust. J. Grape Wine Res. 2010, 16, 56–69. [Google Scholar] [CrossRef]
- Palliotti, A.; Panara, F.; Sivestroni, O.; Lanari, V.; Sabbatini, P.; Howell, G.S.; Gatti, M.; Poni, S. Influence of mechanical postveraison leaf removal apical to the cluster zone on delay of fruit ripening in Sangiovese (Vitis vinifera L.) grapevines. Aust. J. Grape Wine Res. 2013, 19, 369–377. [Google Scholar] [CrossRef]
- Poni, S.; Gatti, M.; Bernizzoni, F.; Civardi, S.; Bobeica, N.; Magnanini, E.; Palliotti, A. Late leaf removal aimed at delaying ripening in cv. Sangiovese: Physiological assessment and vine performance. Aust. J. Grape Wine Res. 2013, 19, 378–387. [Google Scholar] [CrossRef]
- Filippetti, I.; Movahed, N.; Allegro, G.; Valentini, G.; Pastore, C.; Colucci, E.; Intrieri, C. Effect of post-veraison source limitation on the accumulation of sugar, anthocyanins and seed tannins in Vitis vinifera cv. Sangiovese berries. Aust. J. Grape Wine Res. 2015, 21, 90–100. [Google Scholar] [CrossRef]
- Bergqvist, J.; Dokoozlian, N.; Ebisuda, N. Sunlight exposure and temperature effects on berry growth and composition of Cabernet Sauvignon and Grenache in the central San Joaquin Valley of California. Am. J. Enol. Vitic. 2001, 52, 1–7. [Google Scholar]
- Chorti, E.; Guidoni, S.; Ferrandino, A.; Novello, V. Effect of different cluster sunlight exposure levels on ripening and anthocyanin accumulation in Nebbiolo grapes. Am. J. Enol. Vitic. 2010, 61, 23–30. [Google Scholar]
- Sternad Lemut, M.; Sivilotti, P.; Butinar, L.; Laganis, J.; Vrhovsek, U. Pre-flowering leaf removal alters grape microbial population and offers good potential for a more sustainable and cost-effective management of a Pinot Noir vineyard. Aust. J. Grape Wine Res. 2015, 21, 439–450. [Google Scholar] [CrossRef]
