Recent Advances in Modified Cellulose for Tissue Culture Applications
Abstract
1. Introduction
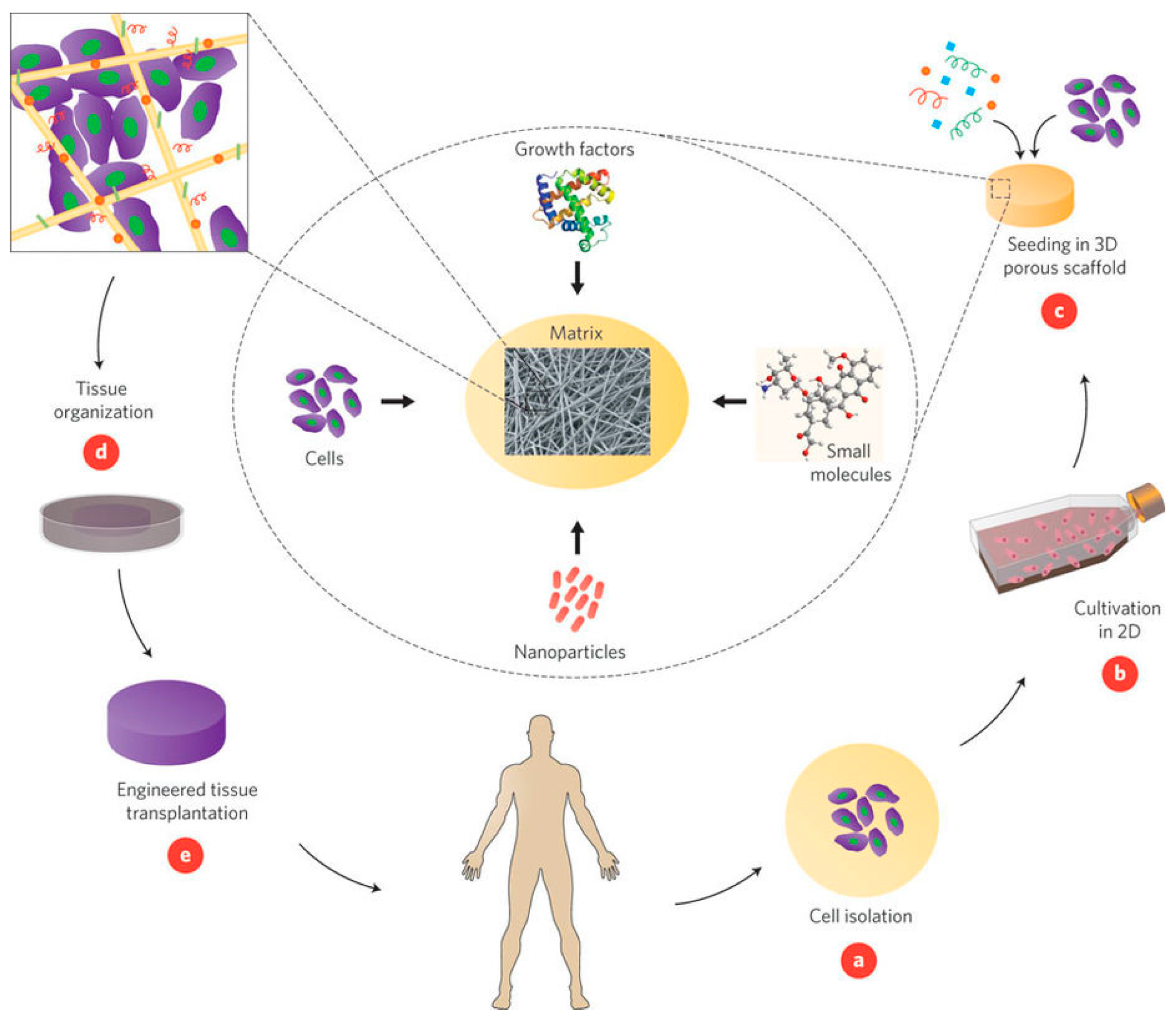
1.1. Principles of Tissue Engineering
- Desired cells are extracted from the patient;
- The isolated cells are cultured and expanded in vitro on a 2D scaffold;
- The cell culture is seeded into a 3D scaffold support and additional biomolecules, such as matrix ligands, are added to promote growth;
- A bioreactor is often used to develop the cell/scaffold construct into functioning tissue; and
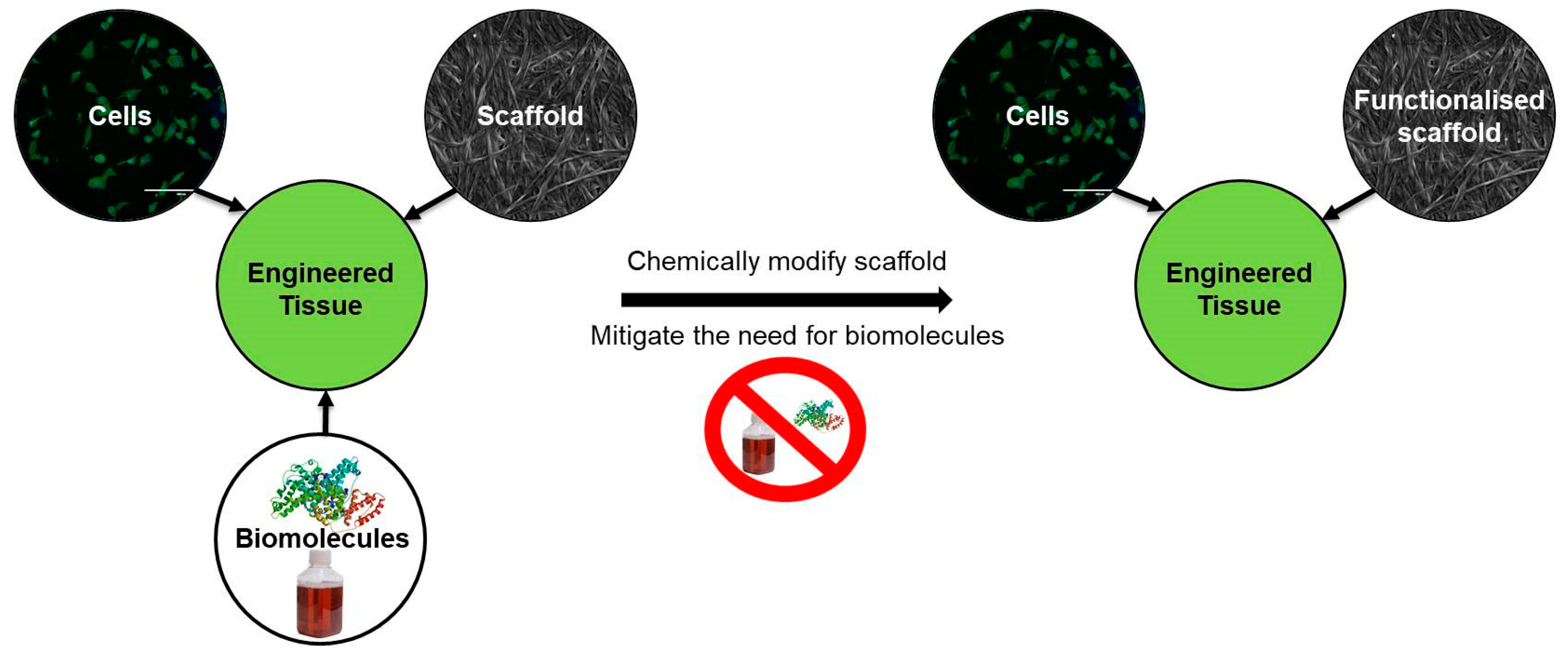
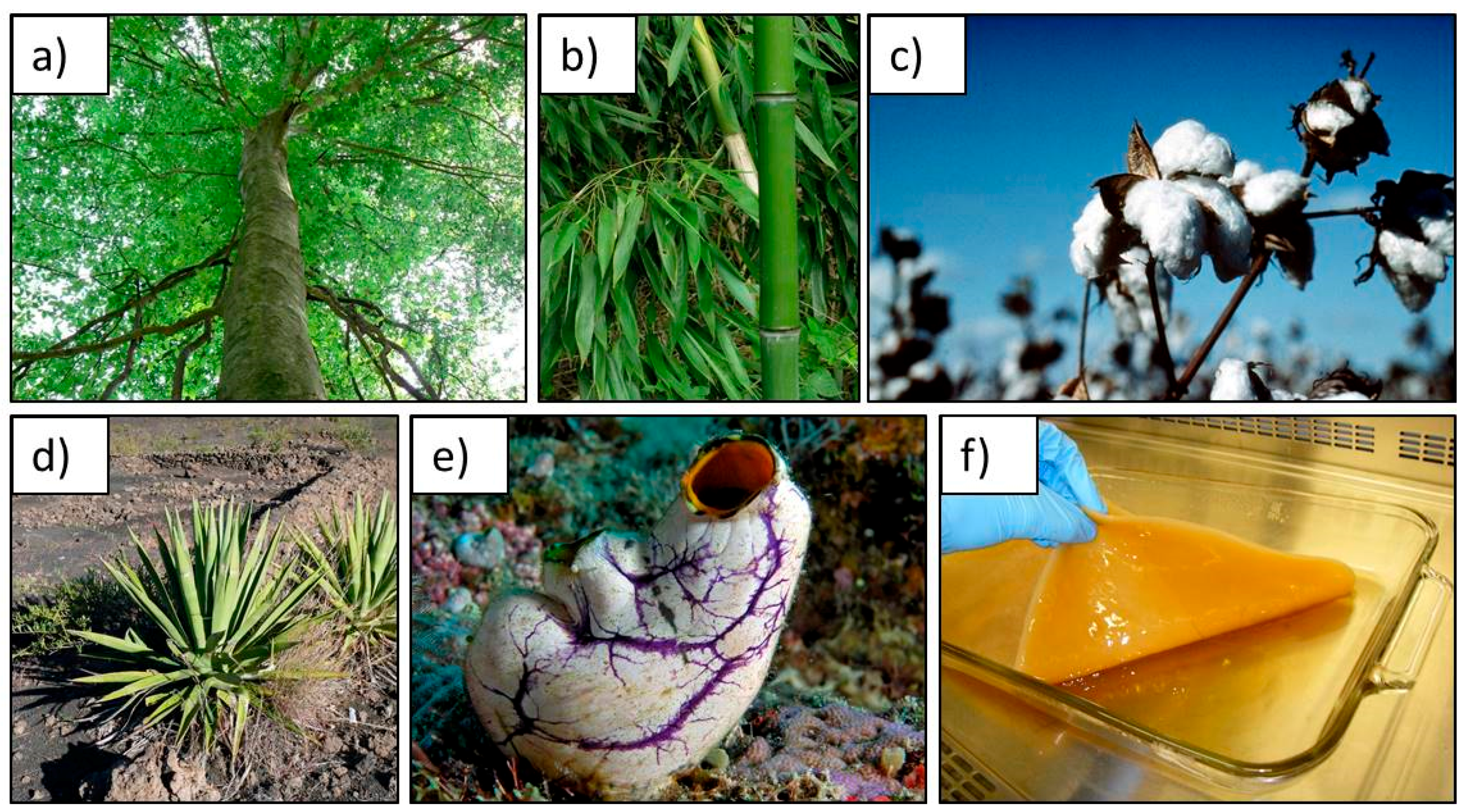
1.2. Cellulose as a Sustainable Scaffold for Tissue Engineering
2. Methods of Scaffold Modification
- Physical modifications—composites and blends;
- Biochemical modifications—grafting of biomolecules onto the surface;
- Chemical modifications—introducing new functional groups.
2.1. Physical Modifications
