Synthesis of Novel Amino Acid–Fipronil Conjugates and Study on Their Phloem Loading Mechanism
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterizations
2.2. Phloem Mobility in R. communis Seedlings
2.3. Prediction of Phloem Mobility Using Log Cf Values
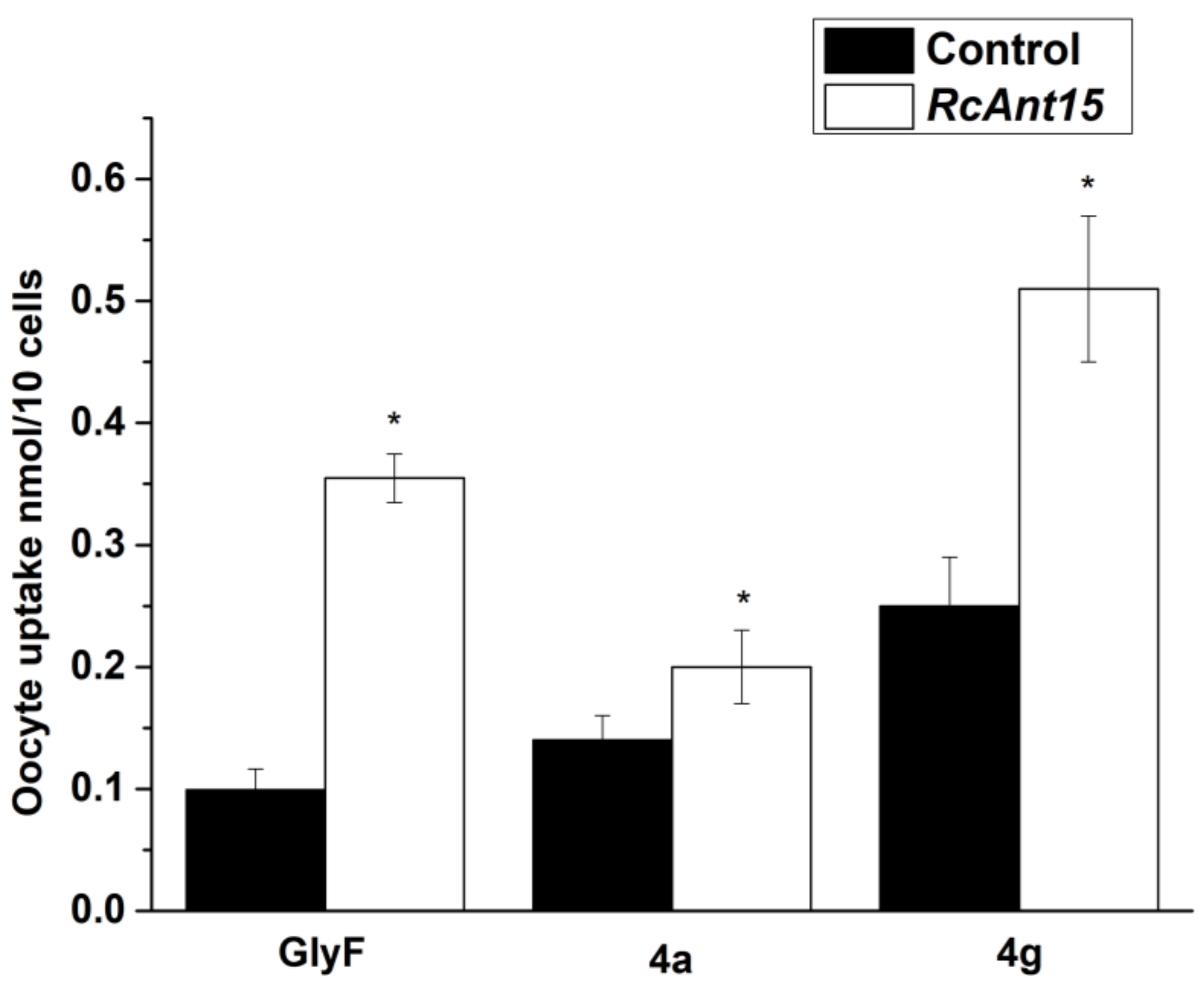
2.4. Uptake Experiments with Xenopus Oocytes
3. Materials and Methods
3.1. Synthesis
3.1.1. General Information
3.1.2. Synthesis of 5-Amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl)]-4-[(trifluoromethyl) sulfinyl)]-1H-pyrazole-3-carboxylic acid (2)
3.1.3. Synthesis of Compound Series 3
3.1.4. Synthesis of Compound Series 4
3.2. Physicochemical Properties
3.3. Plant Materials
3.4. Phloem Sap Collection
3.5. Animal Materials
3.6. Xenopus Heterologous Expression System and Drug Uptake Experiment
3.7. Analytical Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hsu, F.C.; Kleier, D.A.; Melander, W.R. Phloem mobility of xenobiotics II. Bioassay testing of the unified mathematical mode. Plant Physiol. 1988, 86, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Kleier, D.A. Phloem mobility of xenobiotics. V. Structural requirements for phloem-systemic pesticides. Pest Manag. Sci. 1994, 42, 1–11. [Google Scholar] [CrossRef]
- Lichtner, F. Phloem mobility of crop protection products. Aust. J. Plant Physiol. 2000, 27, 609–614. [Google Scholar] [CrossRef]
- Kleier, D.A.; Hsu, F.C. Phloem mobility of xenobiotics. VII. The design of phloem systemic pesticides. Weed Sci. 1996, 44, 749–756. [Google Scholar]
- Dufaud, A.; Chollet, J.F.; Rudelle, J.; Miginiac, L.; Bonnemain, J.L. Derivatives of pesticides with acid function: Synthesis and an α-amino effect on threonine uptake. Pestic. Sci. 1994, 41, 297–304. [Google Scholar] [CrossRef]
- Wu, H.X.; Marhadour, S.; Lei, Z.W.; Yang, W.; Marivingt-Mounir, C.; Bonnemain, J.; Chollet, J. Vectorization of agrochemicals: Amino acid carriers are more efficient than sugar carriers to translocate phenylpyrrole conjugates in the Ricinus system. Environ. Sci. Pollut. Res. 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Marhadour, S.; Wu, H.X.; Yang, W.; Marivingt-Mounir, C.; Bonnemain, J.; Chollet, J. Vectorisation of agrochemicals via amino acid carriers: Influence of the spacer arm structure on the phloem mobility of phenylpyrrole conjugates in the Ricinus system. Pest Manag. Sci. 2017, 73, 1972–1982. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P. Propesticides and their use as agrochemicals. Pest Manag. Sci. 2016, 72, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wu, H.X.; Xu, H.H.; Hu, A.L.; Lu, M.L. Synthesis of glucose–fipronil conjugate and its phloem mobility. J. Agric. Food Chem. 2011, 59, 12534–12542. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.K.; Wen, Y.J.; Zhao, C.; Xu, H.H. Novel amino acid ester-chlorantraniliprole conjugates: Design, synthesis, phloem accumulation and bioactivity. Pest Manag. Sci. 2017, 73, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lei, Z.W.; Zhang, Y.; Yang, W.; Liu, H.F.; Zhou, Y.F.; Yang, M.F. Influence of pyranose and spacer arm structures on phloem mobility and insecticidal activity of new tralopyril derivatives. Molecules 2017, 22, 1058. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.X.; Zheng, X.H.; Shao, G.; Ling, Z.; Xu, H.H. Discovery of a novel series of phenyl pyrazole inner salts based on fipronil as potential dual-target insecticides. J. Agric. Food Chem. 2014, 62, 3577–3583. [Google Scholar] [CrossRef] [PubMed]
- Sammelson, R.E.; Caboni, P.; Durkin, K.A.; Casida, J.E. GABA receptor antagonists and insecticides: Common structural features of 4-alkyl-1-phenylpyrazoles and 4-alkyl-1-phenyltrioxabicyclooctanes. Bioorgan. Med. Chem. 2004, 12, 3345–3355. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.X.; Yang, W.; Zhang, Z.X.; Huang, T.; Yao, G.K.; Xu, H.H. Uptake and phloem transport of glucose-fipronil conjugate in Ricinus communis involve a carrier-mediated mechanism. J. Agric. Food Chem. 2012, 60, 6088–6094. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhao, J.L.; Wang, C.W.; Yu, A.X.; Liu, N.; Chen, L.; Lin, F.; Xu, H.H. Glycinergic–fipronil uptake is mediated by an amino acid carrier system and induces the expression of amino acid transporter genes in Ricinus communis seedlings. J. Agric. Food Chem. 2016, 64, 3810–3818. [Google Scholar] [CrossRef] [PubMed]
- Delétage-Grandon, C.; Chollet, J.F.; Faucher, M.; Rocher, F.; Komor, E.; Bonnemain, J.L. Carrier-mediated uptake and phloem systemy of a 350-dalton chlorinated xenobiotic with an α-amino acid function. Plant Physiol. 2001, 125, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.W.; Wang, J.; Mao, G.L.; Wen, Y.J.; Tian, Y.X.; Wu, H.W.; Li, Y.F.; Xu, H.H. Glucose positions affect the phloem mobility of glucose–fipronil conjugates. J. Agric. Food Chem. 2014, 62, 6065–6071. [Google Scholar] [CrossRef] [PubMed]
