Abstract
A series of novel fused heterocyclic compounds bearing benzo[4,5]imidazo[1,2-d][1,2,4]triazine 4a–4w were designed and conveniently synthesized via the intermediates 2-(halogenated alkyl)-1H-benzo[d]imidazoles 2a, 2b, and 2-((1-(substituted phenyl)hydrazinyl)alkyl)-1H-benzo[d]imidazoles 3a–3g. The structures of all target compounds were characterized by FT-IR, 1H NMR, 13C NMR, and EI-MS, of which, the structure of compound 4n was further determined by the single crystal X-ray diffraction. The crystal structure of 4n was crystallized in the triclinic crystal system, space group P with a = 9.033 (6) Å, b = 10.136 (7) Å, c = 10.396 (7) Å, α = 118.323 (7)°, β = 91.750 (8)°, γ = 104.198 (7)°, Z = 2, V = 800.2 (9) Å3; total R indices: R1 = 0.0475, wR2 = 0.1284. The antifungal activity of title compounds 4a–4w in vitro against the phytopathogenic fungi Botrytis cinerea (B. cinerea), Rhizoctonia solani (R. solani) and Colletotrichum capsici (C. capsici) were evaluated, the bioassay results demonstrated that most of the title compounds exhibited obvious fungicidal activities at 50 μg/mL. This work indicated that benzo[4,5]imidazo[1,2-d][1,2,4]triazine derivatives could be considered as a new leading structure in searching for novel agricultural fungicides.
1. Introduction
Searching for novel fungicides with different structures is a promising way to overcome resistant fungi and plays a key role in preventing loss of agricultural output [1]. Over the past decades, efforts for developing highly efficient fungicides have been made by chemists. For example, some 1,4-pentadien-3-one [2], 1,2,3-triazole [3], quinazoline [4], quinazolin-4(3H)-one [5], 1,3,4-oxadiazole [6], coumarin [7], and tetronic acid [8] derivatives were well reported for their fungicidal activities against plant pathogenic fungi. However, their use as novel fungicides in agriculture was largely limited due to unsatisfactory curative rates, extremely complex synthetic routes, and structural instability in the farmland. Therefore, searching for the novel, eco-friendly, and highly efficient fungicides remains an urgent challenge in agriculture.
Benzimidazole derivatives have received considerable attention in medicinal and agrochemical chemistry due to their various bioactivities, such as antibacterial [9], anti-inflammatory [10], insecticidal [11], anti-cancer [12], and antiviral activities [13]. Some benzimidazole derivatives, such as carbendazim (Figure 1A), thiabendazole (Figure 1B), and Hoe-22845 (Figure 1C), were developed as high efficiency agricultural fungicides that made outstanding contributions for improving the yield and quality of crops [14]. Owing to their various antifungal activities, studies on the related synthesis and bioactivities of novel benzimidazole derivatives have attracted considerable attention [15,16]. Among these benzimidazole derivatives, the agricultural fungicide Hoe-22845 (Figure 1C) containing a unique benzo[4,5]imidazo[1,2-a][1,3,5]triazine substructure is interesting due to its broader antifungal spectra than other agricultural benzimidazole fungicides. Meanwhile, featuring a similar substructure, 3-phenyl-benzo[4,5]imidazo[2,1-b][1,3,5]thiadiazin-4-one (Figure 1D) was also reported for its wide and potent antifungal activity [17]. In addition, the 1,2,4-triazine fragment, an important scaffold of some natural products, including toxoflavin (Figure 1E) and fervenulin (Figure 1F) [18], has been widely studied due to its promising bioactivities, such as antifungal [19], anti-HIV [20], antimicrobial, and anti-cancer [21] activities. Recently, the antifungal activities of 1,2,4-triazines derivatives against Candida albicans, Aspergillus niger, and Cryptococcus neoformans were found by chemists [22]. Obviously, based on the structures of benzimidazole and triazine, further study to find novel effective fungicides is of great significance.
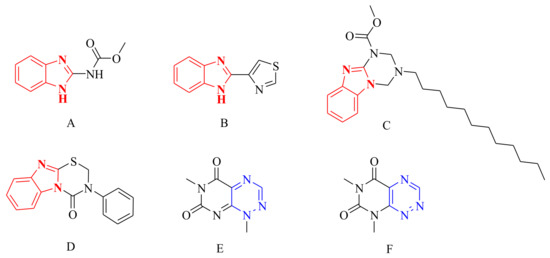
Figure 1.
Compounds bearing a benzimidazole or triazine fragment. (A) Carbendazim; (B) Thiabendazole; (C) Hoe-22845; (D) 3-Phenyl-benzo[4,5]imidazo[2,1-b][1,3,5]thiadiazin-4-one; (E) Toxoflavin; (F) Fervenulin.
In continuation of our research on searching for novel agricultural fungicides, the above-mentioned active substructures in different compounds were comprehensively considered to design and synthesize a series of novel benzo[4,5]imidazo[1,2-d][1,2,4]triazine derivatives. The obtained target compounds were confirmed by FT-IR, 1H NMR, 13C NMR, EI-MS, and single crystal X-ray diffraction. Meanwhile, their agricultural antifungal activities against Botrytis cinerea (B. cinerea), Rhizoctonia solani (R. solani), and Colletotrichum capsici (C. capsici) were evaluated. To the best of our knowledge, this is the first report on the agricultural antifungal activity of benzo[4,5]imidazo[1,2-d][1,2,4]triazine derivatives.
2. Results and Discussion
2.1. Synthesis Strategy for Intermediate 3
The target compounds 4a–4w and their intermediates 2a, 2b, and 3a–3g were successfully achieved, and the synthetic route is illustrated in Scheme 1. The compounds 2a and 2b were synthesized by the reactions of 1,2-diaminobenzene with 2-chloroacetic acid or 2-bromopropionic acid in 70–74% yields, respectively. Then, using Et3N as catalyst, the yellow powders 3a–3g were obtained in 51–64% yields by reaction of compound 2 with substituted phenylhydrazine in refluxing MeOH.
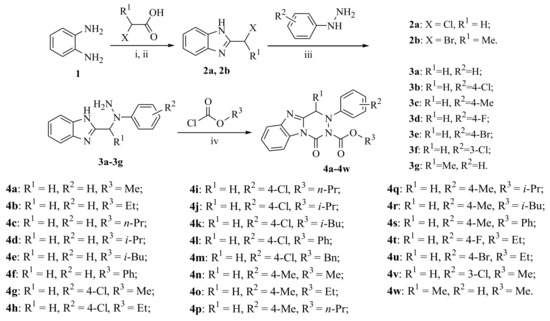
Scheme 1.
Synthetic route to the target compounds 4a–4w. Reagents and conditions: (i) 2-Chloroacetic acid or 2-bromopropionic acid, 0.15 g/mL hydrochloric acid/r.t., reflux, 4 h; (ii) NaHCO3/r.t.; (iii) Substituted phenylhydrazines, Et3N, MeOH/reflux, 4 h; (iv) Et3N, chloroformates, THF/0–5 °C, 2–8 h.
According to the procedure reported in the literature [23,24], the nucleophilic substitution of intermediate 2 with substituted phenylhydrazines was carried out in refluxed methanol for 5 h to obtain 2-((2-(substituted phenyl)hydrazinyl)methyl)-1H-benzo[d]imidazole. However, in the present work, the intermediates 3a–3f (2-((1-(substituted phenyl)hydrazinyl)methyl)-1H-benzo[d]imidazole) and 3g (2-(1-(1-phenylhydrazinyl)ethyl)-1H-benzo[d]imidazole) were successfully prepared using similar materials in refluxed methanol containing Et3N for 4 h. It is an interesting phenomenon that using similar materials and procedures to permit different nucleophilic substitutions happened at different N-positions of the substituted phenylhydrazine groups (Scheme 2). Noteworthily, their only difference is the existence of excess Et3N as the catalyst. In the present work, Et3N was used as an acid binding agent to promote the nucleophilic substitution reaction at the secondary nitrogen position of the hydrazine group. This synthetic strategy in the present work is quite practical and plays a significant role in synthesis of the novel fused heterocycle benzo[4,5]imidazo[1,2-d][1,2,4]triazine derivatives. The above phenomenon was clearly confirmed by the crystal structure of compound 4n. As illustrated in Figure 2, the presence of 4-methylphenyl group at N-3 position of compound 4n indirectly proves the structural correctness of the compounds 3a–3g.
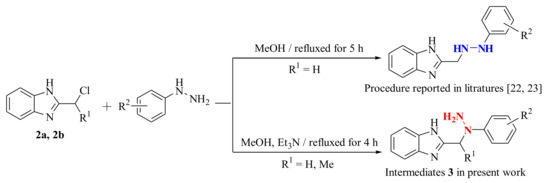
Scheme 2.
Two nucleophilic substitutions of intermediate 2 with substituted phenylhydrazines.
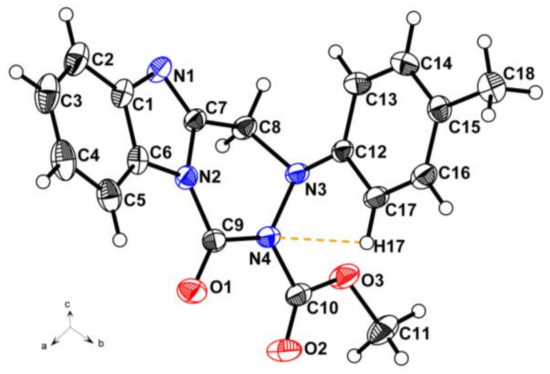
Figure 2.
Crystal structure diagram of compound 4n with intramolecular hydrogen bond.
2.2. Synthesis and Characterization of Title Compounds 4
The compounds 3a–3g were reacted with different chloroformates to gain the target compounds 4a–4w with 26–38% yields. All the synthesized compounds 4a–4w were confirmed via IR, 1H NMR, 13C NMR, and EI-MS. The characteristic absorption at 1740–1790 cm−1 was attributed to the presence of C=O groups. In the 1H NMR spectra, the aromatic protons and the 1,2,4-triazine methylene protons of compounds 4a–4w ranged 6.94–8.02 and 5.12–5.84 ppm, respectively. In the 13C NMR spectra, the absorption peaks at 150.24–153.56 and 113.83–149.01 ppm revealed the presences of C=O groups and aromatic carbons of compounds 4a–4w. Meanwhile, the signals at 55.01–73.73 and 48.07–53.90 ppm signified the carbons in the 2-position of benzimidazole and the 6-position of 1,2,4-triazine, respectively. The mass spectral data of the target compounds 4a–4w were consistent with their chemical structures.
2.3. X-ray Crystal Structure of Compound 4n
As a representative example, the structure of compound 4n was further studied using single crystal X-ray analysis. The obtained crystal data was solved, refined, and is summarized in Table 1. The corresponding single crystal structure and crystal packing diagrams are shown by Figure 2, Figure 3 and Figure 4, respectively.

Table 1.
Crystal data of title compound 4n.
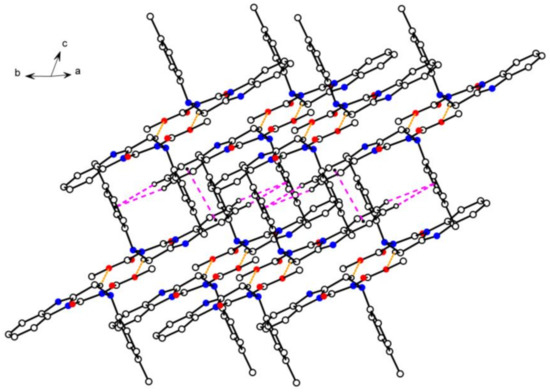
Figure 3.
Crystal packing diagram of compound 4n. Yellow dashed lines show intermolecular hydrogen bonds. Pink dashed lines show π···π interactions.
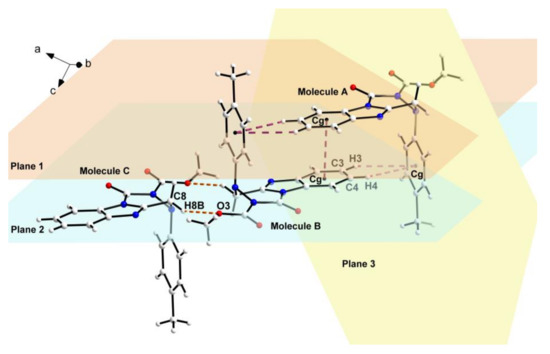
Figure 4.
Partial crystal parking diagram of compound 4n. Planes 1, 2, and 3 indicate respectively three planes containing benzimidazole ring of molecule A, benzimidazole ring of molecule B, and benzene ring of molecule A.
As shown in the crystal structure of compound 4n (Figure 2), the aromatic benzimidazole and benzene rings and the nonaromatic hexatomic ring containing C7, C8, N2, N3, N4, and C9 atoms construct the skeletal structure of benzo[4,5]imidazo[1,2-d][1,2,4]triazine derivatives. In the single crystal structure, the intramolecular hydrogen bond C17–H17···N4 combines with the N3 and C12 atoms to form a latent penta-heterocycle. In the above penta-heterocycle, the angle of C17–H17···N4 and the distance between H17 and N4 were 100° and 2.450 Å, respectively. Meanwhile, owing to the electron delocalization, the single bonds N1–C1 (1.410(2) Å), N2–C6 (1.406(2) Å), N2–C7 (1.401(2) Å), N2–C9 (1.402(2) Å), N4–C9 (1.408(2) Å) and N4–C10 (1.417(2) Å) were obviously shorter than the normal bond N–C (1.47–1.50 Å), and the double bond C7=N1 (1.293(2) Å) was also shorter than the normal bond C=N (1.34–1.38 Å). The relevant data of all the bond lengths and angles in the crystal structure of compound 4n are listed in the Supporting Information.
As shown in the crystal parking diagram of compound 4n (Figure 3), the space network structure with many cavities was obviously constituted by the intermolecular hydrogen bond (C8–H8B···O3) and the intermolecular π–π stacking interactions. In order to further understand the crystal structure of the title compounds, the partial crystal parking diagram of 4n containing three single molecules were obtained and are shown in Figure 4. In this Figure, planes 1, 2, and 3 indicate three planes containing the benzimidazole ring of molecule A, the benzimidazole ring of molecule B, and the benzene ring of molecule A, respectively. The Cg means the centers of all the benzene rings in the single crystal structure of compound 4n. As shown in Figure 4, molecules B and C were connected by the intermolecular hydrogen bond C8–H8B···O3, and the molecules A and B were combined by the two kinds of π–π stacking interactions. These π–π stacking interactions contain an offset face-to-face stacking interaction between two benzimidazole rings and an edge-to-face stacking interaction between benzimidazole and benzene rings in different molecules. In the face-to-face stacking interaction, the centroid-centroid and centroid-plane distances were 3.5279(18) Å and 3.3624(4) Å between the parallel Planes 1 and 2, respectively, which indicates a strong π–π stacking interactions existing in the crystal structure of compound 4n. In the edge-to-face stacking interaction, the distances of C3···Cg, H3···Cg, C4···Cg, and H4···Cg were 3.7844(33) Å, 3.0998(17) Å, 3.8439(29) Å, and 3.2161(14) Å, respectively. Moreover, the angles of C3–H3···Cg and C4–H4···Cg were 131.905(150)° and 126.606(126)°, respectively.
2.4. Antifungal Activity
Using the mycelium growth rate method on PSA (potato sucrose agar) medium [25], the inhibition effects of title compounds 4a–4w against the phytopathogenic fungi including B. cinerea, R. solani, and C. capsici were tested in vitro at 50 μg/mL. The agricultural fungicide carbendazim was used as the standard antifungal reference. Antifungal results in Table 2 indicated that compounds 4a, 4g–4o, 4q, 4u, and 4v shown good activities toward B. cinerea with inhibitory rates of 62.1%, 70.5%, 68.6%, 68.4%, 51.5%, 50.8%, 50.5%, 50.8%, 69.2%, 76.7%, 52.1%, 64.4% and 62.1%, respectively. The compounds 4b, 4f–4i, 4n–4p, and 4s–4v obviously inhibited the mycelium growth of R. solani, with inhibitory rates of 63.5%, 57.3%, 58.0%, 53.7%, 50.6%, 53.9%, 57.8%, 52.8%, 61.4%, 50.2%, 60.6% and 61.1%, respectively. The mycelium growth inhibition rates of compounds 4a, 4g, 4l, 4n, and 4t–4v against C. capsici reached respectively 52.1%, 60.3%, 57.8%, 58.9%, 66.8%, 51.7% and 60.1%. Noteworthily, the inhibitory rates of compounds 4g, 4n, 4u, and 4v against the above all tested fungi exceeded 50%. Meanwhile, compounds 4o against B. cinerea, 4b against R. solani, and 4t against C. capsici showed the highest antifungal activities with inhibitory rates of 76.7%, 63.5%, and 66.8%, respectively.

Table 2.
Antifungal activity of compounds 4 against three test fungi at 50 μg/mL.
2.5. Structure–Activity Relationships
Most of the title compounds showed obvious antifungal activities against B. cinerea, R. solani, and C. capsici, and some structure–activity relationships can be analyzed and summarized. First, the presence of electron-withdrawing groups, such as fluorine, chlorine, and bromine atoms, at R2 position contributes to the enhancement of antifungal activities. For example, the inhibitory rates of compound 4g against B. cinerea, R. solani, and C. capsici were 70.5%, 58.0%, and 60.3%, respectively, which were better than that of compounds 4a (62.1%, 32.0%, and 52.1%) and 4n (69.2%, 53.9%, and 58.9%). Second, the introduction of small alkyls, such as methyl, ethyl, n-propyl, and i-propyl groups at the R3 position plays a positive effect on the antifungal activities of title compounds. For instance, compounds 4g (R3 = Me), 4h (R3 = Et), 4i (R3 = n-Pr), and 4j (R3 = i-Pr) shown higher activities than 4k (R3 = i-Bu), 4l (R3 = Ph), and 4m (R3 = Bn) against almost all tested phytopathogenic fungi. Third, a methyl group at the R1 position of the title compounds weakened their antifungal activities. For example, the inhibitory rates of compound 4a against B. cinerea, R. solani, and C. capsici were respectively 62.1, 32.0, and 52.1%, which were higher than that of the compounds 4w (45.9, 28.1, and 26.6%, respectively).
3. Experimental Section
3.1. General
All melting points of the title compounds were determined on an uncorrected WRS-1B digital melting point apparatus (Jingmi Science, Shanghai, China). 1H NMR and 13C NMR spectra were measured on Bruker 400 spectrometer (Bruker, Karlsruhe, Germany) using DMSO-d6 as solvent and TMS as internal standard. Mass spectra were recorded on a TRACE 2000 spectrometer (Finnigan, Silicon Valley, CA, USA). The compound samples prepared with KBr disk were gauged on a Thermo Nicolet 380 FT-IR spectrometer to obtain the corresponding infrared spectra. Progress of the reactions was monitored by thin layer chromatography on silica gel GF254 (Yuhua, Gongyi, China). All reagents and solvents purchased from commercial suppliers were analytically or chemically pure and were not further purified.
3.2. Chemistry
3.2.1. General Procedure for Synthesis of Compounds 2
The intermediate 2a was synthesized according to the literature [16] with minor modification. The mixture of o-phenylenediamine (20 mmol) and chloroacetic acid (22 mmol) in 0.15 g/mL hydrochloric acid (120 mL) was refluxed under 108 °C for 4 h. Then, the obtained mixture was cooled to room temperature and neutralized with sodium bicarbonate until the pH value was over 7.0 to generate yellow solid 2a. The 2-(chloromethyl)-1H-benzo[d]imidazole 2a was obtained with the 70.3% yield, m.p. 147.6 °C (Ref. 146–148 °C [16]).
Using the similar method, o-phenylenediamine was reacted with 2-bromopropionic acid to obtain the intermediate 2b. 2-(1-Bromoethyl)-1H-benzo[d]imidazole (2b): white powder; yield 73.7%; m.p. 130.8–132.4 °C; IR (KBr, cm−1) v: 2982, 2818, 2731, 1430, 1272, 1226, 745, 690, 617; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 10.17 (s, 1H), 6.74 (dd, J = 17.2, 7.7 Hz, 2H), 6.67 (d, J = 7.3 Hz, 1H), 6.59 (d, J = 7.5 Hz, 1H), 3.76 (q, J = 5.3 Hz, 1H), 1.24 (d, J = 6.6 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 153.73, 138.72, 122.79, 115.85, 114.69, 51.76, 23.54.
3.2.2. General Procedure for Synthesis of Compounds 3
Triethylamine (6.3 mL, 45 mmol) was added to a mixture of compounds 2a (5.0 g, 30 mmol) and substituted hydrazinobenzene (33 mmol) in methanol (50 mL). After refluxing for 4 h, the mixture was poured into cooled water, and then precipitated to obtain the yellow solids 3a–3f. Using the same procedure and intermediate 2b as a material, the yellow solid 3g was synthesized with 60.7% yield.
2-((1-Phenylhydrazinyl)methyl)-1H-benzo[d]imidazole (3a): yellow powder; yield 51.4%; m.p. 138.8–139.9 °C; IR (KBr, cm−1) v: 3255, 3146, 3055, 1596, 1493, 1420, 1198, 767, 738, 689; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 12.37 (br, 1H), 7.50 (s, 2H), 7.21–7.09 (m, 4H), 7.06 (d, J = 8.0 Hz, 2H), 6.65 (t, J = 6.7 Hz, 1H), 4.82 (s, 2H), 4.79 (br, 2H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 152.81, 151.99, 129.08, 121.89, 117.80, 113.50, 53.80.
2-((1-(4-Chlorophenyl)hydrazinyl)methyl)-1H-benzo[d]imidazole (3b): yellow powder; yield 60.2%; m.p. 246.6–247.8 °C; IR (KBr, cm−1) v: 3319, 3054, 2995, 1592, 1491, 1422, 1032, 1023, 857, 807, 739, 747; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 12.39 (br, 1H), 7.51 (s, 2H), 7.30–7.06 (m, 6H), 4.86 (br, 2H), 4.84 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 152.28, 150.86, 128.70, 121.96, 121.27, 114.98, 53.63.
2-((1-(4-Tolyl)hydrazinyl)methyl)-1H-benzo[d]imidazole (3c): yellow powder; yield 63.6%; m.p. 157.2–159.1 °C; IR (KBr, cm−1) v: 3313, 3029, 2915, 1513, 1424, 1310, 899, 805, 745; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 12.16 (br, 1H), 7.49 (dd, J = 5.0, 3.2 Hz, 2H), 7.12 (dd, J = 5.9, 3.1 Hz, 2H), 6.96 (q, J = 8.8 Hz, 4H), 4.78 (br, 2H), 4.77 (s, 2H), 2.15 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 152.85, 150.01, 129.51, 126.54, 121.84, 113.93, 54.14, 20.45.
2-((1-(4-Fluorophenyl)hydrazinyl)methyl)-1H-benzo[d]imidazole (3d): yellow powder; yield 58.7%; m.p. 246.0–247.4 °C; IR (KBr, cm−1) v: 3349, 3047, 2982, 1503, 1430, 1216, 821, 746, 699; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 12.55 (s, 1H), 7.50 (s, 2H), 7.13 (dd, J = 5.8, 3.1 Hz, 2H), 7.07 (dd, J = 8.9, 4.6 Hz, 2H), 6.98 (t, J = 8.8 Hz, 2H), 4.81 (br, 2H), 4.78 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 152.52, 148.87, 121.94, 115.84, 115.52, 115.16, 54.34.
2-((1-(4-Bromophenyl)hydrazinyl)methyl)-1H-benzo[d]imidazole (3e): yellow powder; yield 55.4%; m.p. 225.7–227.7 °C; IR (KBr, cm−1) v: 3221, 3060, 2914, 1590, 1487, 1451, 1437, 1319, 1023, 804, 747; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 12.28 (s, 1H), 7.51 (s, 2H), 7.27 (d, J = 8.7 Hz, 2H), 7.13 (dd, J = 5.8, 3.1 Hz, 2H), 7.01 (d, J = 8.8 Hz, 2H), 4.90 (s, 2H), 4.84 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 152.19, 151.17, 131.51, 121.99, 115.50, 108.81, 53.48.
2-((1-(3-Chlorophenyl)hydrazinyl)methyl)-1H-benzo[d]imidazole (3f): yellow powder; yield 60.8%; m.p. 253.6–255.4 °C; IR (KBr, cm−1) v: 3334, 2983, 2605, 1590, 1495, 1420, 1364, 1271, 990, 857, 739, 687; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 12.30 (s, 1H), 7.50 (s, 2H), 7.14–7.10 (m, 4H), 6.94 (d, J = 8.4 Hz, 1H), 6.65 (d, J = 7.7 Hz, 1H), 4.85 (s, 2H), 4.81 (br, 2H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 153.26, 152.17, 134.01, 130.53, 121.96, 116.90, 112.92, 111.61, 53.31.
2-(1-(1-Phenylhydrazinyl)ethyl)-1H-benzo[d]imidazole (3g): yellow powder; yield 60.7%; m.p. 232.9–233.4 °C; IR (KBr, cm−1) v: 3325, 2869, 2737, 1589, 1480, 1435, 1416, 1325, 876, 811, 759, 746, 677; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 12.18 (s, 1H), 7.49 (s, 2H), 7.28–7.01 (m, 6H), 6.68 (t, J = 6.5 Hz, 1H), 5.32 (q, J = 6.6 Hz, 1H), 4.30 (br, 2H), 1.61 (d, J = 6.7 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ(ppm): 156.22, 151.97, 129.11, 121.79, 118.04, 114.14, 54.39, 15.89.
3.2.3. General Procedure for Synthesis of Compounds 4
The chloroformates (8.2 mmol) were added dropwise to a mixture of compound 3 (4 mmol), Et3N (1.8 mL, 13 mmol) and tetrahydrofuran (10 mL) at 0 °C. Then, the obtained mixture was stirred at 0–5 °C for 2–8 h and monitored by thin layer chromatography on silica gel GF254 using the mixture of ethyl acetate and petroleum ether (V:V = 1:1). After completion of the reaction, the mixture was filtered, concentrated in vacuo and purified on a silica gel column to afford the title compounds 4.
Methyl 1-oxo-3-phenyl-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4a): white powder; yield 33.3%; m.p. 161.2–163.4 °C; IR (KBr, cm−1) v: 1745, 1565, 1498, 1442; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.00–7.93 (m, 1H), 7.72–7.65 (m, 1H), 7.41–7.34 (m, 2H), 7.29 (dd, J = 8.7, 7.4 Hz, 2H), 7.10 (d, J = 8.0 Hz, 2H), 6.95 (t, J = 7.3 Hz, 1H), 5.57 (s, 1H), 5.18 (s, 1H), 3.91 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 152.10, 151.08, 147.47, 145.58, 142.76, 131.22, 130.19, 125.69, 123.11, 120.35, 115.26, 114.40, 55.06, 48.20; EI-MS (m/z): 322, found 322 [M]+.
Ethyl 1-oxo-3-phenyl-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4b): white powder; yield 32.1%; m.p. 177.1–179.3 °C; IR (KBr, cm−1) v: 1740, 1568, 1497, 1451; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.00–7.93 (m, 1H), 7.72–7.65 (m, 1H), 7.41–7.34 (m, 2H), 7.29 (t, J = 8.0 Hz, 2H), 7.10 (d, J = 8.0 Hz, 2H), 6.95 (t, J = 7.3 Hz, 1H), 5.57 (s, 1H), 5.18 (s, 1H), 4.36 (q, J = 7.1 Hz, 2H), 1.30 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.54, 151.13, 147.51, 145.62, 142.77, 131.23, 130.19, 125.66, 123.07, 120.33, 115.22, 114.41, 64.29, 48.16, 14.58; EI-MS (m/z): 336, found 336 [M]+.
Propyl 1-oxo-3-phenyl-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4c): white powder; yield 34.2%; m.p. 161.4–162.9 °C; IR (KBr, cm−1) v: 1788, 1709, 1624, 1596; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.96 (d, J = 8.0 Hz, 1H), 7.67 (d, J = 7.0 Hz, 1H), 7.44–7.33 (m, 2H), 7.29 (t, J = 7.6 Hz, 2H), 7.07 (d, J = 8.0 Hz, 2H), 6.94 (t, J = 7.3 Hz, 1H), 5.55 (s, 1H), 5.17 (s, 1H), 4.28 (t, J = 6.4 Hz, 2H), 1.76–1.62 (m, 2H), 0.92 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.59, 151.15, 147.50, 145.64, 131.23, 130.17, 125.65, 123.06, 120.31, 115.20, 114.42, 69.51, 48.19, 21.99, 10.58; EI-MS (m/z): 350, found, 350 [M]+.
Isopropyl 1-oxo-3-phenyl-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4d): yellow powder; yield 31.3%; m.p. 199.1–200.5 °C; IR (KBr, cm−1) v: 1757, 1731, 1570, 1497; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.95 (d, J = 7.0 Hz, 1H), 7.67 (d, J = 7.3 Hz, 1H), 7.42–7.33 (m, 2H), 7.28 (t, J = 7.6 Hz, 2H), 7.05 (d, J = 8.1 Hz, 2H), 6.94 (t, J = 7.1 Hz, 1H), 5.53 (s, 1H), 5.18 (s, 1H), 5.11 (dt, J = 12.3, 6.1 Hz, 1H), 1.32 (d, J = 3.3 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.10, 147.54, 145.64, 142.77, 131.24, 130.16, 125.62, 123.01, 120.30, 115.17, 114.41, 72.61, 48.14, 22.00; EI-MS (m/z): 350, found, 350 [M]+.
Isobutyl 1-oxo-3-phenyl-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4e): white powder; yield 30.3%; m.p. 171.3–174.5 °C; IR (KBr, cm−1) v: 1743, 1572, 1496, 1448; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.97 (d, J = 6.7 Hz, 1H), 7.68 (d, J = 7.6 Hz, 1H), 7.42–7.33 (m, 2H), 7.29 (t, J = 7.8 Hz, 2H), 7.08 (d, J = 8.1 Hz, 2H), 6.94 (t, J = 7.2 Hz, 1H), 5.57 (s, 1H), 5.18 (s, 1H), 4.12 (d, J = 6.2 Hz, 2H), 2.06–1.87 (m, 1H), 0.92 (d, J = 6.6 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.59, 151.14, 147.52, 145.66, 142.80, 131.25, 130.16, 125.65, 123.07, 120.32, 115.22, 114.45, 73.63, 48.27, 27.83, 19.09; EI-MS (m/z): 364, found 364 [M]+.
Phenyl 1-oxo-3-phenyl-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4f): yellow powder; yield 37.3%; m.p. 185.7–189.2 °C; IR (KBr, cm−1) v: 1750, 1567, 1496, 1485; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.98 (d, J = 7.3 Hz, 1H), 7.71 (d, J = 7.6 Hz, 1H), 7.49 (q, J = 7.7 Hz, 2H), 7.43–7.38 (m, 2H), 7.37–7.28 (m, 5H), 7.21 (d, J = 8.5 Hz, 2H), 6.98 (t, J = 7.2 Hz, 1H), 5.64 (s, 1H), 5.33 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.09, 150.73, 150.41, 147.31, 145.43, 142.79, 131.20, 130.29, 130.20, 127.03, 125.86, 123.30, 122.07, 121.75, 120.45, 115.34, 114.46, 48.09; EI-MS (m/z): 384, found 384 [M]+.
Methyl 1-oxo-3-(4-chlorophenyl)-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4g): yellow powder; yield 32.3%; m.p. 144.5–146.8 °C; IR (KBr, cm−1) v: 1789, 1613, 1559, 1490; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.99–7.90 (m, 1H), 7.73–7.66 (m, 1H), 7.44–7.37 (m, 2H), 7.34 (d, J = 9.1 Hz, 2H), 7.13 (d, J = 9.1 Hz, 2H), 5.57 (s, 1H), 5.18 (s, 1H), 3.91 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.97, 150.86, 146.44, 145.33, 142.75, 131.23, 129.98, 127.20, 125.74, 120.37, 117.14, 114.43, 60.24, 55.13, 48.22; EI-MS (m/z): 356, found 356 [M]+.
Ethyl 1-oxo-3-(4-chlorophenyl)-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4h): yellow powder; yield 34.8%; m.p. 162.1–163.8 °C; IR (KBr, cm−1) v: 1790, 1747, 1570, 1492; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.02–7.92 (m, 1H), 7.73–7.66 (m, 1H), 7.44–7.30 (m, 4H), 7.12 (d, J = 9.0 Hz, 2H), 5.58 (s, 1H), 5.18 (s, 1H), 4.37 (q, J = 7.0 Hz, 2H), 1.31 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.39, 150.90, 146.47, 145.37, 142.76, 131.22, 129.99, 127.13, 125.73, 120.37, 117.09, 114.43, 64.41, 48.19, 14.56; EI-MS (m/z): 370, found 370 [M]+.
Propyl 1-oxo-3-(4-chlorophenyl)-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4i): yellow powder; yield 34.1%; m.p. 150.3–152.1 °C; IR (KBr, cm−1) v: 1785, 1711, 1596, 1574; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.00–7.94 (m, 1H), 7.70 (dd, J = 6.3, 2.4 Hz, 1H), 7.43–7.37 (m, 2H), 7.37–7.31 (m, 2H), 7.13 (dd, J = 9.7, 2.7 Hz, 2H), 5.59 (s, 1H), 5.19 (s, 1H), 4.29 (t, J = 6.5 Hz, 2H), 1.77–1.60 (m, 2H), 0.93 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.44, 150.91, 146.48, 145.39, 142.77, 131.23, 129.98, 127.12, 125.72, 120.36, 117.08, 114.46, 69.63, 48.24, 21.98, 10.59; EI-MS (m/z): 384, found 384 [M]+.
Isopropyl 1-oxo-3-(4-chlorophenyl)-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4j): white powder; yield 35.3%; m.p. 165.5–168.3 °C; IR (KBr, cm−1) v: 1787, 1713, 1569, 1494; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.98–7.93 (m, 1H), 7.73–7.63 (m, 1H), 7.43–7.37 (m, 2H), 7.34 (d, J = 8.8 Hz, 2H), 7.09 (d, J = 8.9 Hz, 2H), 5.56 (s, 1H), 5.14 (s, 1H), 5.15–5.02 (m, 1H), 1.33 (d, J = 3.2 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 150.91, 146.51, 145.40, 142.77, 131.23, 129.99, 127.09, 125.70, 120.35, 117.05, 114.45, 72.78, 48.21, 22.00; EI-MS (m/z): 384, found 384 [M]+.
Isobutyl 1-oxo-3-(4-chlorophenyl)-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4k): white powder; yield 32.3%; m.p.158.0–159.7 °C; IR (KBr, cm−1) v: 1786, 1707, 1619, 1573; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.00–7.93 (m, 1H), 7.72–7.65 (m, 1H), 7.43–7.37 (m, 2H), 7.35 (d, J = 7.9 Hz, 2H), 7.11 (d, J = 8.9 Hz, 2H), 5.57 (s, 1H), 5.17(s, 1H), 4.12 (d, J = 6.3 Hz, 2H), 1.98 (tq, J = 12.7, 6.3 Hz, 1H), 0.93 (d, J = 6.7 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.43, 150.90, 146.49, 145.41, 142.78, 131.24, 129.96, 127.13, 125.69, 120.34, 117.08, 114.48, 73.73, 48.30, 27.80, 19.08; EI-MS (m/z): 398, found 398 [M]+.
Phenyl 1-oxo-3-(4-chlorophenyl)-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4l): yellow powder; yield 36.0%; m.p. 175.5–177.6 °C; IR (KBr, cm−1) v: 1751, 1570, 1491, 1450; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.06–7.94 (m, 1H), 7.74 (d, J = 6.8 Hz, 1H), 7.52 (t, J = 7.8 Hz, 2H), 7.41 (dt, J = 17.1, 7.6 Hz, 7H), 7.29 (d, J = 9.1 Hz, 2H), 5.68 (s, 1H), 5.37 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 150.76, 150.24, 146.25, 145.17, 142.76, 131.19, 130.17, 127.37, 127.05, 125.90, 122.05, 120.47, 117.25, 114.49, 48.13; EI-MS (m/z): 418, found 418 [M]+.
Benzyl 1-oxo-3-(4-chlorophenyl)-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4m): white powder; yield 33.7%; m.p. 158.5–161.2 °C; IR (KBr, cm−1) v: 1747, 1567, 1492, 1449; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.97 (dd, J = 6.3, 2.6 Hz, 1H), 7.70 (dd, J = 6.2, 2.5 Hz, 1H), 7.51–7.27 (m, 9H), 7.14 (d, J = 9.1 Hz, 2H), 5.59 (s, 1H), 5.42 (s, 2H), 5.20 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.40, 150.90, 146.43, 145.35, 142.77, 135.66, 131.21, 129.98, 128.97, 128.78, 128.27, 127.21, 125.76, 120.38, 117.16, 114.48, 69.33, 48.25; EI-MS (m/z): 432, found 432 [M]+.
Methyl 1-oxo-3-(4-tolyl)-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4n): white powder; yield 37.3%; m.p. 171.0–173.2 °C; IR (KBr, cm−1) v: 1792, 1574, 1508, 1454; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.95 (d, J = 7.8 Hz, 1H), 7.68 (d, J = 7.0 Hz, 1H), 7.38 (p, J = 7.3 Hz, 2H), 7.08 (d, J = 8.3 Hz, 2H), 6.96 (d, J = 8.3 Hz, 2H), 5.48 (s, 1H), 5.14 (s, 1H), 3.89 (s, 3H), 2.16 (d, J = 19.4 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 152.12, 151.12, 145.65, 145.18, 142.77, 132.22, 131.22, 130.56, 125.65, 120.31, 115.32, 114.36, 55.01, 48.39, 20.35; EI-MS (m/z): 336, found 336 [M]+.
Ethyl 1-oxo-3-(4-tolyl)-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4o): white powder; yield 35.3%; m.p. 178.1–180.0 °C; IR (KBr, cm−1) v: 1783, 1613, 1565, 1508; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.95 (dd, J = 6.3, 2.5 Hz, 1H), 7.67 (dd, J = 6.3, 2.5 Hz, 1H), 7.40–7.32 (m, 2H), 7.08 (d, J = 8.5 Hz, 2H), 6.96 (d, J = 8.7 Hz, 2H), 5.48 (s, 1H), 5.14 (s, 1H), 4.36 (q, J = 7.1 Hz, 2H), 2.13 (s, 3H), 1.30 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.57, 151.15, 145.68, 145.23, 142.77, 132.18, 131.22, 130.56, 125.62, 120.30, 115.28, 114.38, 64.22, 48.37, 20.36, 14.58; EI-MS (m/z): 350, found 350 [M]+.
Propyl 1-oxo-3-(4-tolyl)-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4p): white powder; yield 34.2%; m.p. 145.0–146.8 °C; IR (KBr, cm−1) v: 1790, 1617, 1569, 1514; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.01–7.90 (m, 1H), 7.75–7.62 (m, 1H), 7.44–7.29 (m, 2H), 7.08 (d, J = 8.4 Hz, 2H), 6.96 (d, J = 8.7 Hz, 2H), 5.49 (s, 1H), 5.12 (s, 1H), 4.28 (t, J = 6.5 Hz, 2H), 2.13 (s, 3H), 1.77–1.60 (m, 2H), 0.92 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.65, 151.17, 145.70, 145.25, 142.78, 132.17, 131.23, 130.55, 125.61, 120.30, 115.28, 114.40, 69.45, 48.40, 22.00, 20.36, 10.59; EI-MS (m/z): 364, found 364 [M]+.
Isopropyl 1-oxo-3-(4-tolyl)-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4q): yellow powder; yield 28.6%; m.p. 165.7–167.6 °C; IR (KBr, cm−1) v: 1784, 1713, 1613, 1569; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.96 (d, 1H), 7.67 (d, J = 6.1, 2.5 Hz, 1H), 7.46–7.30 (m, 2H), 7.08 (d, J = 8.4 Hz, 2H), 6.95 (d, J = 8.5 Hz, 2H), 5.47 (s, 1H), 5.21–4.98 (m, 2H), 2.13 (s, 3H), 1.32 (d, J = 5.2 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.18, 151.10, 145.72, 145.29, 142.80, 132.13, 131.25, 130.55, 125.58, 120.29, 115.25, 114.40, 72.52, 48.38, 22.01, 20.36; EI-MS (m/z): 364, found 364 [M]+.
Isobutyl 1-oxo-3-(4-tolyl)-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4r): white powder; yield 35.3%; m.p. 115.7–116.8 °C; IR 1785, 1751, 1617, 1572; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.96 (dd, J = 6.3, 2.6 Hz, 1H), 7.67 (dd, J = 6.1, 2.6 Hz, 1H), 7.37 (pd, J = 7.4, 3.7 Hz, 2H), 7.08 (d, J = 8.5 Hz, 2H), 6.95 (d, J = 8.7 Hz, 2H), 5.49 (s, 1H), 5.13 (s, 1H), 4.11 (d, J = 6.5 Hz, 2H), 2.14 (s, 3H), 2.03–1.89 (m, 1H), 0.93 (d, J = 6.7 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.64, 151.17, 145.72, 145.26, 142.79, 132.18, 131.24, 130.55, 125.62, 120.29, 115.28, 114.42, 73.57, 48.46, 27.83, 20.37, 19.43, 19.11; EI-MS (m/z): 378, found 378 [M]+.
Phenyl 1-oxo-3-(4-tolyl)-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4s): white powder; yield 37.3%; m.p. 184.2–185.6 °C; IR (KBr, cm−1) v: 1749, 1569, 1510, 1453; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.98 (dd, J = 6.5, 2.5 Hz, 1H), 7.71 (dd, J = 6.2, 2.2 Hz, 1H), 7.54–7.46 (m, 2H), 7.40 (ddd, J = 5.9, 5.3, 3.7 Hz, 2H), 7.35 (t, J = 7.0 Hz, 3H), 7.16–7.07 (m, 4H), 5.58 (s, 1H), 5.32 (s, 1H), 2.16 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.12, 150.73, 145.49, 145.04, 142.79, 132.43, 131.79, 130.70, 130.26, 127.01, 125.82, 122.06, 120.42, 115.40, 114.44, 48.29, 20.40; EI-MS (m/z): 398, found 398 [M]+.
Ethyl 1-oxo-3-(4-fluorophenyl)-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4t): yellow powder; yield 31.1%; m.p. 159.9–161.5 °C; IR (KBr, cm−1) v: 1784, 1709, 1615, 1569; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.96 (d, J = 7.0 Hz, 1H), 7.67 (t, J = 13.3 Hz, 1H), 7.49–7.29 (m, 2H), 7.23–6.99 (m, 4H), 5.52 (s, 1H), 5.16 (s, 1H), 4.37 (q, J = 6.9 Hz, 2H), 1.40–1.24 (m, 3H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.49, 150.99, 145.52, 143.96, 142.78, 131.28, 125.70, 120.36, 117.13, 116.90, 116.68, 114.44, 64.35, 48.54, 14.57; EI-MS (m/z): 354, found 354 [M]+.
Ethyl 1-oxo-3-(4-bromophenyl)-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4u): yellow powder; yield 34.2%; m.p. 151.5–153.4 °C; IR (KBr, cm−1) v: 1590, 1487, 1451, 1437; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.96 (d, J = 7.2 Hz, 1H), 7.69 (d, J = 7.1 Hz, 1H), 7.47 (d, J = 8.0 Hz, 2H), 7.43–7.33 (m, 2H), 7.07 (d, J = 8.0 Hz, 2H), 5.57 (s, 1H), 5.18 (s, 1H), 4.38 (dd, J = 13.8, 6.8 Hz, 2H), 1.31 (t, J = 6.9 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.35, 150.86, 146.92, 145.34, 142.76, 132.86, 131.22, 125.72, 120.36, 117.50, 115.03, 114.43, 64.42, 48.15, 14.56; EI-MS (m/z): 416, found 416 [M]+.
Methyl 1-oxo-3-(3-chlorophenyl)-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4v): yellow powder; yield 28.9%; m.p. 196.2–198.5 °C; IR (KBr, cm−1) v: 1786, 1706, 1616, 1571; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.96 (d, J = 6.8 Hz, 1H), 7.70 (d, J = 7.0 Hz, 1H), 7.40 (td, J = 12.4, 7.9 Hz, 2H), 7.35–7.27 (m, 1H), 7.23 (d, J = 1.8 Hz, 1H), 7.03 (t, J = 9.4 Hz, 2H), 5.63 (s, 1H), 5.14 (s, 1H), 3.90 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 151.87, 150.85, 149.01, 145.27, 142.75, 134.90, 131.90, 131.20, 125.80, 123.00, 120.42, 115.17, 114.47, 113.83, 55.21, 48.07; EI-MS (m/z): 358, found 358 [M]+.
Methyl 1-oxo-3-phenyl-4-methyl-3,4-dihydrobenzo[4,5]imidazo[1,2-d][1,2,4]triazine-2(1H)-carboxylate (4w): yellow powder; yield 26.1%; m.p. 120.0–121.9 °C; IR (KBr, cm−1) v: 1787, 1706, 1616, 1571; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.00 (d, J = 7.8 Hz, 1H), 7.68 (d, J = 7.0 Hz, 1H), 7.44–7.35 (m, 2H), 7.30 (t, J = 7.9 Hz, 2H), 7.13 (d, J = 8.1 Hz, 2H), 6.97 (t, J = 7.2 Hz, 1H), 5.84 (dd, J = 13.4, 6.5 Hz, 1H), 3.93 (s, 3H), 1.70 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 153.56, 152.70, 147.58, 145.33, 142.70, 131.06, 130.23, 125.82, 123.46, 120.42, 115.69, 114.63, 55.31, 53.90, 17.83; EI-MS (m/z): 336, found 336 [M]+.
3.3. Single Crystal X-ray Diffraction Analysis
The single crystal of compound 4n was recrystallized from tetrahydrofuran to obtain a suitable single crystal. The X-ray single crystal diffraction data were collected on a Bruker Smart APEX Ⅱ CCD Single-crystal diffractometer (Bruker, German) with graphite monochromatized Mo Kα radiation (λ = 0.71073 Å) using a φ-ω scan mode at 296(2)K. The obtained crystal structure solved directly and was refined by full-matrix least-squares method via SHELXTL [26]. The crystallographic data for the compound 4n have been deposited in CCDC-1543178. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033; E-mail: deposit@ccdc.cam.ac.uk).
3.4. Antifungal Bioassay
The antifungal activity of all the title compounds was evaluated in vitro with the mycelium growth rate method [25]. The tested strains B. cinerea, R. solani, and C. capsici, which were, respectively, isolated from infected strawberries, rice, and peppers in disease outbreak regions in Jiangsu Province, were provided by the Laboratory of Plant Disease Control at Nanjing Agricultural University. Every sample (4.5 mg) was dissolved in 0.5 mL methanol and evenly mixed with 89.5 mL PSA (potato sucrose agar) medium. An equal volume of methanol in 89.5 mL medium was used as the blank control. Meanwhile, the commercial agricultural fungicide carbendazim was tested as the positive control at the same concentration. Each 15 mL medium was poured into a 9 cm petri plate with 3 replicates. The fungi were inoculated to the center of the medium and cultured at 25 ± 1 °C for 3–5 days in a dark environment. After a certain incubation period (2.5 d for B. cinerea, 1.5 d for R. solani, and 4 d for C. capsici), the diameters of the mycelium colonies were measured, and the inhibitory percentages were calculated.
4. Conclusions
In continuation of our research searching for novel agricultural fungicides, a series of novel benzo[4,5]imidazo[1,2-d][1,2,4]triazine derivatives were synthesized by a practical and convenient reaction route. These compounds were well supported by spectroscopic data and single crystal X-ray diffraction analysis. The bioassays indicated that most of the title compounds exhibited obvious antifungal activities in vitro at 50 μg/mL. Further studies on structure optimization of benzo[4,5]imidazo[1,2-d][1,2,4]triazines as a leading structure of novel agricultural fungicides are well underway.
Supplementary Materials
The supplementary materials containing FT-IR, 1H NMR, 13C NMR, EI-MS spectra of the title compounds and the crystal structure data of compound 4n can be accessed online.
Acknowledgments
The authors gratefully acknowledge the financial support of the Fundamental Research Funds for the Central Universities of China (KYTZ201604) and the Fund for Independent Innovation of Agricultural Sciences in Jiangsu Province of China (CX(15)1001).
Author Contributions
C.-L.Y. conceived and designed the experiments; L.-X.L. and J.J. carried out the synthesis and characterization of all compounds; M.C. performed the X-ray analysis; X.-B.W., X.-C.F. and W.-J.S. preformed the antifungal activity assay; C.-L.Y. and X.-B.W. prepared the manuscript for publication; All authors discussed the contents of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, W.; He, J.; He, M.; Han, F.; Chen, X.; Pan, Z.; Wang, J.; Tong, M. Synthesis and antifungal activity of novel sulfone derivatives containing 1,3,4-oxadiazole moieties. Molecules 2011, 16, 9129–9141. [Google Scholar] [CrossRef] [PubMed]
- Li, S.B.; Hu, D.Y.; Song, B.A.; Yang, S.; Jin, L.H.; Xue, W.; Zeng, S.; Wang, J.; Chen, Z.; Lu, P.; et al. Synthesis and antifungal activity of novel 1,5-diphenyl-1,4-pentadien-3-one oxime esters. Chin. J. Org. Chem. 2008, 28, 311–316. [Google Scholar]
- Dai, Z.C.; Chen, Y.F.; Zhang, M.; Li, S.K.; Yang, T.T.; Shen, L.; Wang, J.X.; Qian, S.S.; Zhu, H.L.; Ye, Y.H. Synthesis and antifungal activity of 1,2,3-triazole phenylhydrazone derivatives. Org. Biomol. Chem. 2015, 13, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ge, S.; Huang, J.; Bao, X. Synthesis of novel (E)-2-(4-(1H-1,2,4-triazol-1-yl)styryl)-4-(alkyl/arylmethyleneoxy)quinazoline derivatives as antimicrobial agents. Mol. Divers. 2018, 22, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, P.; Li, Z.; Yin, J.; He, M.; Xue, W.; Chen, Z.; Song, B.A. Synthesis and bioactivity evaluation of novel arylimines containing a 3-aminoethyl-2-[(p-trifluoromethoxy)anilino]-4(3H)-quinazolinone moiety. J. Agric. Food Chem. 2013, 61, 9575–9582. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, S.; Bhadury, P.; He, J.; He, M.; Gao, L.; Hu, D.; Song, B.A. Synthesis and bioactivity of novel sulfone derivatives containing 2,4-dichlorophenyl substituted 1,3,4-oxadiazole/thiadiazole moiety as chitinase inhibitors. Pestic. Biochem. Phys. 2011, 101, 6–15. [Google Scholar] [CrossRef]
- Zhang, R.R.; Liu, J.; Zhang, Y.; Hou, M.Q.; Zhang, M.Z.; Zhou, F.; Zhang, W.H. Microwave-assisted synthesis and antifungal activity of novel coumarin derivatives: Pyrano[3,2-c]chromene-2,5-diones. Eur. J. Med. Chem. 2016, 116, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, J.; Lu, A.; Yang, C.L. Synthesis, characterization, antifungal evaluation and 3D-QSAR study of phenylhydrazine substituted tetronic acid derivatives. Bioorg. Med. Chem. Lett. 2014, 24, 3772–3776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Z.; Xu, W.; Sun, E.; Tang, L.; Wang, J. Design, synthesis and antibacterial activity of novel actinonin derivatives containing benzimidazole heterocycles. Eur. J. Med. Chem. 2009, 44, 2202–2210. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, S.M.; Singh, N.; Kumar, A.; Lozach, O.; Meijer, L. Synthesis, anti-inflammatory, analgesic and kinase (CDK-1, CDK-5 and GSK-3) inhibition activity evaluation of benzimidazole/benzoxazole derivatives and some Schiff’s bases. Bioorg. Med. Chem. 2006, 14, 3758–3765. [Google Scholar] [CrossRef] [PubMed]
- Webster, L.M.; Johnson, P.C.; Adam, A.; Mable, C.D.; Keller, L.F. Absence of three known benzimidazole resistance mutations in Trichostrongylus tenuis, a nematode parasite of avian hosts. Vet. Parasitol. 2008, 158, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Lukowska-Chojnacka, E.; Wińska, P.; Wielechowska, M.; Poprzeczko, M.; Bretner, M. Synthesis of novel polybrominated benzimidazole derivatives-potential CK2 inhibitors with anticancer and proapoptotic activity. Bioorg. Med. Chem. 2016, 24, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Novelli, F.; Tasso, B.; Vazzana, I.; Sparatore, A. Antiviral activity of benzimidazole derivatives III Novel anti-CVB-5, anti-RSV and anti-Sb-1 agents. Bioorg. Med. Chem. 2014, 22, 4893–4909. [Google Scholar] [CrossRef] [PubMed]
- Lezcano, M.; Al-Soufi, W.; Novo, M.; Rodríguez-Núñez, E.; Tato, J.V. Complexation of several benzimidazole-type fungicides with α-and β-cyclodextrins. J. Agric. Food Chem. 2002, 50, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.F.; Yan, W.; Cao, L.L.; Ye, Y.H. Synthesis and biological evaluation of benzimidazole phenylhydrazone derivatives as antifungal agents against phytopathogenic fungi. Molecules 2016, 21, 1574. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.B.; Zhang, A.L.; Tang, J.J.; Gao, J.M. Synthesis and antifungal activity of 2-chloromethyl-1H-benzimidazole derivatives against phytopathogenic fungi in vitro. J. Agric. Food Chem. 2013, 61, 2789–2795. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qian, X.; Chen, J.; Song, G. Novel fused heterocycles: Synthesis and activity of 5,6-dihydro-7-thia-1,3,3a,5-tetraazainden-4-one and 1-thia-3,4a,9-triazafluoren-4-one derivatives. Monatsh. Chem. 2000, 131, 953–957. [Google Scholar] [CrossRef]
- Davison, E.K.; Sperry, J. Natural products with heteroatom-rich ring systems. J. Nat. Prod. 2017, 80, 3060–3079. [Google Scholar] [CrossRef] [PubMed]
- Reich, M.F.; Fabio, P.F.; Lee, V.J.; Kuck, N.A.; Testa, R.T. Pyrido [3,4-e]-1,2,4-triazines and related heterocycles as potential antifungal agents. J. Med. Chem. 1989, 32, 2474–2485. [Google Scholar] [CrossRef] [PubMed]
- El-Barbary, A.A.; Sakran, M.A.; El-Madani, A.M.; Nielsen, C. Synthesis, characterization and biological activity of some 1,2,4-triazine derivatives. J. Heterocycl. Chem. 2005, 42, 935–941. [Google Scholar] [CrossRef]
- El-All, A.S.A.; Osman, S.A.; Roaiah, H.M.; Abdalla, M.M.; El Aty, A.A.A.; AbdEl-Hady, W.H. Potent anticancer and antimicrobial activities of pyrazole, oxazole and pyridine derivatives containing 1,2,4-triazine moiety. Med. Chem. Res. 2015, 24, 4093–4104. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Shinde, D.B. One pot synthesis and SAR of some novel 3-substituted 5,6-diphenyl-1,2,4-triazines as antifungal agents. Bioorg. Med. Chem. Lett. 2010, 20, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Venkataramana, H.S.; Singh, A.; Tiwari, A.; Tiwari, V. Synthesis of phenyl hydrazine substituted benzimidazole derivatives and their biological activity. Int. J. Pharm. Sci. Res. 2010, 1, 34–38. [Google Scholar]
- Srivastava, S.; Pandeya, S.N.; Yadav, M.K.; Singh, B.K. Synthesis and analgesic activity of novel derivatives of 1,2-substituted benzimidazoles. J. Chem. 2012, 2013. [Google Scholar] [CrossRef]
- Wang, X.F.; Si, T.F.; Li, Q.B.; Zhu, Z.Y.; Zhu, X.J.; Qiang, S.; Yang, C.L. Synthesis, characterization and biological activity of novel (5-RS,6-S)-5-sec-butyl-3-(1-substituted-amino) ethylidene-1H-pyrrolidine-2,4-diones. ARKIVOC 2010, 2, 31–48. [Google Scholar]
- Xu, W.Q.; Chen, M.; Wang, K.Y.; Ren, Z.J.; Lu, A.M.; Yang, C.L. Synthesis, characterization, and antifungal activity of phenylpyrrole-substituted tetramic acids bearing carbonates. Molecules 2016, 21, 355. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 3a–3g and 4a–4w are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).