Anti-Cancer Activity of Lobaric Acid and Lobarstin Extracted from the Antarctic Lichen Stereocaulon alpnum
Abstract
:1. Introduction
2. Results
2.1. Effects of Lobaric Acid and Lobarstin on Cell Viability
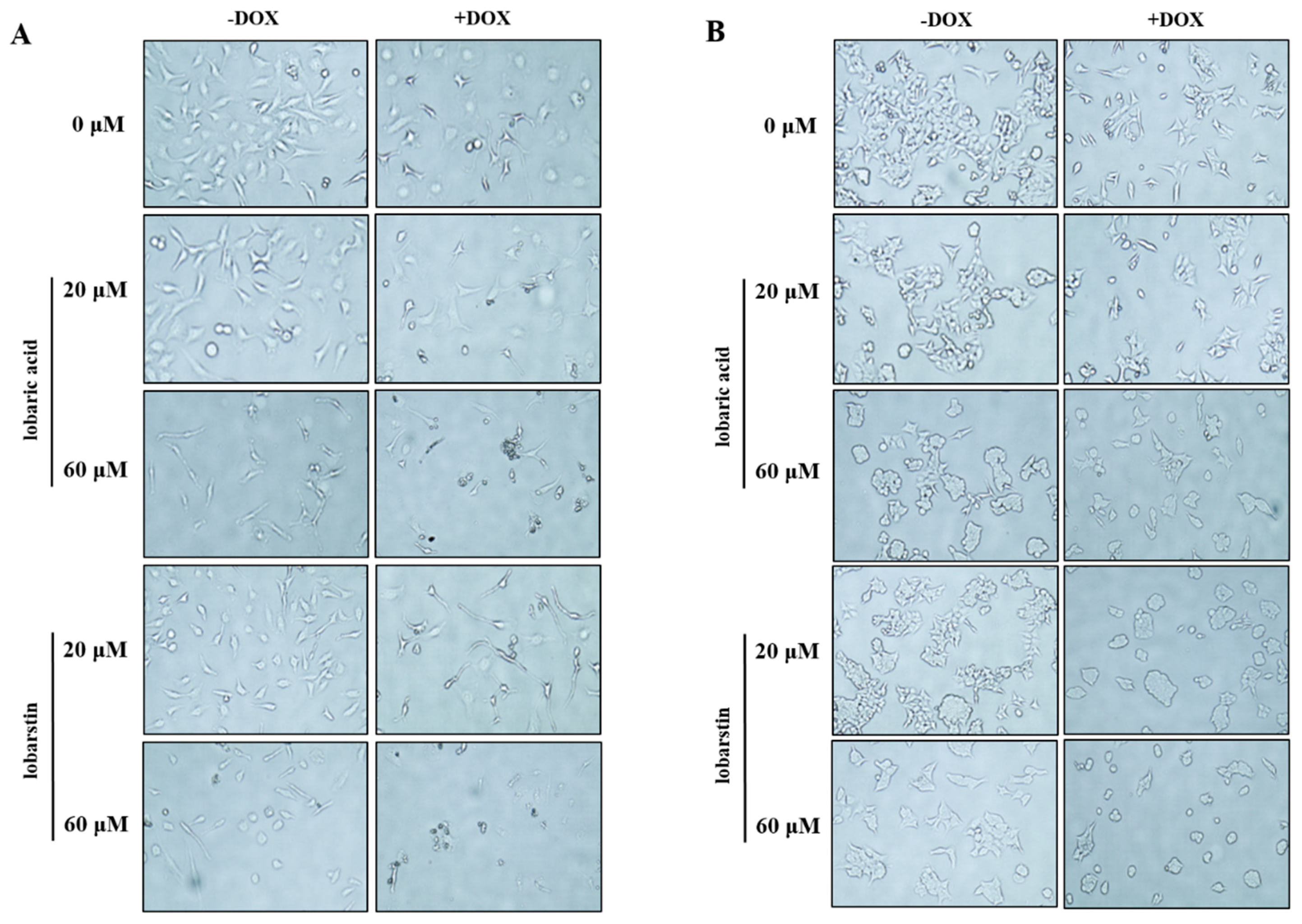
2.2. Effect of Lobaric Acid and Lobarstin on Cell Morphology
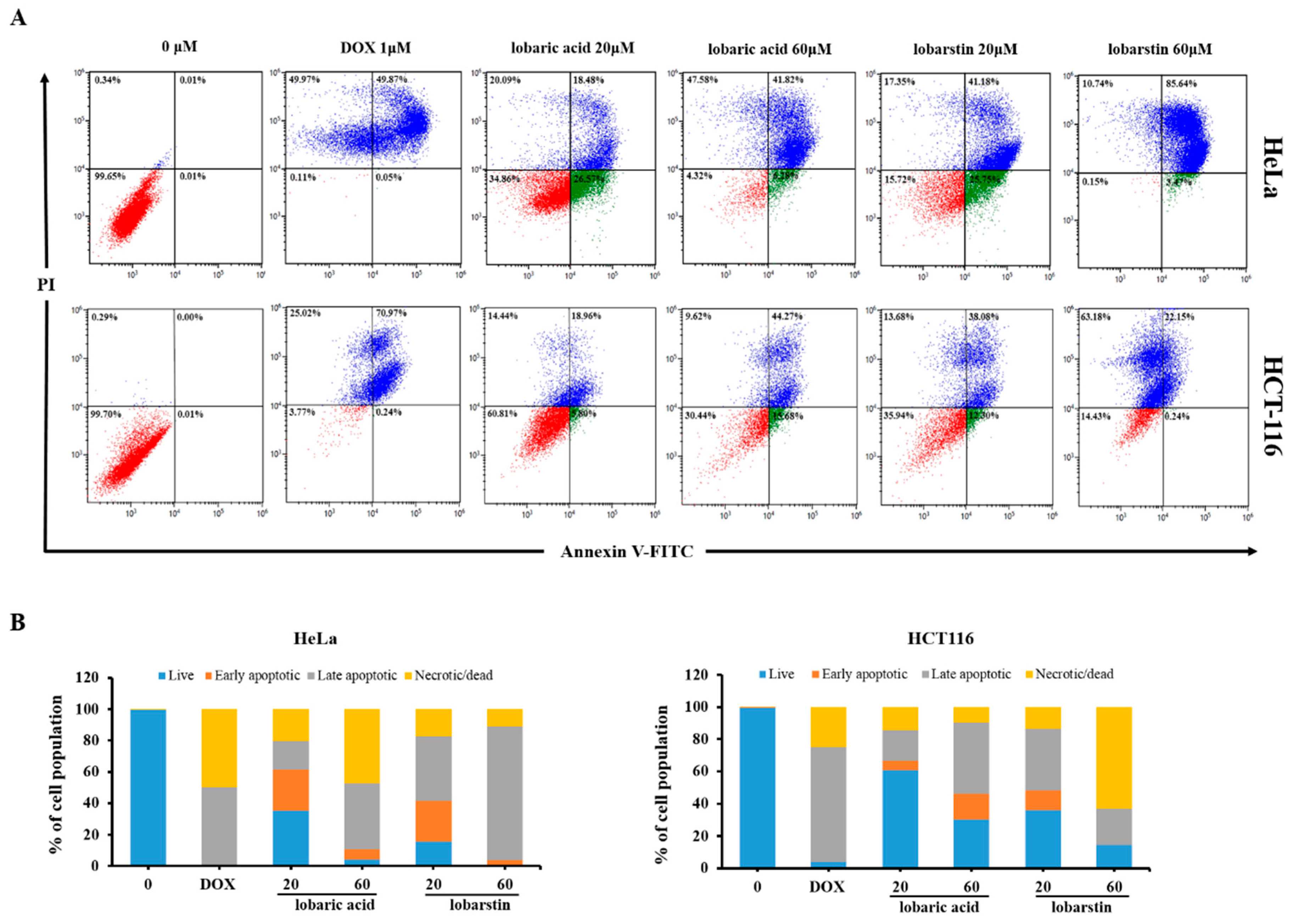
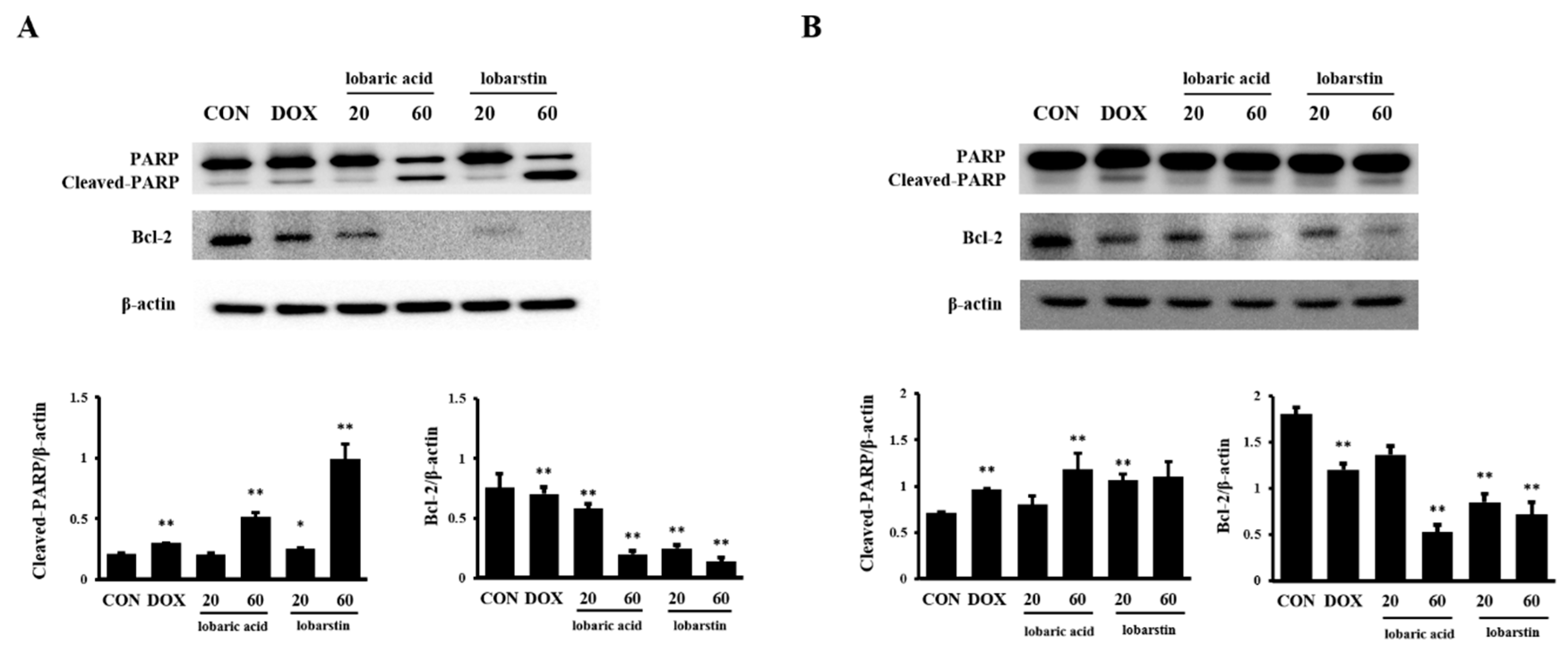
2.3. Effect of Lobaric Acid and Lobarstin on Apoptosis
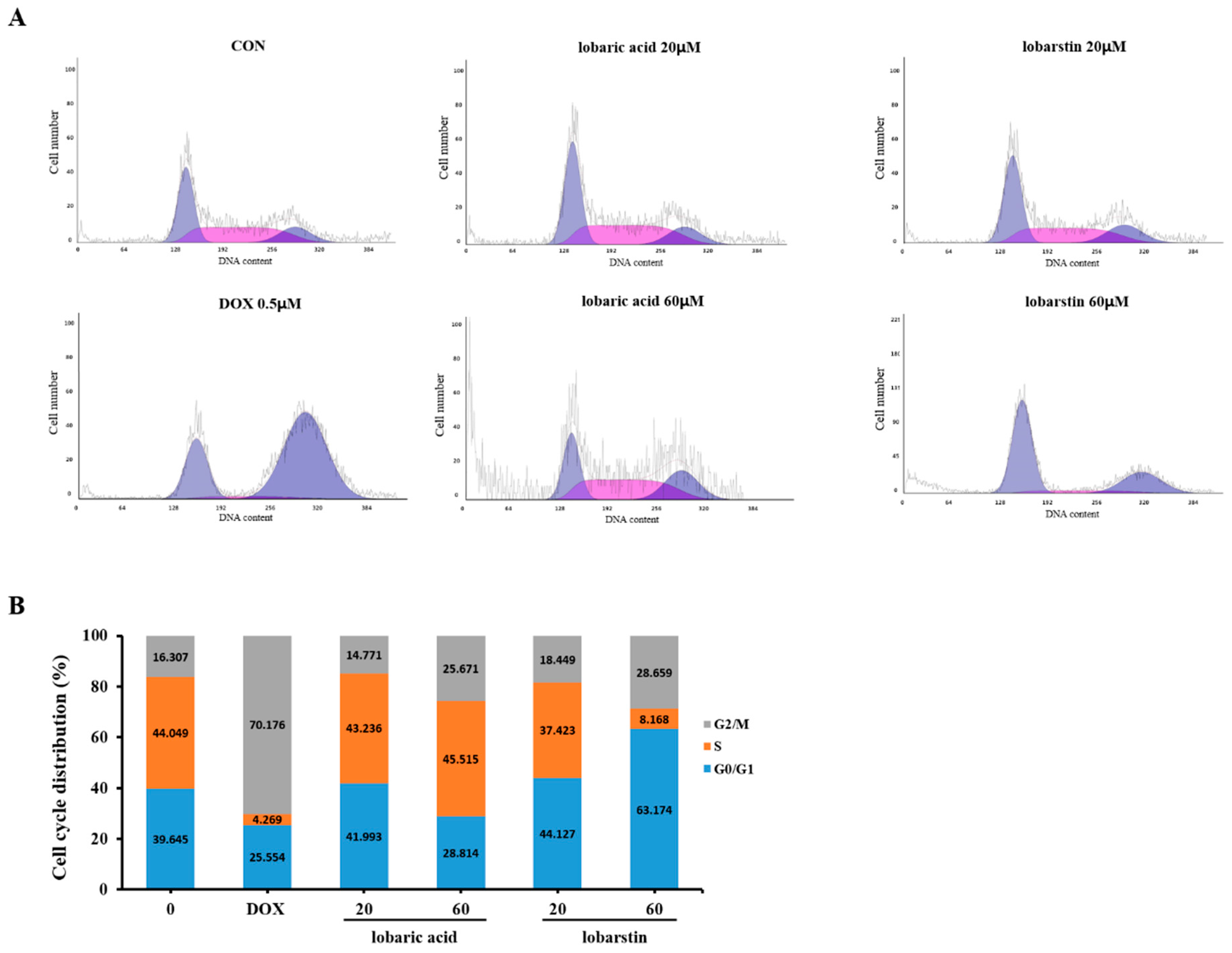
2.4. Effect of Lobaric Acid and Lobarstin on the Cell Cycle
3. Discussion
4. Materials and Methods
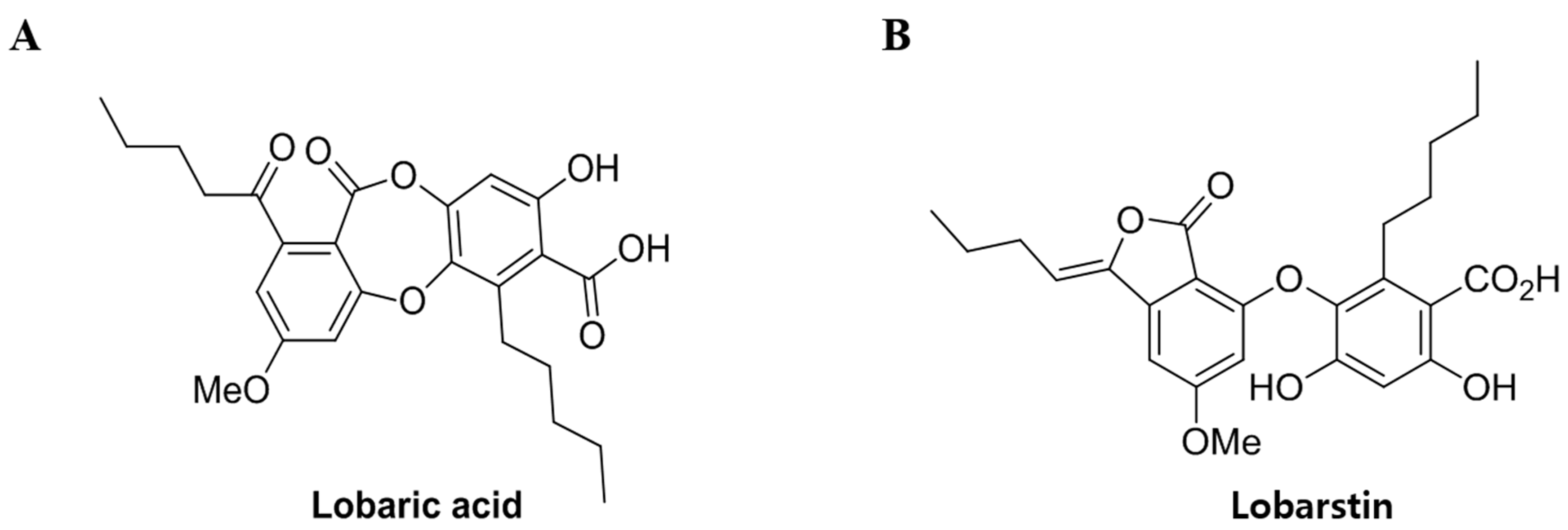
4.1. Preparation of Compounds
4.2. Cell Culture
4.3. Cytotoxicity Assay
4.4. Morphological Analysis
4.5. Apoptosis Assays
4.6. Immunoblot Analysis
4.7. Cell Cycle Analysis
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heron, M.; Tejada-Vera, B. Deaths: Leading causes for 2005. Natl. Vital Stat. Rep. 2009, 58, 1–97. [Google Scholar] [PubMed]
- Michael, J.T.; John, O.D.; Melissa, M.C.; Ahmedin, J.; Elizabeth, M.W. The global burden of cancer: Priorities for prevention. Carcinogenesis 2010, 31, 100–110. [Google Scholar]