2.2. Biochemical Modifications
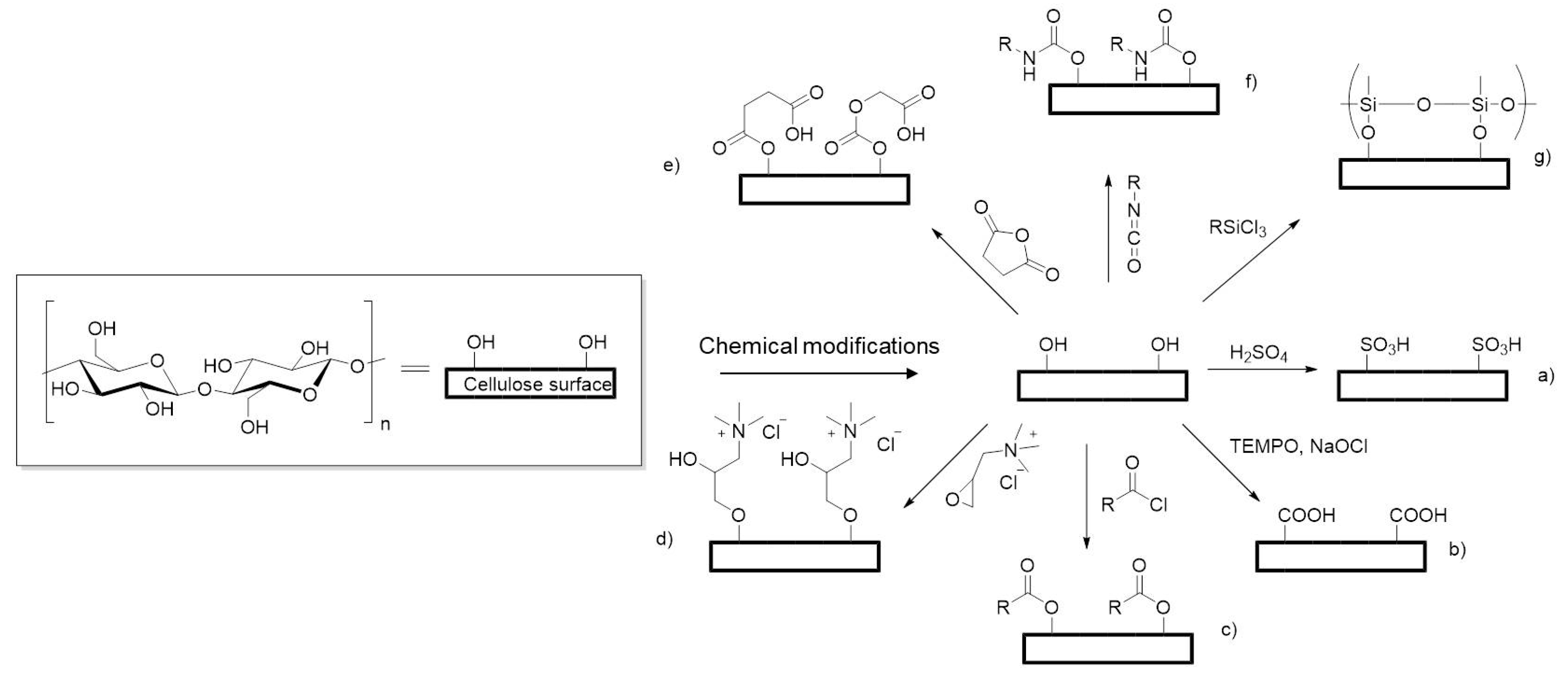
2.3. Chemical Modifications
2.4. Cellulose Bioresorbability and Biodegradability In Vivo
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Salgado, A.J.; Oliveira, J.M.; Martins, A.; Teixeira, F.G.; Silva, N.A.; Neves, N.M.; Sousa, N.; Reis, R.L. Tissue engineering and regenerative medicine: Past, present, and future. Int. Rev. Neurobiol. 2013, 108, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.; Dunnill, P. A brief definition of regenerative medicine. Regen. Med. 2008, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lanza, R.; Langer, R.; Vacanti, J. Principles of Tissue Engineering, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Meyer, U.; Meyer, T.; Handschel, J.; Wiesmann, H.P. The History of Tissue Engineering and Regenerative Medicine in Perspective; Meyer, U., Meyer, T., Handschel, J., Wiesmann, H.P., Eds.; Springer: Berlin, Germany, 2009; ISBN 978-3-540-77754-0. [Google Scholar]