- Kotseridis, Y.; Georgiadou, A.; Tikos, P.; Kallithraka, S.; Koundouras, S. Effects of severity of post-flowering leaf removal on berry growth and composition of three red Vitis vinifera L. cultivars grown under semiarid conditions. J. Agric. Food Chem. 2012, 60, 6000–6010. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Yuan, F.; Skinkis, P.A.; Qian, M.C. Influence of cluster zone leaf removal on Pinot noir grape chemical and volatile composition. Food Chem. 2015, 173, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.-Z.; Cheng, G.; Li, Q.; He, Y.-N.; Wang, Y.; Lan, Y.-B.; Li, S.-Y.; Zhu, Y.-R.; Song, W.-F.; Zhang, X.; et al. Light-induced variation in phenolic compounds in Cabernet Sauvignon grapes (Vitis vinifera L.) involves extensive transcriptome reprogramming of biosynthetic enzymes, transcription factors, and phytohormonal regulators. Front. Plant Sci. 2017, 8, 547. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A.; Mandery, H. Wine aroma. Cell. Mol. Life Sci. 1986, 42, 873–884. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Q.; Cheng, G.; Duan, L.-L.; Jiang, R.; Pan, Q.-H.; Duan, C.-Q.; Wang, J. Effect of training systems on fatty acids and their derived volatiles in Cabernet Sauvignon grapes and wines of the north foot of Mt. Tianshan. Food Chem. 2015, 181, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Gregan, S.M.; Wargent, J.J.; Liu, L.; Shinkle, J.; Hofmann, R.; Winefield, C.; Trought, M.; Jordan, B. Effects of solar ultraviolet radiation and canopy manipulation on the biochemical composition of Sauvignon Blanc grapes. Aust. J. Grape Wine Res. 2012, 18, 227–238. [Google Scholar] [CrossRef]
- Friedel, M.; Stoll, M.; Patz, C.; Will, F.; Dietrich, H. Impact of light exposure on fruit composition of white ‘Riesling’ grape berries (Vitis vinifera L.). Vitis 2015, 54, 107–116. [Google Scholar]
- Kozina, B.; Karoglan, M.; Herjavec, S.; Jeromel, A.; Orlić, S. Influence of basal leaf removal on the chemical composition of Sauvignon Blanc and Riesling wines. J. Food Agric. Environ. 2008, 6, 28–33. [Google Scholar]
- Hernandez-Orte, P.; Concejero, B.; Astrain, J.; Lacau, B.; Cacho, J.; Ferreira, V. Influence of viticulture practices on grape aroma precursors and their relation with wine aroma. J. Sci. Food Agric. 2015, 95, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Šuklje, K.; Antalick, G.; Buica, A.; Langlois, J.; Coetzee, Z.A.; Gouot, J.; Schmidtke, L.M.; Deloire, A. Clonal differences and impact of defoliation on Sauvignon blanc (Vitis vinifera L.) wines: A chemical and sensory investigation. J. Sci. Food Agric. 2016, 96, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Skinkis, P.A.; Qian, M.C. Pinot noir wine volatile and anthocyanin composition under different levels of vine fruit zone leaf removal. Food Chem. 2017, 214, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Verzera, A.; Tripodi, G.; Dima, G.; Condurso, C.; Scacco, A.; Cincotta, F.; Giglio, D.M.L.; Santangelo, T.; Sparacio, A. Leaf removal and wine composition of Vitis vinifera L. cv. Nero d’Avola: The volatile aroma constituents. J. Sci. Food Agric. 2016, 96, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Diago, M.P.; Genisheva, Z.; Oliveira, J.M.; Tardaguila, J. Early leaf removal impact on volatile composition of Tempranillo wines. J. Sci. Food Agric. 2012, 92, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; Mentana, A.; Quinto, M.; Centonze, D.; Previtali, M.A.; Varva, G.; Del Nobile, M.A.; De Palma, L. Volatile composition and sensory profile of wines obtained from partially defoliated vines: The case of Nero di Troia wine. Eur. Food Res. Technol. 2017, 243, 247–261. [Google Scholar] [CrossRef]