- Soumaya, B.J.; Nolwenn, H.; Soumaya, B.; Ahmed, J.; Mokhtar, L.; Christian, M.; Riadh, K. Antioxidant and selective anticancer activities of two Euphorbia species in human acute myeloid leukemia. Biomed. Pharmacother. 2017, 90, 375–385. [Google Scholar]
- Yadav, N.; Kumar, P.; Chhikara, A.; Chopra, M. Development of 1,3,4-oxadiazole thione based novel anticancer agents: Design, synthesis and in-vitro studies. Biomed. Pharmacother. 2017, 95, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, G.; Ebrahimi, S.A.; Rahbar-Roshandel, N.; Foroumadi, A. Antiproliferative activity of flavonoids: Influence of the sequential methoxylation state of the flavonoid structure. Phytother. Res. 2012, 26, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.X.; Nguyen, B.; Jia, S.; Herich, J.; Guastella, J.; Reddy, S.; Tseng, B.; Drewe, J.; Kasibhatla, S. Discovery of substituted N-phenyl nicotinamides as potent inducers of apoptosis using a cell-and caspase-based high throughput screening assay. J. Med. Chem. 2003, 46, 2474–2481. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.D. Lichens used in traditional medicine. In Lichen Secondary Metabolites; Rankovic, B., Ed.; Springer International Publishing: New York, NY, USA, 2015; pp. 27–80. ISBN 978-3-319-13374-4. [Google Scholar]
- Agnieszka, F.; Alicja, P.S.; Anna, P.; Krystyna, B.; Anna, H.A.; Beata, G.K. Antibacterial and anticancer activities of acetone extracts from in vitro cultured lichen-forming fungi. BMC Complement. Altern. Med. 2017, 17, 300. [Google Scholar]
- Huneck, S. The significance of lichens and their metabolites. Naturwissenschaften 1999, 86, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Huneck, S.; Yoshimura, I. Identification of Lichen Substances; Springer: Berlin/Heidelberg, Germany, 1996; pp. 11–123. [Google Scholar]
- Scirpa, P.; Scambia, G.; Masciullo, V.; Battaglia, F.; Foti, E.; Lopez, R.; Villa, P.; Malecore, M.; Mancuso, S. Terapia adiuvante con un preparato a base di zinco solfato e acido usnico delle lesioni genitali da Human Papilloma Virus (HPV) dopo trattamento chirurgico distruttivo. Minerva Ginecol. 1999, 51, 255–260. [Google Scholar] [PubMed]
- Mayer, M.; O’Neill, M.A.; Murray, K.E.; Santos-Magalhaes, N.S.; Carneiro-Leao, A.M.; Thompson, A.M.; Appleyard, V.C. Usnic acid: A non-genotoxic compound with anti-cancer properties. Anticancer Drugs 2005, 16, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.; Sohn, J.H.; Ahn, J.S.; Yim, J.H.; Lee, H.K.; Oh, H. Protein tyrosine phosphatase 1B inhibitory effects of depsidone and pseudodepsidone metabolites from the Antarctic lichen Stereocaulon alpinum. Bioorg. Med. Chem. Lett. 2009, 19, 2801–2803. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, H.D.; Kim, T.; Oh, H.; Yim, J.H. A new pseudodepsidone from the Antarctic lichen Stereocaulon alpinum and its antioxidant, antibacterial activity. J. Antibiot. (Tokyo) 2013, 66, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Ogmundsdóttir, H.M.; Zoëga, G.M.; Gissurarson, S.R.; Ingólfsdóttir, K. Anti-proliferative effects of lichen-derived inhibitors of 5-lipoxygenase on malignant cell-lines and mitogen-stimulated lymphocytes. J. Pharm. Pharmacol. 1998, 50, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Gissurarson, S.R.; Sigurdsson, S.B.; Hildebert, W.; Kristin, I. Effect of lobaric acid on cysteinyl-leukotriene formation and contractile activity of guinea pig taenia coli. J. Pharmacol. Exp. Ther. 1997, 280, 770–773. [Google Scholar] [PubMed]