- Murail, P.; Girard, L. False teeth of the Roman world. Nature 1998, 391, 7–8. [Google Scholar] [CrossRef]
- Green, W.T.J. Articular cartilage repair. Behavior of rabbit chondrocytes during tissue culture and subsequent allografting. Clin. Orthop. Relat. Res. 1977, 124, 237–250. [Google Scholar]
- Vacanti, C. The history of tissue engineering. J. Cell. Mol. Med. 2006, 1, 569–576. [Google Scholar] [CrossRef]
- Constant, E.; Burke, J.F. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Plast. Reconstr. Surg. 1982, 70, 784. [Google Scholar] [CrossRef]
- Phillips, T.J.; Kehinde, O.; Green, H.; Gilchrest, B.A. Treatment of skin ulcers with cultured epidermal allografts. J. Am. Acad. Dermatol. 1989, 21, 191–199. [Google Scholar] [CrossRef]
- Coulomb, B.; Friteau, L.; Baruch, J.; Guilbaud, J.; Chretien-Marquet, B.; Glicenstein, J.; Lebreton-Decoster, C.; Bell, E.; Dubertret, L. Advantage of the Presence of Living Dermal Fibroblasts within in Vitro Reconstructed Skin for Grafting in Humans. Plast. Reconstr. Surg. 1998, 101, 1891–1903. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Gonfiotti, A.; Jaus, M.O.; Barale, D.; Baiguera, S.; Comin, C.; Lavorini, F.; Fontana, G.; Sibila, O.; Rombolà, G.; Jungebluth, P.; et al. The first tissue-engineered airway transplantation: 5-year follow-up results. Lancet 2014, 383, 238–244. [Google Scholar] [CrossRef]
- Delaere, P.R.; Van Raemdonck, D. The trachea: The first tissue-engineered organ? J. Thorac. Cardiovasc. Surg. 2014, 147, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B. Paralysed Man Darek Fidyka Walks again after Pioneering Surgery. 2004. Available online: http://www.theguardian.com/science/2014/oct/21/paralysed-darek-fidyka-pioneering-surgery (accessed on 21 October 2014).