- Winterhalter, P.; Sefton, M.; Williams, P. Volatile C13-norisoprenoid compounds in Riesling wine are generated from multiple precursors. Am. J. Enol. Vitic. 1990, 41, 277–283. [Google Scholar]
- Mathieu, S.; Terrier, N.; Procureur, J.; Bigey, F.; Günata, Z. A carotenoid cleavage dioxygenase from Vitis vinifera L.: Functional characterization and expression during grape berry development in relation to C13-norisoprenoid accumulation. J. Exp. Bot. 2005, 56, 2721–2731. [Google Scholar] [CrossRef] [PubMed]
- Lashbrooke, J.G.; Young, P.R.; Dockrall, S.J.; Vasanth, K.; Vivier, M.A. Functional characterisation of three members of the Vitis vinifera L. carotenoid cleavage dioxygenase gene family. BMC Plant Biol. 2013, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Pineau, B.; Barbe, J.-C.; Van Leeuwen, C.; Dubourdieu, D. Which impact for β-damascenone on red wines aroma? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Kotseridis, Y.; Baumes, R.L.; Bertrand, A.; Skouroumounis, G.K. Quantitative determination of β-ionone in red wines and grapes of Bordeaux using a stable isotope dilution assay. J. Chromatogr. A 1999, 848, 317–325. [Google Scholar] [CrossRef]
- Kwasniewski, M.T.; Vanden Heuvel, J.E.; Pan, B.S.; Sacks, G.L. Timing of cluster light environment manipulation during grape development affects C13 norisoprenoid and carotenoid concentrations in Riesling. J. Agric. Food Chem. 2010, 58, 6841–6849. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, J. Carotenoid biosynthesis in flowering plants. Curr. Opin. Plant Biol. 2001, 4, 210–218. [Google Scholar] [CrossRef]
- Marais, J.; Van Wyk, C.; Rapp, A. Effect of sunlihgt and shade on norisoprenoid levels in maturing Weisser Riesling and Chenin Blanc grapes and Weisser Riesling wines. S. Afr. J. Enol. Vitic. 1992, 13, 23–32. [Google Scholar] [CrossRef]
- Mateo, J.J.; Jiménez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Marais, J. Terpenes in the aroma of grapes and wines: A review. S. Afr. J. Enol. Vitic. 1983, 4, 49–58. [Google Scholar] [CrossRef]
- Schreier, P.; Jennings, W.G. Flavor composition of wines: A review. Crit. Rev. Food Sci. Nutr. 1979, 12, 59–111. [Google Scholar] [CrossRef]
- Sasaki, K.; Takase, H.; Matsuyama, S.; Kobayashi, H.; Matsuo, H.; Ikoma, G.; Takata, R. Effect of light exposure on linalool biosynthesis and accumulation in grape berries. Biosci. Biotechnol. Biochem. 2016, 80, 2376–2382. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Smart, R.; Wang, H.; Dambergs, B.; Sparrow, A.; Qian, M.C. Effect of grape bunch sunlight exposure and UV radiation on phenolics and volatile composition of Vitis vinifera L. cv. Pinot noir wine. Food Chem. 2015, 173, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Qian, M.C. Quantification of selected aroma-active compounds in Pinot Noir wines from different grape maturities. J. Agric. Food Chem. 2006, 54, 8567–8573. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S.; Mateo, J.J. Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: A review. Appl. Microbiol. Biotechnol. 2005, 67, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Ebeler, S.E.; Williams, L.E.; Matthews, M.A. Fruit ripening in Vitis vinifera: Light intensity before and not during ripening determines the concentration of 2-methoxy-3-isobutylpyrazine in Cabernet Sauvignon berries. Physiol. Plant. 2012, 145, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.S.; Lacey, M.J.; Harris, R.L.N.; Brown, W.V. Contribution of methoxypyrazines to Sauvignon Blanc wine aroma. Am. J. Enol. Vitic. 1991, 42, 109–112. [Google Scholar]
- Hein, K.; Ebeler, S.E.; Heymann, H. Perception of fruity and vegetative aromas in red wine. J. Sens. Stud. 2009, 24, 441–455. [Google Scholar] [CrossRef]
- Ryona, I.; Pan, B.S.; Sacks, G.L. Rapid measurement of 3-alkyl-2-methoxypyrazine content of winegrapes to predict levels in resultant wines. J. Agric. Food Chem. 2009, 57, 8250–8257. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, J.J.; Sacks, G.L.; Pan, B.; Ennahli, S.; Tarlton, L.; Wise, A.; Lerch, S.D.; Heuvel, J.E.V. Impact of severity and timing of basal leaf removal on 3-isobutyl-2-methoxypyrazine concentrations in red winegrapes. Am. J. Enol. Vitic. 2010, 61, 358–364. [Google Scholar]
- Sivilotti, P.; Herrera, J.C.; Lisjak, K.; Baša Česnik, H.; Sabbatini, P.; Peterlunger, E.; Castellarin, S.D. Impact of leaf removal, applied before and after flowering, on anthocyanin, tannin, and methoxypyrazine concentrations in ‘Merlot’ (Vitis vinifera L.) grapes and wines. J. Agric. Food Chem. 2016, 64, 4487–4496. [Google Scholar] [CrossRef] [PubMed]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; Herraiz, M.; Reglero, G.; Martin-Alvarez, P.J.; Cabezudo, M.D. Changes in the composition of alcohols and aldehydes of C6 chain length during the alcoholic fermentation of grape must. J. Agric. Food Chem. 1990, 38, 969–972. [Google Scholar] [CrossRef]
- Moreno, D.; Valdés, E.; Uriarte, D.; Gamero, E.; Talaverano, I.; Vilanova, M. Early leaf removal applied in warm climatic conditions: Impact on Tempranillo wine volatiles. Food Res. Int. 2017, 98, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- De Smidt, O.; du Preez, J.C.; Albertyn, J. The alcohol dehydrogenases of Saccharomyces cerevisiae: A comprehensive review. FEMS Yeast Res. 2008, 8, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.G.; Keyzers, R.A.; Kalua, C.M.; Maffei, S.M.; Nicholson, E.L.; Boss, P.K. Grape contribution to wine aroma: Production of hexyl acetate, octyl acetate, and benzyl acetate during yeast fermentation is dependent upon precursors in the must. J. Agric. Food Chem. 2012, 60, 2638–2646. [Google Scholar] [CrossRef] [PubMed]
- Lilly, M.; Bauer, F.F.; Lambrechts, M.G.; Swiegers, J.H.; Cozzolino, D.; Pretorius, I.S. The effect of increased yeast alcohol acetyltransferase and esterase activity on the flavour profiles of wine and distillates. Yeast 2006, 23, 641–659. [Google Scholar] [CrossRef] [PubMed]
- Estevez, P.; Gil, M.L.; Falque, E. Effects of seven yeast strains on the volatile composition of Palomino wines. Int. J. Food Sci. Technol. 2004, 39, 61–69. [Google Scholar] [CrossRef]
- Torrens, J.; Urpi, P.; Riu-Aurnatell, M.; Vichi, S.; Lopez-Tamames, E.; Buxaderas, S. Different commercial yeast strains affecting the volatile and sensory profile of cava base wine. Int. J. Food Microbiol. 2008, 124, 48–57. [Google Scholar] [CrossRef] [PubMed]
- De-la-Fuente-Blanco, A.; Sáenz-Navajas, M.-P.; Ferreira, V. On the effects of higher alcohols on red wine aroma. Food Chem. 2016, 210, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Hazelwood, L.A.; Daran, J.M.; van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 3920–3920. [Google Scholar] [CrossRef]
- Nisbet, M.A.; Tobias, H.J.; Brenna, J.T.; Sacks, G.L.; Mansfield, A.K. Quantifying the contribution of grape hexoses to wine volatiles by high-precision [U13C]-glucose tracer studies. J. Agric. Food Chem. 2014, 62, 6820–6827. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Cacho, J.F.; Ferreira, V. Relationship between varietal amino acid profile of grapes and wine aromatic composition. Experiments with model solutions and chemometric study. J. Agric. Food Chem. 2002, 50, 2891–2899. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Grose, C.; Fedrizzi, B.; Stuart, L.; Albright, A.; McLachlan, A. Grape cluster microclimate influences the aroma composition of Sauvignon blanc wine. Food Chem. 2016, 210, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry, 1st ed.; John Wiley & Sons: Chichester, UK, 2016; pp. 205–212. ISBN 9781118627808. [Google Scholar]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions, 5th ed.; John Wiley & Sons: Chichester, UK, 2011; pp. 174–175. ISBN 9780470518458. [Google Scholar]
- Rowen, E.; Kaplan, I. Eco-evolutionary factors drive induced plant volatiles: A meta-analysis. New Phytol. 2016, 210, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.G.; Zhang, Y.; Nelson, C.J.; Gambetta, G.; Kennedy, J.A.; Kurtural, S.K. Anthocyanin composition of Merlot is Ameliorated by light microclimate and irrigation in central California. Am. J. Enol. Vitic. 2015. [Google Scholar] [CrossRef]
- Geller, J.P.; Kurtural, S.K. Mechanical canopy and crop-load management of Pinot Gris in a warm climate. Am. J. Enol. Vitic. 2013, 64, 65–73. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
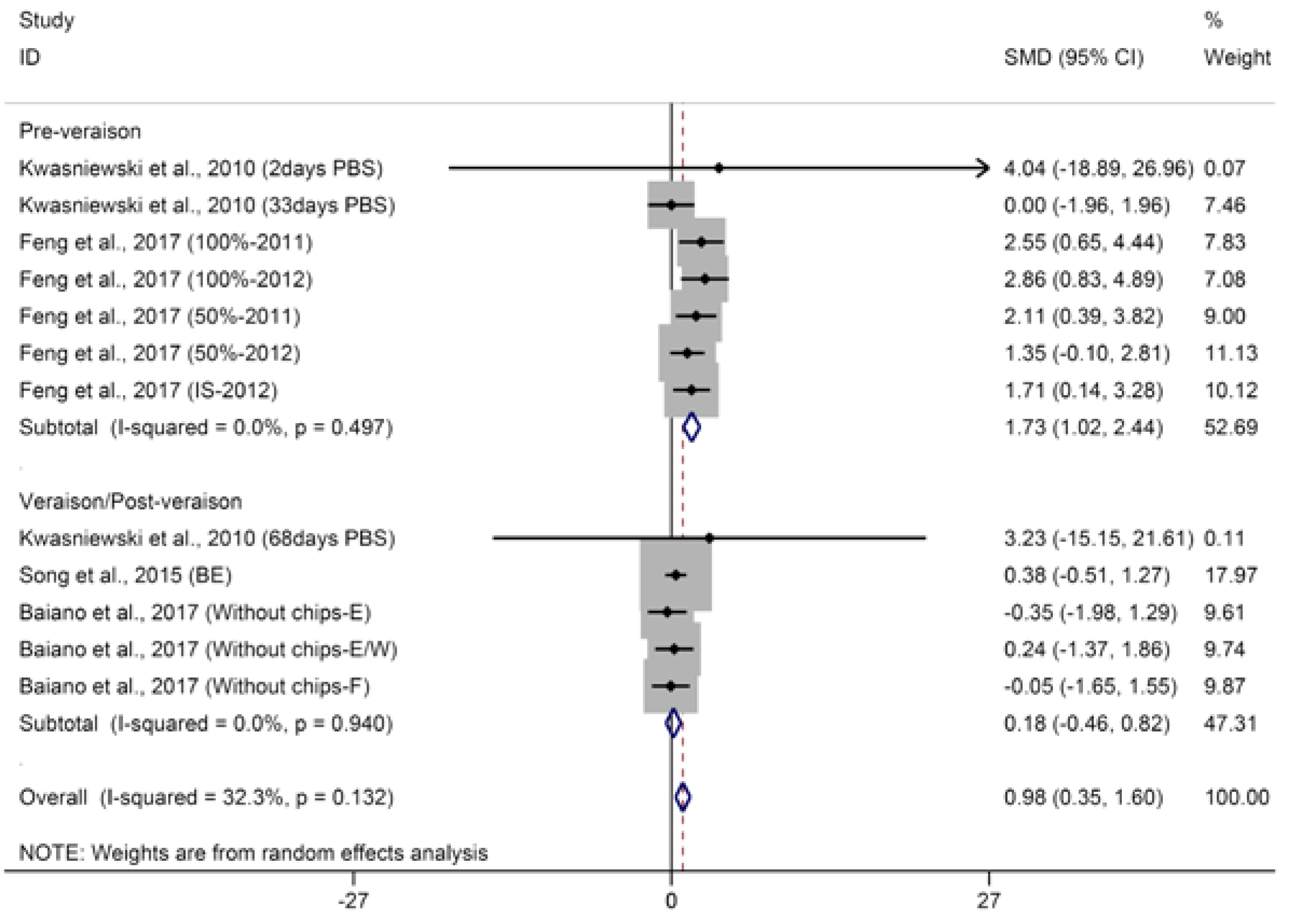
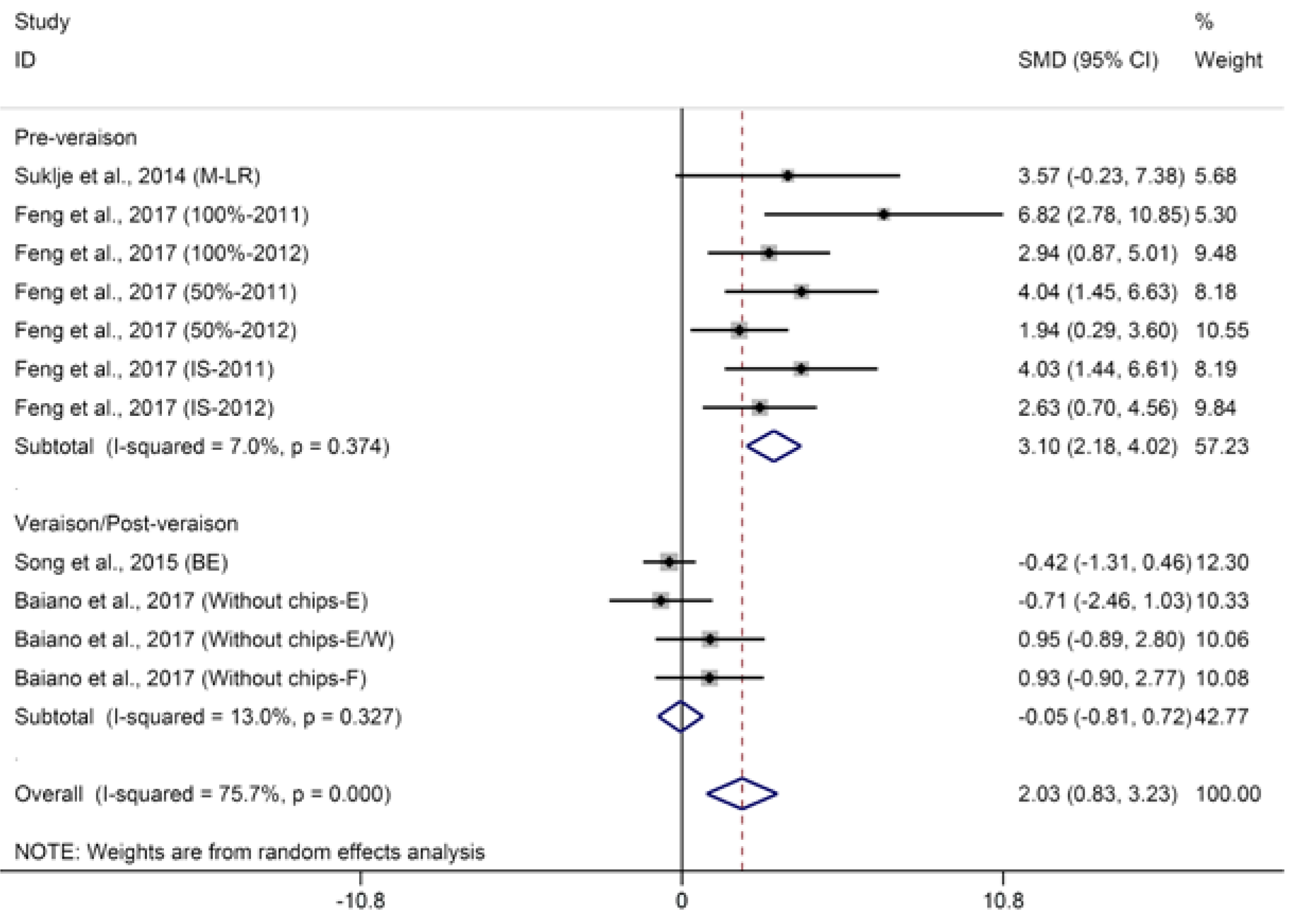
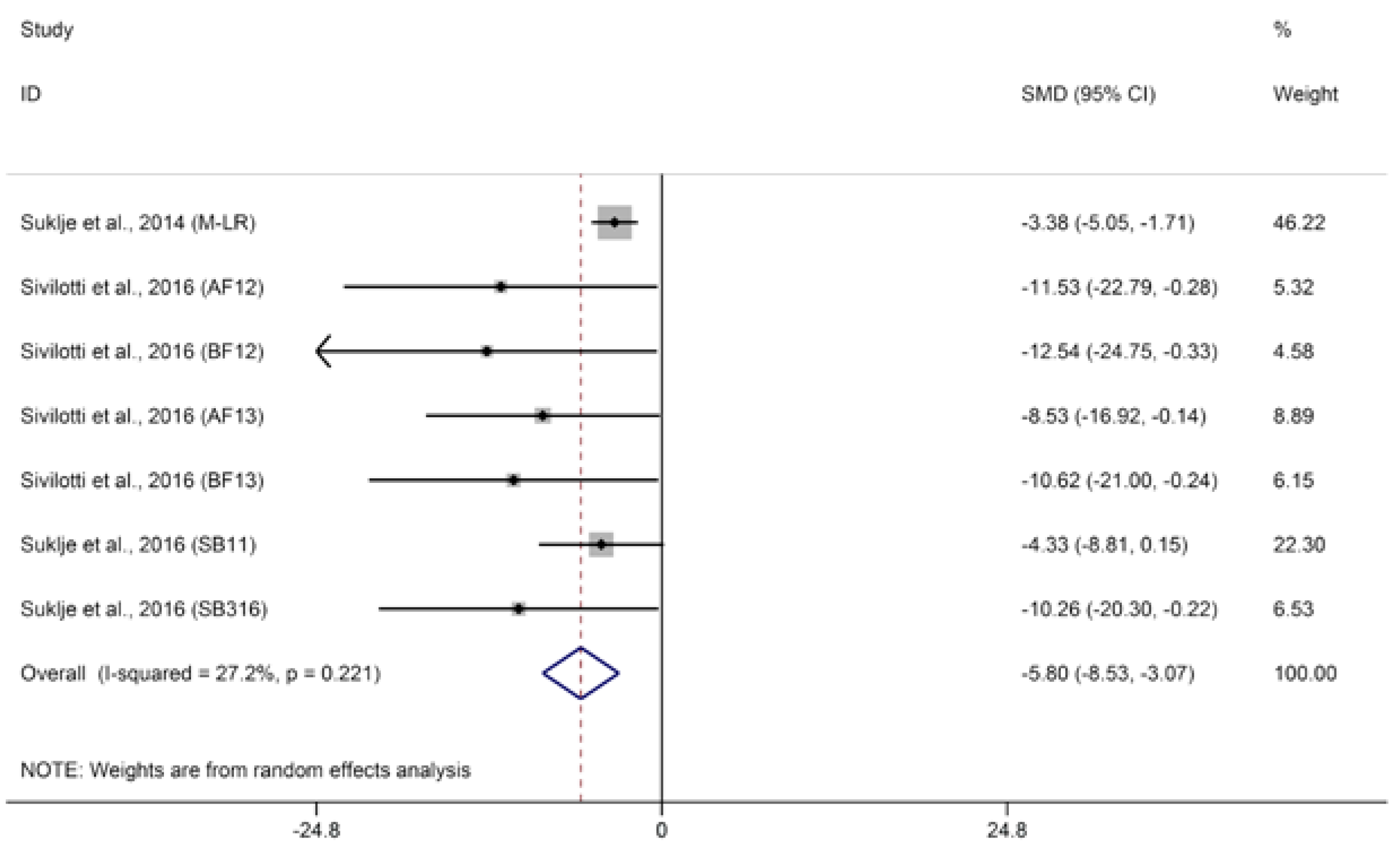
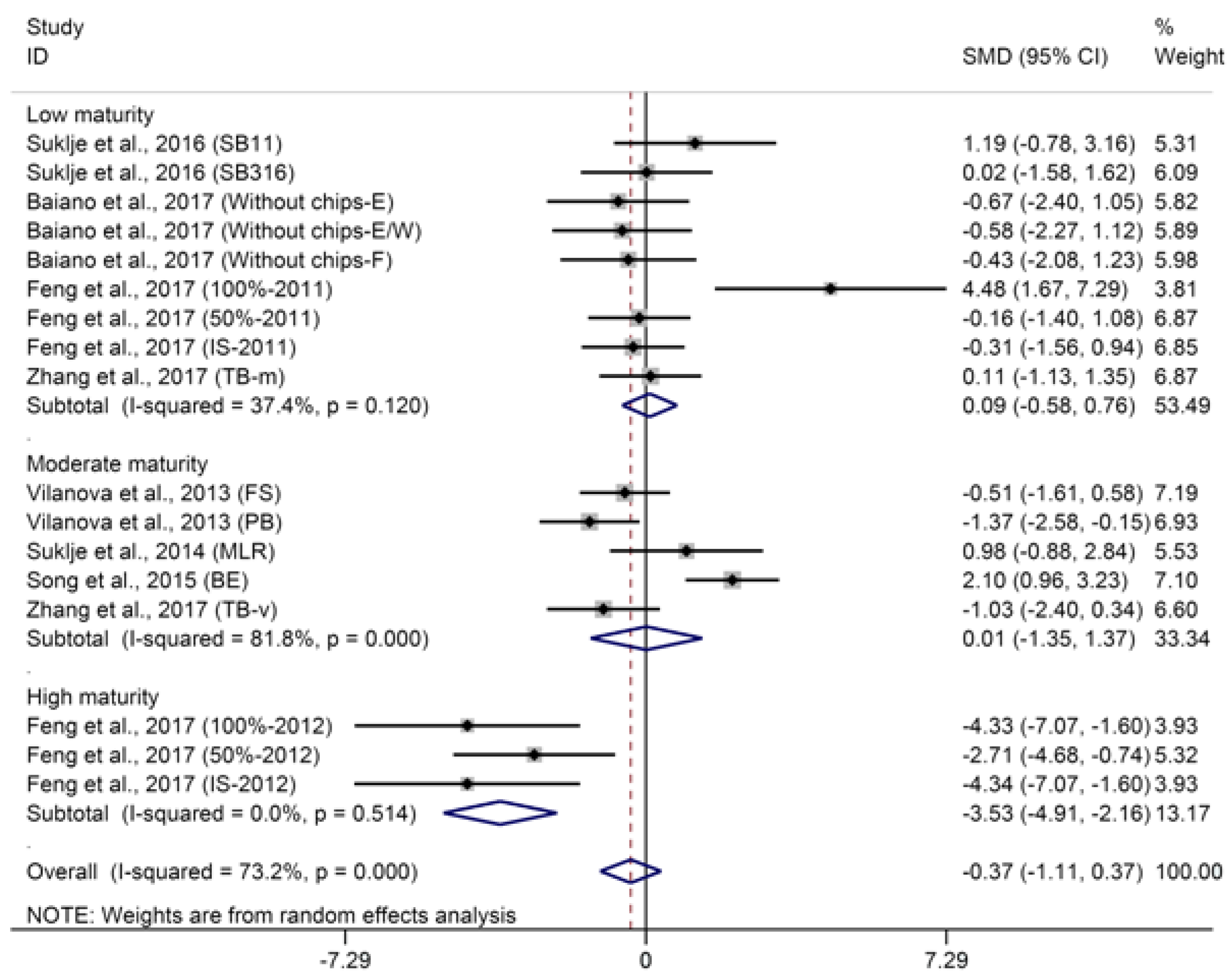
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; He, L.; Pan, Q.; Duan, C.; Wang, J. Effects of Basal Defoliation on Wine Aromas: A Meta-Analysis. Molecules 2018, 23, 779. https://doi.org/10.3390/molecules23040779
Wang Y, He L, Pan Q, Duan C, Wang J. Effects of Basal Defoliation on Wine Aromas: A Meta-Analysis. Molecules. 2018; 23(4):779. https://doi.org/10.3390/molecules23040779
Chicago/Turabian StyleWang, Yu, Lei He, Qiuhong Pan, Changqing Duan, and Jun Wang. 2018. "Effects of Basal Defoliation on Wine Aromas: A Meta-Analysis" Molecules 23, no. 4: 779. https://doi.org/10.3390/molecules23040779
APA StyleWang, Y., He, L., Pan, Q., Duan, C., & Wang, J. (2018). Effects of Basal Defoliation on Wine Aromas: A Meta-Analysis. Molecules, 23(4), 779. https://doi.org/10.3390/molecules23040779