- Hidalgo, M.E.; Bascunan, L.; Quilhot, W.; Fernandez, E.; Rubio, C. Spectroscopic and photochemical properties of the lichen compound lobaric acid. Photochem. Photobiol. 2005, 81, 1447–1449. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jo, S.; Lee, H.; Kim, T.U.; Kim, I.C.; Yim, J.H.; Chung, H. Lobarstin enhances chemosensitivity in human glioblastoma T98G cells. Anticancer Res. 2013, 33, 5445–5451. [Google Scholar] [PubMed]
- Antti, S.; Kari, P. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar]
- Frederik, H.I.; Peter, H.K. Death and anti-death: Tumor resistance to apoptosis. Nat. Rev. Cancer 2002, 2, 277–288. [Google Scholar]
- Mohamed, H.; Hidemichi, W.; Ali, A.A.; Yusuke, O.; Noriaki, S. Apoptosis and Molecular Targeting Therapy in Cancer. Biomed. Res. Int. 2014, 2014, 150845. [Google Scholar]
- Duane, R.S.; William, J.; Harrington, J. Apoptosis: Programmed cell death at a molecular level. Semin. Arthritis Rheum. 2003, 32, 345–369. [Google Scholar]
- Geoffrey, I.S.; Harper, J.W. Anticancer drug targets: Cell cycle and checkpoint control. J. Clin. Investig. 1999, 104, 1645–1653. [Google Scholar]
- Xu, W.; Mi, Y.; He, P.; He, S.; Niu, L. γ-Tocotrienol inhibits proliferation and induces apoptosis via the mitochondrial pathway in human cervical cancer HeLa cells. Molecules 2017, 22, 1299–1313. [Google Scholar]
- Tang, Y.; Xie, M.; Jiang, N.; Huang, F.; Zhang, X.; Li, R.; Lu, J.; Liao, S.; Liu, Y. Icarisid II inhibits the proliferation of human osteosarcoma cells by inducing apoptosis and cell cycle arrest. Tumor Biol. 2017, 39, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Eva, P.; Cecilia, L.; Ben, H.; Ricky, A.S. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer 2008, 8, 193–204. [Google Scholar]
- Brisdelli, F.; Perilli, M.; Sellitri, D.; Piovano, M.; Garbarino, J.A.; Nicoletti, M.; Bozzi, A.; Amicosante, G.; Celenza, G. Cytotoxic activity and antioxidant capacity of purified lichen metabolites: An in vitro study. Phytother. Res. 2013, 27, 431–437. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all the compounds (lobaric acid and lobarstin) are available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.-M.; Suh, S.-S.; Kim, T.K.; Kim, J.E.; Han, S.J.; Youn, U.J.; Yim, J.H.; Kim, I.-C. Anti-Cancer Activity of Lobaric Acid and Lobarstin Extracted from the Antarctic Lichen Stereocaulon alpnum. Molecules 2018, 23, 658. https://doi.org/10.3390/molecules23030658
Hong J-M, Suh S-S, Kim TK, Kim JE, Han SJ, Youn UJ, Yim JH, Kim I-C. Anti-Cancer Activity of Lobaric Acid and Lobarstin Extracted from the Antarctic Lichen Stereocaulon alpnum. Molecules. 2018; 23(3):658. https://doi.org/10.3390/molecules23030658
Chicago/Turabian StyleHong, Ju-Mi, Sung-Suk Suh, Tai Kyoung Kim, Jung Eun Kim, Se Jong Han, Ui Joung Youn, Joung Han Yim, and Il-Chan Kim. 2018. "Anti-Cancer Activity of Lobaric Acid and Lobarstin Extracted from the Antarctic Lichen Stereocaulon alpnum" Molecules 23, no. 3: 658. https://doi.org/10.3390/molecules23030658
APA StyleHong, J.-M., Suh, S.-S., Kim, T. K., Kim, J. E., Han, S. J., Youn, U. J., Yim, J. H., & Kim, I.-C. (2018). Anti-Cancer Activity of Lobaric Acid and Lobarstin Extracted from the Antarctic Lichen Stereocaulon alpnum. Molecules, 23(3), 658. https://doi.org/10.3390/molecules23030658