- Chollet, J.F.; Rocher, F.; Jousse, C.; Delétage-Grandon, C.; Bashiardes, G. Synthesis and phloem mobility of acidic derivatives of the fungicide fenpiclonil. Pest Manag. Sci. 2004, 60, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Bhal, S.K.; Kassam, K.; Peirson, I.G.; Pearl, G.M. The rule of five revisited: Applying log D in place of log P in drug-likeness filters. Mol. Pharm. 2007, 4, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Fustero, S.; Román, R.; Sanz-Cervera, J.F.; Simón-Fuentes, A.; Bueno, J.; Villanova, S. Synthesis of new fluorinated tebufenpyrad analogs with acaricidal activity through regioselective pyrazole formation. J. Org. Chem. 2008, 73, 8545–8552. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.Q.; Li, Y.Q.; Xiong, L.X.; Wang, Q.M. Design, synthesis and insecticidal activity of novel phenylpyrazoles containing a 2,2,2-trichloro-1-alkoxyethyl moiety. J. Agric. Food Chem. 2010, 58, 4992–4998. [Google Scholar] [CrossRef] [PubMed]
- Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. From 2000 to mid-2010: A fruitful decade for the synthesis of pyrazoles. Chem. Rev. 2011, 111, 6984–7034. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.R.; Ning, Y.X.; Shen, C.; Nuermaimaiti, A.; Besenbacher, F.; Linderoth, T.R.; Gothelf, K.V. Oligo(naphthylene-ethynylene) molecular rods. Eur. J. Org. Chem. 2013, 2013, 2813–2822. [Google Scholar] [CrossRef]
- Nakajima, M.; Watanabe, B.; Han, L.Y.; Shimizu, B.; Wada, K.; Fukuyama, K.; Suzuki, H.; Hiratake, J. Glutathione-analogous peptidyl phosphorus esters as mechanism-based inhibitors of γ-glutamyl transpeptidase for probing cysteinyl-glycine binding site. Bioorgan. Med. Chem. 2014, 22, 1176–1194. [Google Scholar] [CrossRef] [PubMed]
- Garg, G.; Zhao, H.P.; Blagg, B.S.J. Design, synthesis, and biological evaluation of ring-constrained novobiocin analogues as Hsp90 C-terminal inhibitors. ACS Med. Chem. Lett. 2015, 6, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.F.; Lai, S.T.; Guo, Y.C.; Chen, M.J. Inhibitory effects of novel synthetic methimazole derivatives on mushroom tyrosinase and melanogenesis. Bioorgan. Med. Chem. 2014, 22, 2809–2815. [Google Scholar] [CrossRef] [PubMed]
- Rocher, F.; Chollet, J.F.; Legros, S.; Jousse, C.; Lemoine, R.; Faucher, M.; Bush, D.R.; Bonnemain, J.L. Salicylic acid transport in Ricinus communis involves a pH-dependent carrier system in addition to diffusion. Plant Physiol. 2009, 150, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, R. Phloem loading: How leaves gain their independence. Bioscience 2006, 56, 15–24. [Google Scholar] [CrossRef]
- Fischer, W.N.; Loo, D.D.F.; Koch, W.; Ludewig, U.; Boorer, K.J.; Tegeder, M.; Rentsch, D.; Wright, E.M.; Frommer, W.B. Low and high affinity amino acid H+-cotransporters for cellular import of neutral and charged amino acids. Plant J. 2002, 29, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Sheng, Q.Q.; Wang, C.W.; Zhao, C.; Xu, H.H. The glycinergic-fipronil conjugate in apoplastic induces RcANT15 in the castor oil plant. J. Agric. Food Chem. 2018. submitted. [Google Scholar]
- Weber, W. Ion currents of Xenopus laevis oocytes: State of the art. Biochim. Biophys. Acta. Gen. Subj. 1999, 1421, 213–233. [Google Scholar] [CrossRef]
- Lau, Y.T.; Reynhout, J.K.; Horowitz, S.B. Membrane permeability changes during Rana oocyte maturation. Experientia 1994, 50, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Van Winkle, L.J. Endogenous amino acid transport systems and expression of mammalian amino acid transport proteins in Xenopus oocytes. BBA Rev. Biomembr. 1993, 1154, 157–172. [Google Scholar] [CrossRef]
- Miller, A.J.; Zhou, J.J. Xenopus oocytes as an expression system for plant transporters. BBA Rev. Biomembr. 2000, 1465, 343–358. [Google Scholar] [CrossRef]
- Zhou, J.J.; Theodoulou, F.L.; Muldin, I.; Ingemarsson, B.; Miller, A.J. Cloning and functional characterization of a Brassica napus transporter that is able to transport nitrate and histidine. J. Biol. Chem. 1998, 273, 12017–12023. [Google Scholar] [CrossRef] [PubMed]
- Boorer, K.J.; Forde, B.G.; Leigh, R.A.; Miller, A.J. Functional expression of a plant plasma membrane transporter in Xenopus oocytes. FEBS Lett. 1992, 302, 166–168. [Google Scholar] [CrossRef]
- Wang, J.; Lei, Z.W.; Wen, Y.J.; Mao, G.L.; Wu, H.X.; Xu, H.H. A novel fluorescent conjugate applicable to visualize the translocation of glucose–fipronil. J. Agric. Food Chem. 2014, 62, 8791–8798. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.W.; Wang, J.; Wang, H.; Wen, Y.J.; Lu, M.L.; Li, Y.F.; Xu, Y.S.; Xu, H.H. Synthesis of rotenone-O-monosaccharide derivatives and their phloem mobility. J. Agric. Food Chem. 2014, 62, 4521–4527. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.G.; Wu, H.X.; Lu, M.L.; Song, G.P.; Xu, H.H. Synthesis of a series of monosaccharide–fipronil conjugates and their phloem mobility. J. Agric. Food Chem. 2013, 61, 4236–4241. [Google Scholar] [CrossRef] [PubMed]
- Nour-Eldin, H.H.; Norholm, M.H.; Halkier, B.A. Screening for plant transporter function by expressing a normalized Arabidopsis full-length cDNA library in Xenopus oocytes. Plant Methods 2006, 2, 17. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 2–4 are available from the authors. |
| Compounds | Conjugated Amino Acid | Concentration in Phloem Sap (μM) a |
|---|---|---|
| 4a | Glycine (Gly) | 15.00 ± 2.50 |
| 4b | Alanine (Ala) | 35.00 ± 4.00 |
| 4c | Valine (Val) | 27.00 ± 4.00 |
| 4d | Leucine (Leu) | 15.00 ± 1.50 |
| 4e | Isoleucine (Ile) | ND b |
| 4f | Threonine (Thr) | 18.00 ± 2.60 |
| 4g | Serine (Ser) | 52.00 ± 5.80 |
| 4h | Phenylalanine (Phe) | 6.11 ± 0.80 |
| 4i | Tyrosine (Tyr) | 12.00 ± 2.00 |
| 4j | Tryptophan (Trp) | 8.70 ± 2.00 |
| 4k | Aspartic acid (Asp) | 41.77 ± 5.00 |
| 4l | Glutamic acid (Glu) | 20.00 ± 3.00 |
| GlyF | Glycine (Gly) | 10.14 ± 0.30 [15] |
| Fipronil Analog | R | pKa b | Molecular Weight (g/mol) | Log Ko/w c | Log Cf d |
|---|---|---|---|---|---|
| 4a | H | 2.8 | 513.2 | 2.65 | −0.36 |
| 4b | CH3 | 2.9 | 527.23 | 3 | 0.11 |
| 4c |  | 3 | 555.28 | 3.88 | −3.44 |
| 4d | 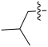 | 3.1 | 569.31 | 4.41 | −18.2 |
| 4e | 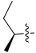 | 3.1 | 569.31 | 4.41 | −18.2 |
| 4f | 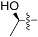 | 2.9 | 557.25 | 2.6 | −0.15 |
| 4g |  | 2.8 | 543.23 | 2.25 | −0.59 |
| 4h | 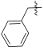 | 3.2 | 603.32 | 4.68 | −39.58 |
| 4i |  | 3.1 | 619.32 | 3.95 | −4.42 |
| 4j |  | 3.4 | 642.36 | 4.61 | −32.43 |
| 4k |  | 2.9 | 571.24 | 3.01 | −0.19 |
| 4l |  | 2.9 | 585.26 | 2.24 | −0.52 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, Q.; Liu, X.; Xie, Y.; Lin, F.; Zhang, Z.; Zhao, C.; Xu, H. Synthesis of Novel Amino Acid–Fipronil Conjugates and Study on Their Phloem Loading Mechanism. Molecules 2018, 23, 778. https://doi.org/10.3390/molecules23040778
Sheng Q, Liu X, Xie Y, Lin F, Zhang Z, Zhao C, Xu H. Synthesis of Novel Amino Acid–Fipronil Conjugates and Study on Their Phloem Loading Mechanism. Molecules. 2018; 23(4):778. https://doi.org/10.3390/molecules23040778
Chicago/Turabian StyleSheng, Qingqing, Xinxin Liu, Yun Xie, Fei Lin, Zhixiang Zhang, Chen Zhao, and Hanhong Xu. 2018. "Synthesis of Novel Amino Acid–Fipronil Conjugates and Study on Their Phloem Loading Mechanism" Molecules 23, no. 4: 778. https://doi.org/10.3390/molecules23040778
APA StyleSheng, Q., Liu, X., Xie, Y., Lin, F., Zhang, Z., Zhao, C., & Xu, H. (2018). Synthesis of Novel Amino Acid–Fipronil Conjugates and Study on Their Phloem Loading Mechanism. Molecules, 23(4), 778. https://doi.org/10.3390/molecules23040778