- Agrawal, C.M.; Ong, J.L.; Appleford, M.R.; Mani, G. Tissue Engineering. In Introduction to Biomaterials—Basic Theory with Engineering Applications; Saltzman, M.W., Chien, S., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 341–374. [Google Scholar]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Keller, G. Embryonic stem cell differentiation: Emergence of a new era in biology and medicine. Genes Dev. 2005, 19, 1129–1155. [Google Scholar] [CrossRef] [PubMed]
- Heath, C.A. Cells for tissue engineering. Trends Biotechnol. 2000, 18, 17–19. [Google Scholar] [CrossRef]
- Gupta, R.; Enver, T.; Medvinsky, A. Cord Blood Stem Cells: Current Uses and Future Challenges. 2015. Available online: http://www.eurostemcell.org/factsheet/cord-blood-stem-cells-current-uses-and-future-challenges (accessed on 5 April 2015).
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Van der Valk, J. Fetal Bovine Serum (FBS): Past—Present—Future. ALTEX 2017, 35, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, J.C.; Johns, M.A.; Galembeck, F.; Deneke, C.; Lanzoni, E.M.; Costa, C.A.; Scott, J.L.; Sharma, R.I. Surface modified cellulose scaffolds for tissue engineering. Cellulose 2017, 24, 253–267. [Google Scholar] [CrossRef]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 2014, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J.; Maddox, R.D.; Taboas, J.M. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials 2002, 23, 4095–4103. [Google Scholar] [CrossRef]
- Agrawal, C.M.; Ray, R.B. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J. Biomed. Mater. Res. Part A 2001, 55, 141–150. [Google Scholar] [CrossRef]
- Peloso, A.; Dhal, A.; Zambon, J.P.; Li, P.; Orlando, G.; Atala, A.; Soker, S. Current achievements and future perspectives in whole-organ bioengineering. Stem Cell Res. Ther. 2015, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Abouna, G.M. Organ Shortage Crisis: Problems and Possible Solutions. Transplant. Proc. 2008, 40, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Patterson, J.; Martino, M.M.; Hubbell, J.A. Biomimetic materials in tissue engineering. Mater. Today 2010, 13, 14–22. [Google Scholar] [CrossRef]
- Benoit, D.S.W.; Anseth, K.S. Heparin functionalized PEG gels that modulate protein adsorption for hMSC adhesion and differentiation. Acta Biomater. 2005, 1, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Post, M.J. Cultured meat from stem cells: Challenges and prospects. Meat Sci. 2012, 92, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, H.L.; De Mattos, M.J.T. Environmental impacts of cultured meat production. Environ. Sci. Technol. 2011, 45, 6117–6123. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Lustri, W.R.; de Oliveira Barud, H.G.; Da Silva Barud, H.; Peres, M.F.S.; Gutierrez, J.; Tercjak, A.; de Oliveira Junior, O.B.; José Lima Ribeiro, S. Microbial Cellulose—Biosynthesis Mechanisms and Medical Applications, In Cellulose—Fundamental Aspects and Current Trends; Poletto, D.M., Ed.; INTECH Open Access Publisher: Rijeka, Croatia, 2015. [Google Scholar]
- Potulski, D.C.; De Muniz, G.I.B.; Klock, U.; De Andrade, A.S. Green composites from sustainable cellulose nanofibrils: A review. Sci. For. Sci. 2014, 40, 345–351. [Google Scholar] [CrossRef]
- Nitta, S.K.; Numata, K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef] [PubMed]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, C.M.; Ong, J.L.; Appleford, M.R.; Mani, G. Natural biomaterials. In Introduction to Biomaterials—Basic Theory with Engineering Applications; Cambrigde University Press: Cambridge, UK, 2014; pp. 198–232. [Google Scholar]
- Klemm, D.; Heublein, B.; Fink, H.-P.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Modulevsky, D.J.; Cuerrier, C.M.; Pelling, A.E. Biocompatibility of Subcutaneously Implanted Plant-Derived Cellulose Biomaterials. PLoS ONE 2016, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Biocompatibility of bacterial cellulose based biomaterials. J. Funct. Biomater. 2012, 3, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [PubMed]
- Jedvert, K.; Heinze, T. Cellulose modification and shaping—A review. J. Polym. Eng. 2017, 37, 845–860. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.A.; Isaksson, P.; Lindstro, T.; Nishino, T. Nanopaper Structures of High Toughness. Biomacromolecules 2008, 9, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Samir, M.A.S.A.; Alloin, F.; Sanchez, J.Y.; Dufresne, A. POE-based nanocomposite polymer electrolytes reinforced with cellulose whiskers. Macromolecules 2004, 37, 4839–4844. [Google Scholar] [CrossRef]
- De Souza Lima, M.M.; Borsali, R. Rodlike Cellulose Microcrystals: Structure, Properties, and Applications. Macromolecules 2004, 25, 771–787. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.; Dahman, Y. Improvements in the production of bacterial synthesized biocellulose nanofibres using different culture methods. J. Chem. Technol. Biotechnol. 2010, 85, 151–164. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 1–19. [Google Scholar] [CrossRef]
- Chao, Y.; Ishida, T.; Sugano, Y.; Shoda, M. Bacterial cellulose production by Acetobacter xylinum in a 50-L internal-loop airlift reactor. Biotechnol. Bioeng. 2000, 68, 345–352. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, J.; Yang, G. Present status and applications of bacterial cellulose-based materials for skin tissue repair. Carbohydr. Polym. 2013, 92, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Nazrul Islam, M.; Mustapha, A.; Sudesh, K.; Dungania, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Afewerki, S.; Alimohammadzadeh, R.; Osong, S.H.; Tai, C.-W.; Engstrand, P.; Córdova, A.; Afewerki, S.; Alimohammadzadeh, R.; Córdova, A.; Osong, S.H.; et al. Sustainable Design for the Direct Fabrication and Highly Versatile Functionalization of Nanocelluloses. Glob. Chall. 2017, 1, 1–6. [Google Scholar] [CrossRef]
- Li, Q.; McGinnis, S.; Sydnor, C.; Wong, A.; Renneckar, S. Nanocellulose life cycle assessment. ACS Sustain. Chem. Eng. 2013, 1, 919–928. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Malinen, M.M.; Lauren, P.; Lou, Y.-R.; Kuisma, S.W.; Kanninen, L.; Lille, M.; Corlu, A.; GuGuen-Guillouzo, C.; Ikkala, O.; et al. Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J. Control. Release 2012, 164, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Ramakrishna, S. Electrospun regenerated cellulose nanofiber affinity membrane functionalized with protein A/G for IgG purification. J. Membr. Sci. 2008, 319, 23–28. [Google Scholar] [CrossRef]
- Torres-Rendon, J.G.; Femmer, T.; De Laporte, L.; Tigges, T.; Rahimi, K.; Gremse, F.; Zafarnia, S.; Lederle, W.; Ifuku, S.; Wessling, M.; et al. Bioactive Gyroid Scaffolds Formed by Sacrificial Templating of Nanocellulose and Nanochitin Hydrogels as Instructive Platforms for Biomimetic Tissue Engineering. Adv. Mater. 2015, 27, 2989–2995. [Google Scholar] [CrossRef] [PubMed]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.K.; Dolivo, D.M.; Yang, T.; Dominko, T.; et al. Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Modulevsky, D.J.; Lefebvre, C.; Haase, K.; Al-Rekabi, Z.; Pelling, A.E. Apple Derived Cellulose Scaffolds for 3D Mammalian Cell Culture. PLoS ONE 2014, 9, e97835. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, J.C.; Ramalhete, S.M.; Skuze, W.J.; Soni, R.; Khimyak, Y.Z.; Edler, K.J.; Scott, J.L. Unravelling cationic cellulose nanofibril hydrogel structure: NMR spectroscopy and small angle neutron scattering analyses. Soft Matter 2018, 14, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Capron, I.; Cathala, B. Surfactant-free high internal phase emulsions stabilized by cellulose nanocrystals. Biomacromolecules 2013, 14, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Braun, B.; Dorgan, J.R. Single-Step Method for the Isolation and Surface Functionalization of Cellulosic Nanowhiskers. Biomacromolecules 2009, 10, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Missoum, K.; Belgacem, M.N.; Bras, J. Nanofibrillated cellulose surface modification: A review. Materials 2013, 6, 1745–1766. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Ogasawara, K.; Kusumoto, R.; Oshima, K.; Hashimoto, H.; Shimizu, Y. Cationic cellulose hydrogels cross-linked by poly(ethylene glycol): Preparation, molecular dynamics, and adsorption of anionic dyes. Carbohydr. Polym. 2016, 152, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.W.; Luong, J.H.T. Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol. 2012, 30, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.J.; Edler, K.J.; Lindhoud, S.; Scott, J.L.; Unali, G. Formation of shear thinning gels from partially oxidised cellulose nanofibrils. Green Chem. 2012, 14, 300–303. [Google Scholar] [CrossRef]
- Birkheur, S.; de Sousa Faria-Tischer, P.C.; Tischer, C.A.; Pimentel, E.F.; Fronza, M.; Endringer, D.C.; Butera, A.P.; Ribeiro-Viana, R.M. Enhancement of fibroblast growing on the mannosylated surface of cellulose membranes. Mater. Sci. Eng. C 2017, 77, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Taokaew, S.; Phisalaphong, M.; Newby, B.Z. Modification of bacterial cellulose with organosilanes to improve attachment and spreading of human fibroblasts. Cellulose 2015, 22, 2311–2324. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Lee, D.; Shin, S.; Hyun, J. Effect of negatively charged cellulose nanofibers on the dispersion of hydroxyapatite nanoparticles for scaffolds in bone tissue engineering. Colloids Surf. B Biointerfaces 2015, 130, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Bodin, A.; Ahrenstedt, L.; Fink, H.; Brumer, H.; Risberg, B.; Gatenholm, P. Modification of Nanocellulose with a Xyloglucan—RGD Conjugate Enhances Adhesion and Proliferation of Endothelial Cells: Implications for Tissue Engineering. Biomacromolecules 2007, 8, 3697–3704. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lv, X.; Chen, S.; Li, Z.; Yao, J.; Peng, X.; Feng, C.; Xu, Y.; Wang, H. Use of heparinized bacterial cellulose based scaffold for improving angiogenesis in tissue regeneration. Carbohydr. Polym. 2018, 181, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Pertile, R.; Moreira, S.; Andrade, F.; Domingues, L.; Gama, M. Bacterial cellulose modified using recombinant proteins to improve neuronal and mesenchymal cell adhesion. Biotechnol. Prog. 2012, 28, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Saha, N.; Bandyopadhyay, S.; Saha, P. Rheological performance of bacterial cellulose based nonmineralized and mineralized hydrogel scaffolds. AIP Conf. Proc. 2017, 1843, 050008. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Yang, F.; Shao, Y.; Zhang, X.; Dai, K. Modification and evaluation of micro-nano structured porous bacterial cellulose scaffold for bone tissue engineering. Mater. Sci. Eng. C 2017, 75, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, J.V.; Jadhav, S.H.; Bodas, D.S.; Barhanpurkar-Naik, A.; Wani, M.R.; Paknikar, K.M.; Rrajwade, J.M. In vitro and in vivo studies of a novel bacterial cellulose-based acellular bilayer nanocomposite scaffold for the repair of osteochondral defects. Int. J. Nanomed. 2017, 12, 6437–6459. [Google Scholar] [CrossRef] [PubMed]
- Kirdponpattara, S.; Khamkeaw, A.; Sanchavanakit, N.; Pavasant, P.; Phisalaphong, M. Structural modification and characterization of bacterial cellulose-alginate composite scaffolds for tissue engineering. Carbohydr. Polym. 2015, 132, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Pietrucha, K.; Marzec, E.; Kudzin, M. Pore structure and dielectric behaviour of the 3D collagen-DAC scaffolds designed for nerve tissue repair. Int. J. Biol. Macromol. 2016, 92, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Weber, D.E.; Sharon, S.; Lapidot, S.; Shoseyov, O. Multifunctional cellulosic scaffolds from modified cellulose nanocrystals. ACS Appl. Mater. Interfaces 2017, 9, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- Petreus, T.; Stoica, B.A.; Petreus, O.; Goriuc, A.; Cotrutz, C.E.; Antoniac, I.V.; Barbu-Tudoran, L. Preparation and cytocompatibility evaluation for hydrosoluble phosphorous acid-derivatized cellulose as tissue engineering scaffold material. J. Mater. Sci. Mater. Med. 2014, 25, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Shukry, N.; El-Gendy, A.; El-Sakhawy, M. Bioactive cellulose grafted soy protein isolate towards biomimetic calcium phosphate mineralization. Ind. Crops Prod. 2017, 95, 170–174. [Google Scholar] [CrossRef]
- Kashani Rahimi, S.; Aeinehvand, R.; Kim, K.; Otaigbe, J.U. Structure and Biocompatibility of Bioabsorbable Nanocomposites of Aliphatic-Aromatic Copolyester and Cellulose Nanocrystals. Biomacromolecules 2017, 18, 2179–2194. [Google Scholar] [CrossRef] [PubMed]
- Pal, N.; Dubey, P.; Gopinath, P.; Pal, K. Combined effect of cellulose nanocrystal and reduced graphene oxide into poly-lactic acid matrix nanocomposite as a scaffold and its anti-bacterial activity. Int. J. Biol. Macromol. 2017, 95, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, J.C.; Deneke, C.; Lanzoni, E.M.; Costa, C.A.; Bae, Y.; Scott, J.L.; Sharma, R.I. Modulating cell response on cellulose surfaces; tuneable attachment and scaffold mechanics. Cellulose 2017, 1–16. [Google Scholar] [CrossRef]
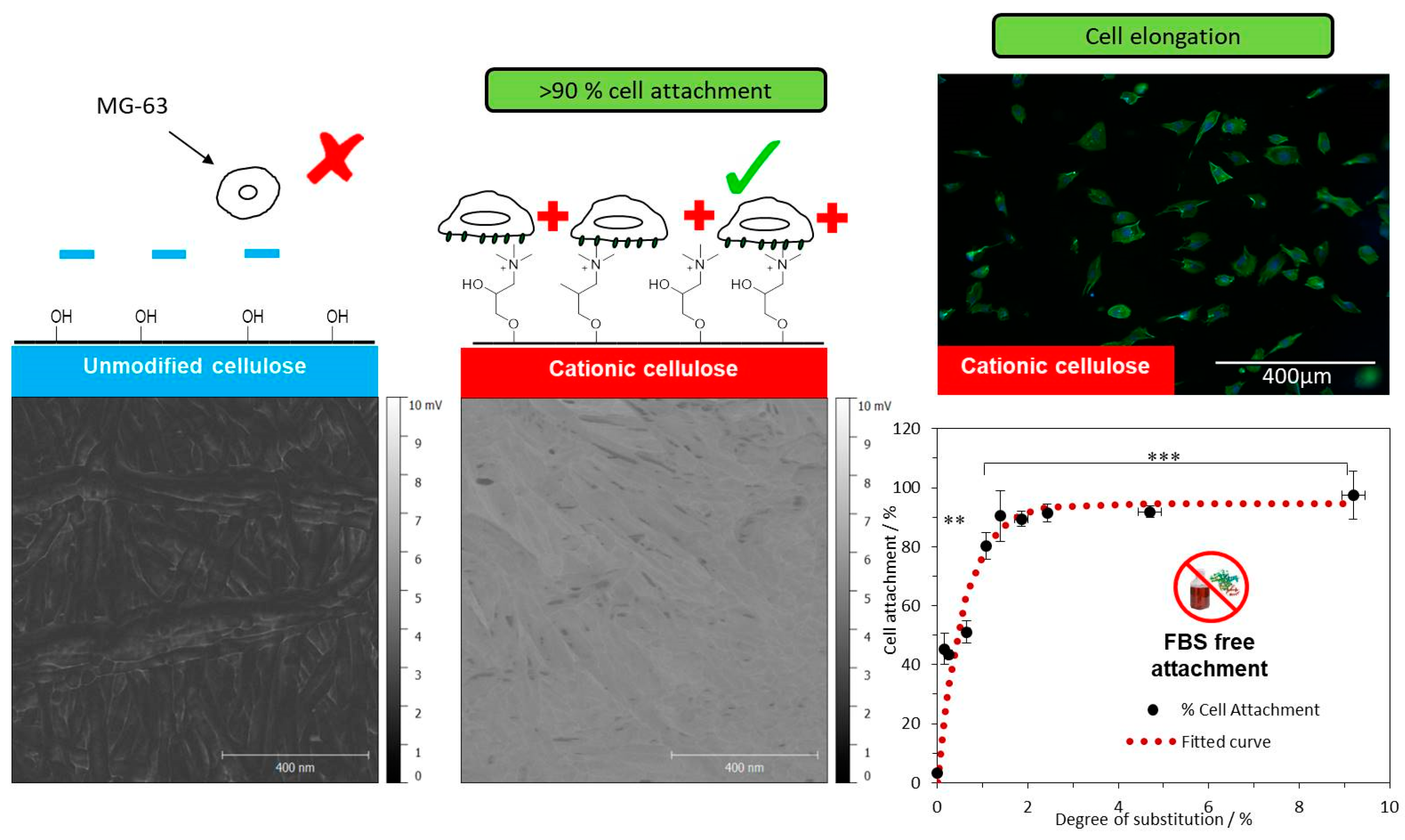
- Johns, M.A.; Bae, Y.; Guimarães, F.E.G.; Lanzoni, E.M.; