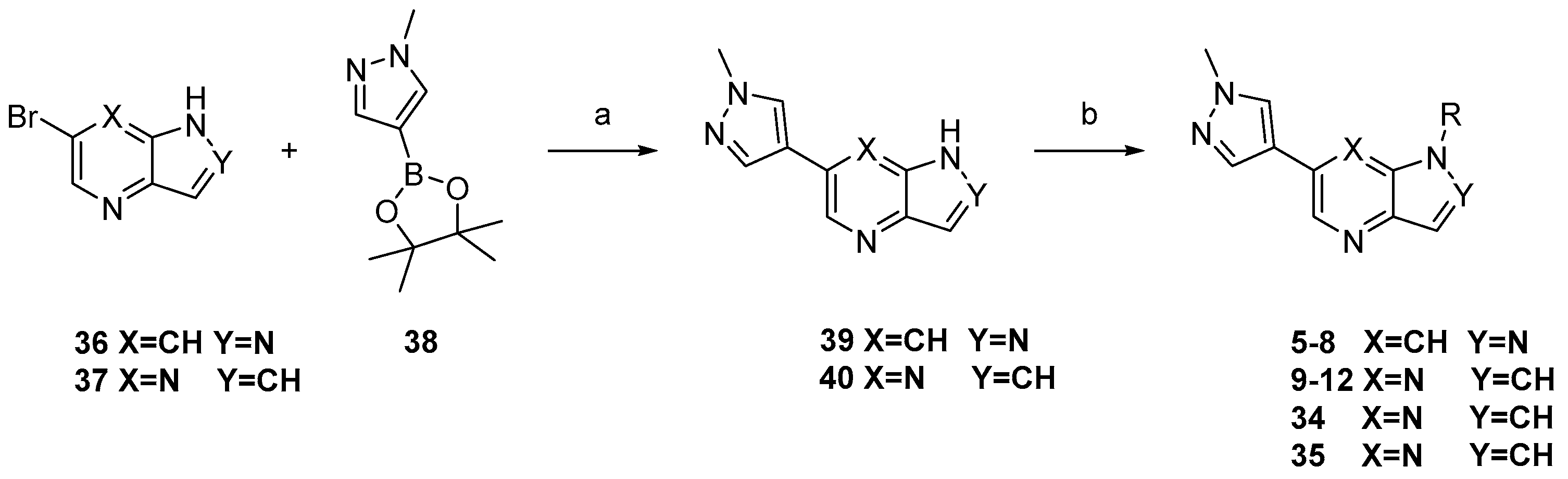
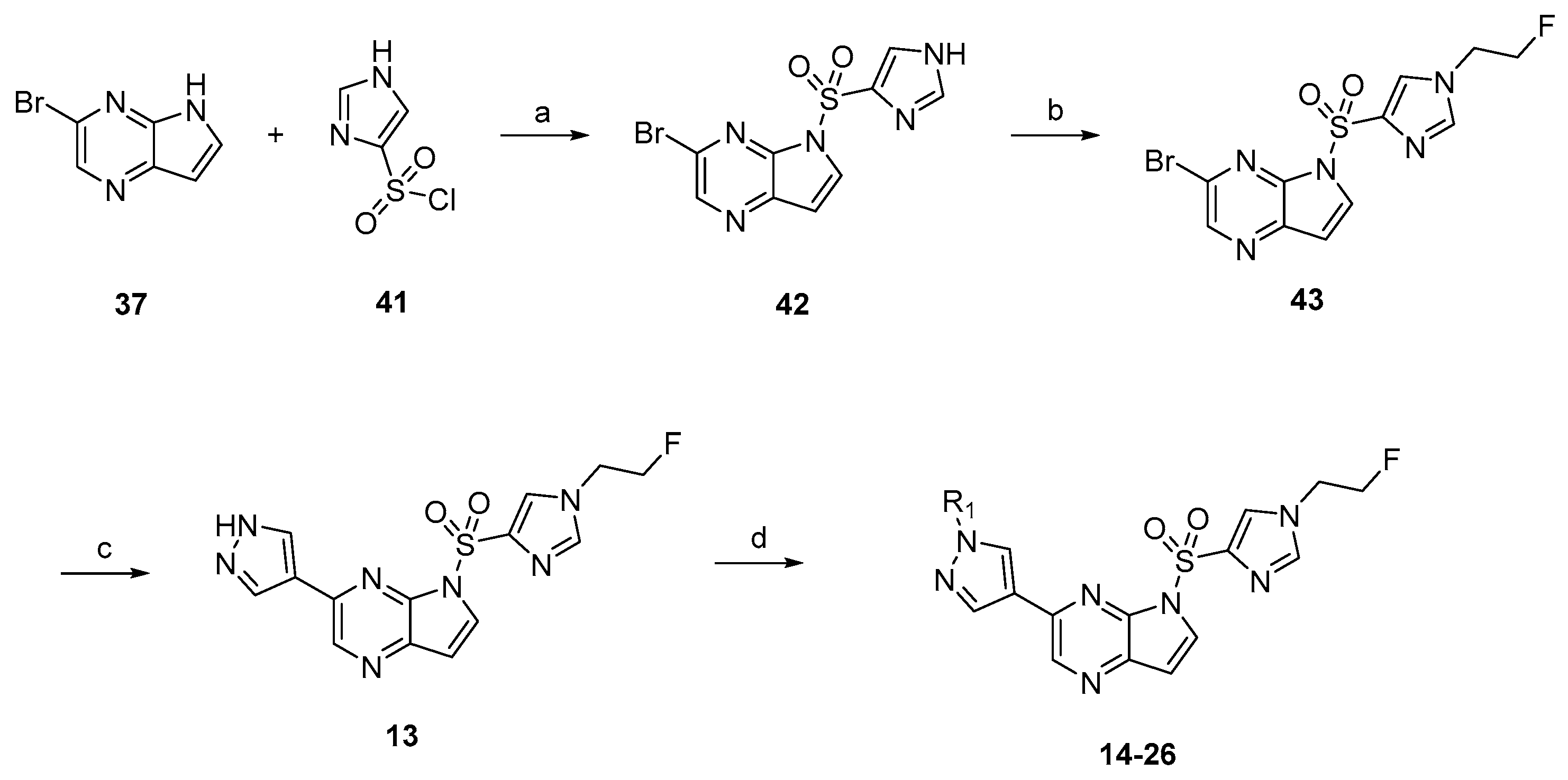
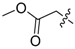
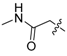
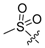
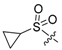
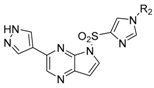
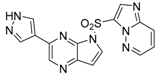
3.1.2. Compound 40 Was Prepared as Described for the Synthesis of Compound 39
3-(1-methyl-1H-pyrazol-4-yl)-5H-pyrrolo[2,3-b]pyrazine (40). Yield: 86%; yellow solid. 1H-NMR (400 MHz, DMSO-d6) δ 9.30 (s, 1H), 8.72 (s, 1H), 8.11 (s, 1H), 7.97 (s, 1H), 7.28 (s, 1H), 6.74 (dd, J = 3.7, 1.9 Hz, 1H), 4.02 (s, 3H). ESI-MS: C10H9N5, Exact Mass: 199.09, m/z 200.1 (M + 1)+. Retention time 2.56 min, >98% purity.
General Procedure for the Synthesis of 6-chloro-5-((6-(1-methyl-1H-pyrazol-4-yl)-1H-pyrazolo[4,3-b]pyridin-1-yl)sulfonyl)imidazo[2,1-b]thiazole (5). NaH (6.7 mg, 0.28 mmol) was suspended in dry DMF. 6-(1-methyl-1H-pyrazol-4-yl)-1H-pyrazolo[4,3-b]pyridine (39, 50 mg, 0.25 mmol) was added into the above solution slowly at 0 °C. Then, the cooling solution was stirred for 0.5 h. Subsequently, 6-chloroimidazo[2,1-b]thiazole-5-sulfonyl chloride (77 mg, 0.3 mmol) was added and stirred for another 4 h at room temperature. Then, water (30 mL) was added to the reaction system. The reaction mixture was extracted with ethyl acetate (3 × 40 mL). The combined organic phase was washed with saturated salt water (3 × 40 mL), dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give the corresponding crude target product, which was purified by flash column chromatography with dichloromethane/methanol to afford compound 5 as a white hairy solid with the yield of 78%. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.88 (d, J = 1.8 Hz, 1H), 8.54 (s, 1H), 8.35 (s, 1H), 8.21 (d, J = 4.6 Hz, 1H), 7.93 (s, 1H), 7.84 (s, 1H), 7.17 (d, J = 4.5 Hz, 1H), 4.03 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 151.78, 147.08, 142.45, 141.49, 140.84, 137.30, 134.38, 129.09, 128.07, 121.12, 119.20, 116.39, 116.32, 115.13, 39.41. ESI-MS: C15H10ClN7O2S2, Exact Mass: 419.0, m/z 420.0 (M + 1)+. HRMS-ESI m/z calcd. for C15H11ClN7O2S2 [M + H]+ 420.0099, found 420.0135. Retention time 2.53 min, >98% purity.
The compounds
6–
12,
34,
35 were prepared as described for the synthesis of compound
5 (
Scheme 1).
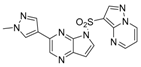
6-(1-Methyl-1H-pyrazol-4-yl)-1-(pyrazolo[1,5-a]pyrimidin-3-ylsulfonyl)-1H-pyrazolo[4,3-b]pyridine (6). Yield: 79%; white solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.83 (d, J = 1.8 Hz, 1H), 8.76 (dd, J = 7.0, 1.7 Hz, 1H), 8.71 (d, J = 4.1 Hz, 2H), 8.66 (s, 1H), 8.35 (s, 1H), 7.97 (s, 1H), 7.88 (s, 1H), 7.11 (dd, J = 6.9, 4.3 Hz, 1H), 4.03 (s, 3H). 13C-NMR (126 MHz, DMSO-d6) δ 155.14, 146.36, 146.16, 145.60, 141.25, 140.73, 138.19, 136.83, 133.71, 129.34, 128.34, 118.21, 115.37, 112.00, 105.05, 38.81. ESI-MS: C16H13N8O2S, Exact Mass: 380.08, m/z 381 (M + 1)+. HRMS-ESI m/z calcd. for C16H13N8O2S [M+H]+ 381.0877, found 381.0891. Retention time 2.55 min, >98% purity.
1-(Imidazo[1,2-b]pyridazin-3-ylsulfonyl)-6-(1-methyl-1H-pyrazol-4-yl)-1H-pyrazolo[4,3-b]pyridine (7). Yield: 80%; yellow solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.87 (d, J = 1.9 Hz, 1H), 8.64 (dd, J = 1.9, 0.9 Hz, 1H), 8.58 (s, 1H), 8.35 (d, J = 0.9 Hz, 1H), 8.30 (dd, J = 4.6, 1.6 Hz, 1H), 8.07 (dd, J = 9.3, 1.6 Hz, 1H), 7.98 (d, J = 0.8 Hz, 1H), 7.94–7.87 (m, 1H), 7.26–7.22 (m, 1H), 4.04 (s, 3H). 13C-NMR (126 MHz, DMSO-d6, ppm) δ 146.58, 146.08, 142.87, 142.14, 141.13, 140.46, 136.89, 134.52, 129.44, 128.66, 126.90, 122.26, 121.22, 118.12, 115.41, 38.81. ESI-MS: C16H13N8O2S, Exact Mass: 380.08, m/z 381.08 (M + 1)+. HRMS-ESI m/z calcd. for C16H13N8O2S [M + H]+ 381.0877, found 381.0974. Retention time 2.60 min, >98% purity.
1-((6-Bromoimidazo[1,2-a]pyridin-3-yl)sulfonyl)-6-(1-methyl-1H-pyrazol-4-yl)-1H-pyrazolo[4,3-b]pyridine (8). Yield: 78%; white solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.90 (d, J = 1.9 Hz, 1H), 8.73 (d, J = 1.0 Hz, 1H), 8.56 (s, 1H), 8.41–8.36 (m, 1H), 8.05–7.98 (m, 2H), 7.93 (s, 1H), 7.22 (d, J = 9.6 Hz, 1H), 4.05 (s, 3H). ESI-MS: C16H11ClN8O2S, Exact Mass: 414.04, m/z 415.10 (M + 1)+. HRMS-ESI m/z calcd. for C16H12ClN8O2S [M + H]+ 415.0487, found 415.0555.
6-Chloro-5-((3-(1-methyl-1H-pyrazol-4-yl)-5H-pyrrolo[2,3-b]pyrazin-5-yl)sulfonyl)imidazo[2,1-b]thiazole (9). Yield: 80%; white solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.68 (s, 1H), 8.42 (d, J = 4.5 Hz, 1H), 8.02 (d, J = 4.1 Hz, 1H), 7.78 (d, J = 9.0 Hz, 2H), 7.23 (d, J = 4.6 Hz, 1H), 6.83 (d, J = 4.1 Hz, 1H), 3.97 (s, 3H). ESI-MS: C15H10ClN7O2S2, Exact Mass: 419.00, m/z 420.02 (M + 1)+. HRMS-ESI m/z calcd. for C15H11ClN7O2S2 [M + H]+ 420.0099, found 420.0121. Retention time 2.58 min, >98% purity.
3-(1-Methyl-1H-pyrazol-4-yl)-5-(pyrazolo[1,5-a]pyrimidin-3-ylsulfonyl)-5H-pyrrolo[2,3-b]pyrazine (10). Yield: 79%; pale white solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.76 (s, 1H), 8.67 (s, 1H), 8.50 (dd, J = 4.4, 1.4 Hz, 1H), 8.17 (d, J = 4.1 Hz, 1H), 8.07 (dd, J = 9.2, 1.4 Hz, 1H), 7.96 (d, J = 11.6 Hz, 2H), 7.33–7.27 (m, 1H), 6.81 (d, J = 4.2 Hz, 1H), 4.01 (s, 3H). ESI-MS: C16H12N8O2S, Exact Mass: 380.08, m/z 381.05 (M + 1)+. HRMS-ESI m/z calcd. for C16H13N8O2S [M + H]+ 381.0877, found 381.0976. Retention time 2.67 min, >98% purity.
5-((1-Methyl-1H-imidazol-4-yl)sulfonyl)-3-(1-methyl-1H-pyrazol-4-yl)-5H-pyrrolo[2,3-b]pyrazine (11). Yield: 75%; white solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.71 (s, 1H), 8.06 (s, 1H), 8.04–8.02 (m, 1H), 8.01 (s, 1H), 7.90 (s, 1H), 7.43 (d, J = 1.3 Hz, 1H), 6.82 (d, J = 4.1 Hz, 1H), 4.02 (s, 3H), 3.75 (s, 3H). ESI-MS: C14H13N7O2S, Exact Mass: 343.09, m/z 343.07 (M + 1)+. HRMS-ESI m/z calcd. for C14H14N7O2S [M + H]+ 344.0924, found 344.1037. Retention time 2.60 min, >98% purity.
5-((1-(2-Fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-3-(1-methyl-1H-pyrazol-4-yl)-5H-pyrrolo[2,3-b]pyrazine (12). Yield: 85%; yellow solid. 1H-NMR (400 MHz, DMSO-d6, ppm) δ 8.92 (s, 1H), 8.66 (s, 1H), 8.41 (s, 1H), 8.14 (s, 1H), 8.10 (d, J = 4.1 Hz, 1H), 7.86 (s, 1H), 6.93 (d, J = 4.0 Hz, 1H), 4.77 (t, J = 4.8 Hz, 1H), 4.65 (t, J = 4.6 Hz, 1H), 4.43 (t, J = 4.5 Hz, 1H), 4.36 (t, J = 4.7 Hz, 1H), 3.92 (s, 3H). ESI-MS: C15H15FN7O2S, Exact Mass: 375.09, m/z 376.15 (M + 1)+. HRMS-ESI m/z calcd. for C15H15FN7O2S [M + H]+ 376.0986, found 376.1195. Retention time 2.51 min, >98% purity.
3-(1H-Pyrazol-4-yl)-5-(pyrazolo[1,5-a]pyrimidin-3-ylsulfonyl)-5H-pyrrolo[2,3-b]pyrazine (34). Yield: 81%; white solid. 1H-NMR (400 MHz, DMSO-d6, ppm) δ 13.24 (s, 1H), 9.03 (s, 1H), 8.93 (s, 1H), 8.71 (dd, J = 4.5, 1.5 Hz, 1H), 8.44 (s, 1H), 8.34 (dd, J = 9.3, 1.5 Hz, 1H), 8.27 (d, J = 4.2 Hz, 1H), 8.12 (s, 1H), 7.51 (dd, J = 9.3, 4.6 Hz, 1H), 6.97 (d, J = 4.2 Hz, 1H). ESI-MS: C15H11N8O2S, Exact Mass: 366.06, m/z 367.08 (M + 1)+. HRMS-ESI m/z calcd. for C15H11N8O2S [M + H]+ 367.0720, found 367.0820. Retention time 2.47 min, >98% purity.
5-(Imidazo[1,2-b]pyridazin-3-ylsulfonyl)-3-(1H-pyrazol-4-yl)-5H-pyrrolo[2,3-b]pyrazine (35). Yield: 79%; white solid. 1H-NMR (400 MHz, DMSO-d6, ppm) δ 13.25 (s, 1H), 9.32 (dd, J = 7.0, 1.7 Hz, 1H), 9.25 (s, 1H), 8.93 (s, 1H), 8.84 (dd, J = 4.3, 1.7 Hz, 1H), 8.51 (s, 1H), 8.21 (d, J = 4.1 Hz, 1H), 8.19 (s, 1H), 7.37 (dd, J = 7.0, 4.3 Hz, 1H), 6.90 (d, J = 4.1 Hz, 1H). 13C-NMR (126 MHz, DMSO-d6, ppm) δ 155.46, 148.14, 145.79, 142.58, 139.90, 138.68, 138.63, 138.60, 137.94, 130.35, 128.22, 119.61, 112.35, 105.88, 105.68. ESI-MS: C15H11N8O2S, Exact Mass: 366.06, m/z 367.06(M + 1)+. HRMS-ESI m/z calcd. for C15H11N8O2S [M + H]+ 367.0720, found 367.0791. Retention time 2.40 min, >90% purity.
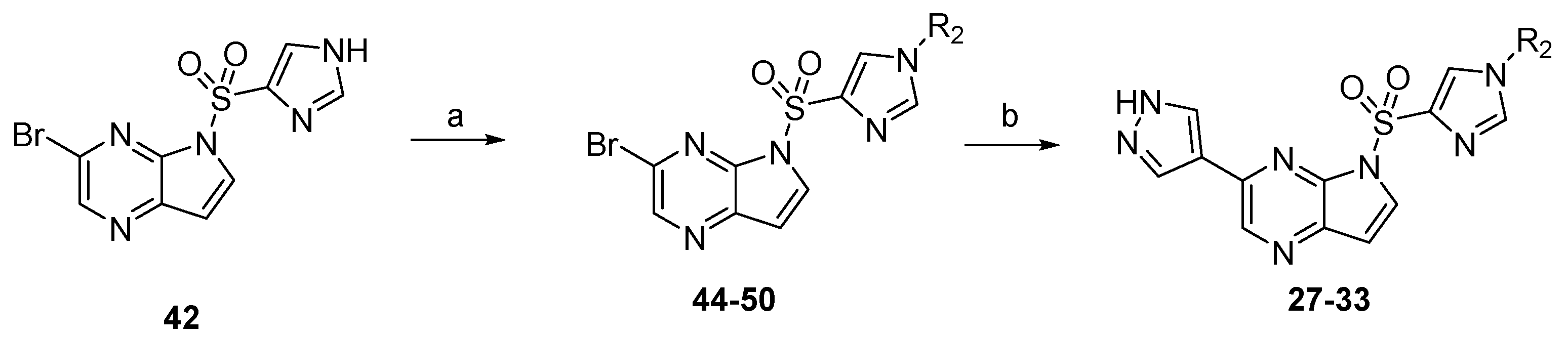
General Procedure for the Synthesis of (5-((1H-imidazol-4-yl)sulfonyl)-3-bromo-5H-pyrrolo[2,3-b]pyrazine) (42). NaH (160 mg, 6.71 mmol) was suspended in dry DMF. 3-bromo-5H-pyrrolo[2,3-b]pyrazine (1.20 g, 6.1 mmol) was added into the above solution slowly at 0 °C. Then, the cooling solution was stirred for 0.5 h. Subsequently, 1H-imidazole-4-sulfonyl chloride (1.22 g, 7.32 mmol) was added and stirred for 4 h at room temperature. Then, water (50 mL) was added to the reaction system. The reaction mixture was extracted with dichloromethane (3 × 40 mL). The combined organic phase was concentrated under reduced pressure to give the crude target product, which was purified by flash column chromatography with dichloromethane/methanol to afford compound 42 as a white solid with the yield of 84%. 1H-NMR (400 MHz, CD3OD, ppm) δ 8.62 (s, 1H), 8.22 (d, J = 1.1 Hz, 1H), 8.16 (d, J = 4.2 Hz, 1H), 7.79 (d, J = 1.0 Hz, 1H), 6.88 (d, J = 4.1 Hz, 1H). ESI-MS: C9H7BrN5O2S, Exact Mass: 326.94, m/z 328.11 (M + 1)+. Retention time 2.42 min, >98% purity.
General Procedure for the Synthesis of (3-bromo-5-((1-(2-fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazine) (43). NaH (130 mg, 5.52 mmol) was suspended in dry DMF. After 5-((1H-imidazol-4-yl)sulfonyl)-3-bromo-5H-pyrrolo[2,3-b]pyrazine (42, 1.5 g, 4.6 mmol) was added into the above solution, the resulting mixture was stirred at room temperature for 0.5 h. Subsequently, 1-fluoro-2-iodoethane (960 mg, 5.52 mmol) was added and stirred at 80 °C for another 4 h. Then, water (30 mL) was added to the reaction system. The reaction mixture was extracted with dichloromethane (3 × 40 mL). The combined organic phase was washed with saturated salt water (3 × 40 mL), dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give the corresponding crude target product, which was purified by flash column chromatography with dichloromethane/methanol to afford compound 43 as a white solid with the yield of 90%. 1H-NMR (400 MHz, DMSO-d6, ppm) δ 8.76 (s, 1H), 8.46 (s, 1H), 8.25 (d, J = 4.2 Hz, 1H), 7.89 (s, 1H), 7.04 (d, J = 4.2 Hz, 1H), 4.78 (t, J = 4.7 Hz, 1H), 4.67 (t, J = 4.7 Hz, 1H), 4.44 (t, J = 4.7 Hz, 1H), 4.37 (t, J = 4.7 Hz, 1H). ESI-MS: C11H10BrFN5O2S, Exact Mass: 372.96, m/z 374.17 (M + 1)+. Retention time 2.84 min, >97% purity.
General Procedure for the Synthesis of (5-((1-(2-fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-3-(1H-pyrazol-4-yl)-5H-pyrrolo[2,3-b]pyrazine) (13). Bromo-5-((1-(2-fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazine (43, 1.2 g, 3.2 mmol), 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole (745 mg, 3.84 mmol), K2CO3 (1.32 g, 9.6 mmol), and Pd(dppf)Cl2 (130 mg, 0.16 mmol) was dissolved in the solvent of 1,4-dioxane/H2O (v/v = 3:1, 20 mL). The resulting mixture was stirred at 80 °C under Ar for 4 h. Then, the reaction mixture was evaporated to dryness. The residue was purified by flash chromatography to give compound 13 as a yellow solid with the yield of 87%. 1H-NMR (400 MHz, DMSO-d6, ppm) δ 13.23 (s, 1H), 8.97 (s, 1H), 8.67 (d, J = 1.2 Hz, 1H), 8.48 (s, 1H), 8.19 (d, J = 1.3 Hz, 1H), 8.10 (d, J = 4.1 Hz, 1H), 7.86 (s, 1H), 6.93 (d, J = 4.1 Hz, 1H), 4.75 (t, J = 4.7 Hz, 1H), 4.64 (t, J = 4.6 Hz, 1H), 4.43 (t, J = 4.6 Hz, 1H), 4.36 (t, J = 4.7 Hz, 1H). ESI-MS: C14H13FN7O2S, Exact Mass: 361.08, m/z 362.24 (M + 1)+. HRMS-ESI m/z calcd. for C14H13FN7O2S [M + H]+ 362.0830, found 362.0905. Retention time 2.29 min, >99% purity.
General Procedure for the Synthesis of (1-(4-(5-((1-(2-fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazin-3-yl)-1H-pyrazol-1-yl)ethan-1-one) (15). 5-((1-(2-fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-3-(1H-pyrazol-4-yl)-5H-pyrrolo[2,3-b]pyrazine (13, 50 mg, 0.14 mmol), acetyl chloride (14 mg, 0.17 mmol), and K2CO3 (38 mg, 0.28 mmol) was dissolved in dry DMF. The resulting mixture was stirred at room temperature for 8 h. Then, water (30 mL) was added to the reaction system. The reaction mixture was extracted with ethyl acetate (3 × 40 mL). The combined organic phase was washed with saturated salt water (3 × 40 mL), dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give the corresponding crude target product, which was purified by flash column chromatography with dichloromethane/methanol to afford compound 15 as a white hairy solid with the yield of 89%. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.79 (s, 1H), 8.69 (s, 1H), 8.29 (s, 1H), 8.11 (d, J = 4.1 Hz, 1H), 8.09 (s, 1H), 7.52 (s, 1H), 6.86 (d, J = 4.1 Hz, 1H), 4.71(t, J = 4.5 Hz, 1H), 4.59 (t, J = 4.6 Hz, 1H), 4.32(t, J = 4.6 Hz, 1H), 4.26 (t, J = 4.5 Hz, 1H), 4.15 (s, 3H). ESI-MS: C16H15FN7O3S, Exact Mass: 403.09, m/z 404.24 (M + 1)+. Retention time 2.65 min, >95% purity.
The compounds
14–
26 were prepared as described for the synthesis of compound
15 (
Scheme 2).
3-(1-Cyclopropyl-1H-pyrazol-4-yl)-5-((1-(2-fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazine (14). Yield: 72%; white solid. 1H-NMR (400 MHz, DMSO-d6, ppm) δ 8.93 (s, 1H), 8.69 (s, 1H), 8.49 (s, 1H), 8.12–8.10 (m, 2H), 7.86 (s, 1H), 6.93 (d, J = 4.1 Hz, 1H), 4.77 (t, J = 4.6 Hz, 1H), 4.65 (t, J = 4.6 Hz, 1H), 4.43 (t, J = 4.7 Hz, 1H), 4.36 (t, J = 4.6 Hz, 1H), 3.82 (m, 1H), 1.15–1.10 (m, 2H), 1.05–1.02 (m, 2H). ESI-MS: C17H17FN7O2S, Exact Mass: 401.11, m/z 402.16 (M + 1)+. HRMS-ESI m/z calcd. for C17H17FN7O2S [M + H]+ 402.1143, found 402.1376. Retention time 2.76 min, >96% purity.
1-(4-(5-((1-(2-Fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazin-3-yl)-1H-pyrazol-1-yl)propan-1-one (16). Yield: 83%; yellow solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.79 (s, 1H), 8.74 (s, 1H), 8.24 (s, 1H), 8.12–8.08 (m, 2H), 7.52 (s, 1H), 6.85 (d, J = 4.1 Hz, 1H), 4.72 (t, J = 4.6 Hz, 1H), 4.61 (t, J = 4.5 Hz, 1H), 4.34 (t, J = 4.4 Hz, 1H), 4.28 (t, J = 4.6 Hz, 1H), 3.21 (q, J = 7.5 Hz, 2H), 1.33 (t, J = 7.3 Hz, 3H). ESI-MS: C17H17FN7O3S, Exact Mass: 417.10, m/z 418.05 (M + 1)+. HRMS-ESI m/z calcd. for C17H17FN7O3S [M + H]+ 418.1092, found 418.1194. Retention time 2.79 min, >96% purity.
Methyl 2-(4-(5-((1-(2-fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazin-3-yl)-1H-pyrazol-1-yl)acetate (17). Yield: 75%; white solid. 1H-NMR (400 MHz, DMSO-d6, ppm) δ 8.96 (s, 1H), 8.65 (s, 1H), 8.52 (s, 1H), 8.21 (s, 1H), 8.12 (d, J = 4.1 Hz, 1H), 7.87 (s, 1H), 6.95 (d, J = 4.1 Hz, 1H), 5.20 (s, 2H), 4.75 (t, J = 4.7 Hz, 1H), 4.63 (t, J = 4.7 Hz, 1H), 4.44 (t, J = 4.6 Hz, 1H), 4.37 (t, J = 4.6 Hz, 1H), 3.73 (s, 3H). ESI-MS: C17H17FN7O4S, Exact Mass: 433.10, m/z 434.16 (M + 1)+. Retention time 2.61 min, >97% purity.
1-(4-(5-((1-(2-Fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazin-3-yl)-1H-pyrazol-1-yl)propan-2-one (18). Yield: 80%; white solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.72 (s, 1H), 8.08 (s, 1H), 8.07 (s, 1H), 8.07 (s, 1H), 8.03 (d, J = 4.2 Hz, 1H), 7.49 (s, 1H), 6.82 (d, J = 4.3 Hz, 1H), 5.03 (s, 2H), 4.68 (d, J = 4.8 Hz, 1H), 4.56 (d, J = 4.4 Hz, 1H), 4.33 (d, J = 4.5 Hz, 1H), 4.26 (d, J = 4.5 Hz, 1H), 2.24 (s, 3H). ESI-MS: C17H17FN7O3S, Exact Mass: 417.10, m/z 418.13 (M + 1)+. HRMS-ESI m/z calcd. for C17H17FN7O3S [M + H]+ 418.1092, found 418.1222. Retention time 2.48 min, >96% purity.
Methyl 2-(4-(5-((1-(2-fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazin-3-yl)-1H-pyrazol-1-yl)acetate (19). Yield: 76%; white solid. 1H-NMR (400 MHz, DMSO-d6, ppm) δ 8.96 (s, 1H), 8.65 (s, 1H), 8.52 (s, 1H), 8.21 (s, 1H), 8.12 (d, J = 4.1 Hz, 1H), 7.87 (s, 1H), 6.95 (d, J = 4.1 Hz, 1H), 5.20 (s, 2H), 4.75 (t, J = 4.7 Hz, 1H), 4.63 (t, J = 4.7 Hz, 1H), 4.44 (t, J = 4.6 Hz, 1H), 4.37 (t, J = 4.6 Hz, 1H), 3.73 (s, 3H). ESI-MS: C17H17FN7O4S, Exact Mass: 433.10, m/z 434.16 (M + 1)+. HRMS-ESI m/z calcd. for C17H17FN7O4S [M + H]+ 434.1041, found 434.1252. Retention time 2.61 min, >97% purity.
Ethyl 2-(4-(5-((1-(2-fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazin-3-yl)-1H-pyrazol-1-yl)acetate (20). Yield: 78%; yellow solid. 1H-NMR (400 MHz, DMSO-d6, ppm) δ 8.95 (s, 1H), 8.63 (d, J = 1.3 Hz, 1H), 8.49 (s, 1H), 8.21 (s, 1H), 8.12 (d, J = 4.1 Hz, 1H), 7.86 (d, J = 1.1 Hz, 1H), 6.94 (d, J = 4.1 Hz, 1H), 5.16 (s, 2H), 4.74 (t, J = 4.0 Hz, 1H), 4.63 (t, J = 4.0 Hz, 1H), 4.43 (t, J = 4.0 Hz, 1H), 4.35 (t, J = 4.1 Hz, 1H), 4.19 (q, J = 7.1 Hz, 2H), 1.24 (t, J = 7.1 Hz, 3H). ESI-MS: C18H19FN7O4S, Exact Mass: 447.11, m/z 448.16 (M + 1)+. HRMS-ESI m/z calcd. for C18H19FN7O4S [M + H]+ 448.1198, found 448.1446. Retention time 2.80 min, >98% purity.
2-(4-(5-((1-(2-Fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazin-3-yl)-1H-pyrazol-1-yl)-N-methylacetamide (21). Yield: 79%; white solid. 1H-NMR (400 MHz, DMSO-d6, ppm) δ 8.96 (s, 1H), 8.65 (s, 1H), 8.46 (s, 1H), 8.18 (s, 1H), 8.12 (d, J = 4.1 Hz, 1H), 8.08–8.04 (m, 1H), 7.86 (s, 1H), 6.94 (d, J = 4.1 Hz, 1H), 4.86 (s, 2H), 4.76 (t, J = 4.6 Hz, 1H), 4.64 (t, J = 4.7 Hz, 1H), 4.44 (t, J = 4.7 Hz, 1H), 4.37 (t, J = 4.7 Hz, 1H), 2.65 (d, J = 4.6 Hz, 3H). ESI-MS: C17H18FN8O3S, Exact Mass: 432.11, m/z 433.16 (M + 1)+. HRMS-ESI m/z calcd. for C17H18FN8O3S [M + H]+ 433.1201, found 433.1226. Retention time 2.36 min, >98% purity.
2-(4-(5-((1-(2-Fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazin-3-yl)-1H-pyrazol-1-yl)-N,N-dimethylacetamide (22). Yield: 81%; yellow solid. 1H-NMR (400 MHz, DMSO-d6, ppm) δ 8.95 (s, 1H), 8.63 (d, J = 1.2 Hz, 1H), 8.39 (s, 1H), 8.15 (s, 1H), 8.11 (d, J = 4.1 Hz, 1H), 7.86 (d, J = 1.1 Hz, 1H), 6.94 (d, J = 4.1 Hz, 1H), 5.20 (s, 2H), 4.75(t, J = 4.7 Hz, 1H), 4.67(t, J = 4.7 Hz, 1H), 4.43 (t, J = 4.7 Hz, 1H), 4.36 (t, J = 4.7 Hz, 1H), 3.08 (s, 3H), 2.89 (s, 3H). ESI-MS: C18H20FN8O3S, Exact Mass: 446.13, m/z 447.21 (M + 1)+. HRMS-ESI m/z calcd. for C18H20FN8O3S [M + H]+ 447.1358, found 447.1574. Retention time 2.45 min, >97% purity.
5-((1-(2-Fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-3-(1-(methylsulfonyl)-1H-pyrazol-4-yl)-5H-pyrrolo[2,3-b]pyrazine (23). Yield: 85%; white solid. 1H-NMR (400 MHz, DMSO-d6, ppm) δ 9.17 (s, 1H), 9.02 (s, 1H), 8.70 (d, J = 1.4 Hz, 1H), 8.59 (s, 1H), 8.22 (d, J = 4.1 Hz, 1H), 7.87 (d, J = 1.2 Hz, 1H), 6.99 (d, J = 4.2 Hz, 1H), 4.76 (t, J = 4.7 Hz, 1H), 4.65 (t, J = 4.7 Hz, 1H), 4.43 (t, J = 4.7 Hz, 1H), 4.36 (t, J = 4.6 Hz, 1H), 3.64 (s, 3H). ESI-MS: C15H15FN7O4S2, Exact Mass: 439.05, m/z 440.14 (M + 1)+. HRMS-ESI m/z calcd. for C15H15FN7O4S2 [M + H]+ 440.0605, found 440.0659. Retention time 2.67 min, >98% purity.
3-(1-(Cyclopropylsulfonyl)-1H-pyrazol-4-yl)-5-((1-(2-fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazine (24). Yield: 82%; white solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.77 (s, 1H), 8.52 (s, 1H), 8.33 (s, 1H), 8.12 (d, J = 4.2 Hz, 1H), 8.08 (s, 1H), 7.52 (s, 1H), 6.86 (d, J = 4.1 Hz, 1H), 4.72 (t, J = 4.6 Hz, 1H), 4.63 (t, J = 4.6 Hz, 1H), 4.34 (t, J = 4.6 Hz, 1H), 4.26 (t, J = 4.6 Hz, 1H), 2.89–2.82 (m, 1H), 1.29–1.24 (m, 2H), 0.86–1.83 (m, 2H). ESI-MS: C17H17FN7O4S2, Exact Mass: 465.07, m/z 466.12 (M + 1)+. HRMS-ESI m/z calcd. for C17H17FN7O4S2 [M + H]+ 466.0762, found 466.0780. Retention time 2.92 min, >96% purity.
Dimethyl 2-(4-(5-((1-(2-fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazin-3-yl)-1H-pyrazol-1-yl)malonate (25). Yield: 82%; yellow solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.74 (s, 1H), 8.38 (s, 1H), 8.12 (s, 1H), 8.08 (s, 1H), 8.05 (d, J = 4.4 Hz, 1H), 7.50 (s, 1H), 6.82 (d, J = 4.0 Hz, 1H), 5.96 (s, 1H), 4.67(t, J = 4.5 Hz, 1H), 4.56 (t, J = 4.5 Hz, 1H), 4.34 (t, J = 4.4Hz, 1H), 4.27 (t, J = 4.5 Hz, 1H), 3.90 (s, 6H). 13C-NMR (126 MHz, CD3OD, ppm) δ 165.08, 141.71, 140.11, 139.04, 138.26, 137.36, 136.82, 130.59, 130.30, 127.91, 121.62, 105.14, 82.54, 81.20, 52.77, 52.72, 29.36, 28.92, 13.02. ESI-MS: C19H19FN7O6S, Exact Mass: 491.10, m/z 492.18 (M + 1)+. HRMS-ESI m/z calcd. for C19H19FN7O6S [M + H]+ 492.1096, found 492.1241. Retention time 2.79 min, >98% purity.
Diethyl 2-(4-(5-((1-(2-fluoroethyl)-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazin-3-yl)-1H-pyrazol-1-yl)malonate (26). Yield: 76%; white solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.73 (s, 1H), 8.39 (s, 1H), 8.12 (s, 1H), 8.08 (s, 1H), 8.04 (d, J = 4.1 Hz, 1H), 7.50 (s, 1H), 6.81 (d, J = 4.1 Hz, 1H), 5.91 (s, 1H), 4.66 (t, J = 4.5 Hz, 1H), 4.55 (t, J = 4.5 Hz, 1H), 4.40–4.30 (m, 5H), 4.27(t, J = 4.5 Hz, 1H), 1.36 (t, J = 7.1 Hz, 6H). ESI-MS: C21H23FN7O6S, Exact Mass: 519.13, m/z 520.24 (M + 1)+. HRMS-ESI m/z calcd. for C21H23FN7O6S [M + H]+ 520.1409, found 520.1667. Retention time 3.11 min, >92% purity.
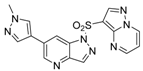
General Procedure for the Synthesis of 3-bromo-5-((1-ethyl-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazine (46). NaH (130 mg, 5.52 mmol) was suspended in dry DMF. 5-((1H-imidazol-4-yl)sulfonyl)-3-bromo-5H-pyrrolo[2,3-b]pyrazine (42, 1 g, 3 mmol) and iodoethane (562 mg, 3.6 mmol) were dissolved in the DMF. Then, the resulting mixture was stirred at 80 °C for 4 h. After the reaction was cooled to the room temperature, water (30 mL) was added to the reaction system. The aqueous layer was extracted with EtOAc (2 × 20 mL). The combined organic layers were dried (Na2SO4), filtered, and concentrated to afford compound 46 as a yellow hairy solid with the yield of 82%. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.57 (s, 1H), 8.09–8.04 (m, 2H), 7.48 (d, J = 1.1 Hz, 1H), 6.82 (d, J = 4.1 Hz, 1H), 4.08 (q, J = 7.3 Hz, 2H), 1.53 (t, J = 7.4 Hz, 3H). ESI-MS: C11H11BrN5O2S, Exact Mass: 354.97, m/z 356.03 (M + 1)+. Retention time 2.80 min, >98% purity.
General Procedure for the Synthesis of 5-((1-ethyl-1H-imidazol-4-yl)sulfonyl)-3-(1H-pyrazol-4-yl)-5H-pyrrolo[2,3-b]pyrazine (29). 3-bromo-5-((1-ethyl-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazine (46, 50 mg, 0.14 mmol), 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole (33 mg, 0.17 mmol), K2CO3 (58 mg, 0.42 mmol) and Pd(dppf)Cl2 (6 mg, 0.007 mmol) was dissolved in the solvent of 1,4-dioxane/H2O (v/v = 3:1, 20 mL). The resulting mixture was stirred at 80 °C under Ar for 4 h. Then, the reaction mixture was evaporated to dryness. The residue was purified by flash chromatography to give compound 29 as a yellow hairy solid with the yield of 87%. 1H-NMR (400 MHz, DMSO-d6, ppm) δ 13.25 (s, 1H), 8.97 (s, 1H), 8.73 (s, 1H), 8.52 (s, 1H), 8.21 (s, 1H), 8.09 (d, J = 4.1 Hz, 1H), 7.84 (s, 1H), 6.92 (d, J = 4.1 Hz, 1H), 4.06 (q, J = 7.3 Hz, 2H), 1.30 (t, J = 7.2 Hz, 3H). ESI-MS: C14H14N7O2S, Exact Mass: 343.09, m/z 344.11 (M + 1)+. HRMS-ESI m/z calcd. for C14H14N7O2S [M + H]+ 344.0924, found 344.0929. Retention time 2.34 min, >98% purity.
The compounds
27–
33 were prepared as described for the synthesis of compound
29 (
Scheme 3).
3-((1H-Imidazol-4-yl)sulfonyl)-3-bromo-5H-pyrrolo[2,3-b]pyrazine (44). Yield: 79%; white solid. 1H-NMR (400 MHz, CD3OD, ppm) δ 8.62 (s, 1H), 8.22 (d, J = 1.1 Hz, 1H), 8.16 (d, J = 4.2 Hz, 1H), 7.79 (d, J = 1.0 Hz, 1H), 6.88 (d, J = 4.1 Hz, 1H). ESI-MS: C9H7BrN5O2S, Exact Mass: 326.94, m/z 328.11 (M + 1)+. Retention time 2.40 min, >98% purity.
5-((1H-Imidazol-4-yl)sulfonyl)-3-(1H-pyrazol-4-yl)-5H-pyrrolo[2,3-b]pyrazine (27). Yield: 80%; white solid. 1H-NMR (400 MHz, DMSO-d6, ppm) δ 13.24 (s, 1H), 13.14 (s, 1H), 8.96 (s, 1H), 8.57 (s, 1H), 8.50 (s, 1H), 8.17 (s, 1H), 8.10 (d, J = 4.1 Hz, 1H), 7.82 (s, 1H), 6.91 (d, J = 4.1 Hz, 1H). ESI-MS: C12H10N7O2S, Exact Mass: 315.05, m/z 316.09(M + 1)+. HRMS-ESI m/z calcd. for C12H10N7O2S [M + H]+ 316.0611, found 316.0607. Retention time 2.18 min, >98% purity.
3-Bromo-5-((1-methyl-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazine (45). Yield: 76%; white solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.58 (s, 1H), 8.07 (d, J = 4.1 Hz, 1H), 7.99 (d, J = 1.4 Hz, 1H), 7.43 (s, 1H), 6.82 (d, J = 4.1 Hz, 1H), 3.79 (s, 3H). ESI-MS: C10H9BrN5O2S, Exact Mass: 340.96, m/z 341.91 (M + 1)+. Retention time 2.23 min, >98% purity.
5-((1-Methyl-1H-imidazol-4-yl)sulfonyl)-3-(1H-pyrazol-4-yl)-5H-pyrrolo[2,3-b]pyrazine (28). Yield: 82%; yellow solid. 1H-NMR (400 MHz, DMSO-d6, ppm) δ 13.25 (s, 1H), 8.97 (s, 1H), 8.59 (s, 1H), 8.50 (s, 1H), 8.20 (s, 1H), 8.08 (d, J = 4.1 Hz, 1H), 7.77 (s, 1H), 6.92 (d, J = 4.1 Hz, 1H), 3.71 (s, 3H). 13C-NMR (126 MHz, DMSO-d6, ppm) δ 142.75, 141.24, 140.13, 138.69, 138.65, 138.01, 135.91, 130.55, 129.73, 128.19, 119.70, 106.08, 34.28. ESI-MS: C13H12N7O2S, Exact Mass: 329.07, m/z 330.08 (M + 1)+. HRMS-ESI m/z calcd. for C13H12N7O2S [M + H]+ 330.0768, found 330.0771. Retention time 2.25 min, >96% purity.
3-Bromo-5-((1-isopropyl-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazine (47). Yield: 83%; white solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.57 (s, 1H), 8.15 (d, J = 1.3 Hz, 1H), 8.08 (d, J = 4.1 Hz, 1H), 7.53 (d, J = 1.2 Hz, 1H), 6.82 (d, J = 4.1 Hz, 1H), 4.48–4.36 (m, 1H), 1.55 (d, J = 6.7 Hz, 6H). ESI-MS: C12H13BrN5O2S, Exact Mass: 368.99, m/z 370.06 (M + 1)+. Retention time 2.96 min, >98% purity.
6-((1-Isopropyl-1H-imidazol-4-yl)sulfonyl)-3-(1H-pyrazol-4-yl)-5H-pyrrolo[2,3-b]pyrazine (30). Yield: 80%; white solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.70 (s, 1H), 8.16 (s, 2H), 8.07 (s, 1H), 8.01 (d, J = 4.1 Hz, 1H), 7.52 (s, 1H), 6.79 (d, J = 4.1 Hz, 1H), 4.41–4.30 (m, 1H), 1.44 (d, J = 6.7 Hz, 6H). 13C-NMR (126 MHz, CD3OD, ppm) δ 142.50, 140.37, 138.60, 138.28, 137.30, 136.41, 130.28, 127.53, 125.78, 119.92, 104.99, 50.90, 22.11(C×2), one signal missing. ESI-MS: C15H16N7O2S, Exact Mass: 357.10, m/z 358.15(M + 1)+. HRMS-ESI m/z calcd. for C15H16N7O2S [M + H]+ 358.1081, found 358.1081. Retention time 2.42 min, >99% purity.
2-Bromo-5-((1-isobutyl-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazine (48). Yield: 76%; white solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.57 (s, 1H), 8.08 (d, J = 4.1 Hz, 1H), 8.03 (s, 1H), 7.42 (s, 1H), 6.82 (d, J = 4.1 Hz, 1H), 3.82 (d, J = 7.1 Hz, 2H), 2.12–2.03 (m, 1H), 0.93 (d, J = 7.0 Hz, 6H). ESI-MS: C13H15BrN5O2S, Exact Mass: 383.01, m/z 406.1 (M + Na)+. Retention time 3.31 min, >96% purity.
5-((1-Isobutyl-1H-imidazol-4-yl)sulfonyl)-3-(1H-pyrazol-4-yl)-5H-pyrrolo[2,3-b]pyrazine (31). Yield: 79%; white solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.76 (s, 1H), 8.60 (s, 1H), 8.25 (s, 1H), 8.10 (d, J = 4.1 Hz, 1H), 7.94 (d, J = 1.3 Hz, 1H), 7.39 (d, J = 1.2 Hz, 1H), 6.84 (d, J = 4.1 Hz, 1H), 3.76 (d, J = 7.2 Hz, 2H), 2.06–1.93 (m, 1H), 0.83 (d, J = 6.7 Hz, 6H). ESI-MS: C16H18N7O2S, Exact Mass: 371.12, m/z 372.16 (M + 1)+. HRMS-ESI m/z calcd. for C16H18N7O2S [M + H]+ 372.1237, found 372.1281. Retention time 3.35 min, >97% purity.
3-Bromo-5-((1-cyclopentyl-1H-imidazol-4-yl)sulfonyl)-5H-pyrrolo[2,3-b]pyrazine (49). Yield: 82%; white solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.57 (s, 1H), 8.11 (d, J = 1.3 Hz, 1H), 8.07 (d, J = 4.1 Hz, 1H), 7.51 (d, J = 1.3 Hz, 1H), 6.81 (d, J = 4.1 Hz, 1H), 4.57–4.49 (m, 1H), 2.30–2.21 (m, 2H), 1.95–1.77 (m, 6H). ESI-MS: C14H15BrN5O2S, Exact Mass: 395.01, m/z (M + 1)+. Retention time 2.35 min, >98% purity.
5-((1-Cyclopentyl-1H-imidazol-4-yl)sulfonyl)-3-(1H-pyrazol-4-yl)-5H-pyrrolo[2,3-b]pyrazine (32). Yield: 86%; white solid. 1H-NMR (400 MHz, CDCl3, ppm) δ 8.74 (s, 1H), 8.18 (s, 2H), 8.03 (d, J = 4.1 Hz, 1H), 8.01 (s, 1H), 7.49 (s, 1H), 6.81 (d, J = 4.1 Hz, 1H), 4.51–4.43 (m, 1H), 2.19 (td, J = 14.9, 8.5 Hz, 4H), 1.77–1.65 (m, 4H). ESI-MS: C17H18N7O2S, Exact Mass: 383.12, m/z 384.15 (M + 1)+. HRMS-ESI m/z calcd. for C17H18N7O2S [M + H]+ 384.1237, found 384.1395. Retention time 2.60 min, >96% purity.
2-(4-((3-(1H-Pyrazol-4-yl)-5H-pyrrolo[2,3-b]pyrazin-5-yl)sulfonyl)-1H-imidazol-1-yl)-N-methylacetamide (33). Yield: 84%; white solid. 1H-NMR (400 MHz, DMSO-d6, ppm) δ 13.22 (s, 1H), 8.97 (s, 1H), 8.52 (s, 1H), 8.49 (s, 1H), 8.18 (s, 1H), 8.14–8.08 (m, 2H), 7.75 (s, 1H), 6.92 (d, J = 4.1 Hz, 1H), 4.77 (s, 2H), 2.57 (d, J = 4.5 Hz, 3H). ESI-MS: C15H15N8O3S, Exact Mass: 386.09, m/z 387.14(M + 1)+. HRMS-ESI m/z calcd. for C15H15N8O3S [M + H]+ 387.0982, found 387.1067. Retention time 2.57 min, >98% purity.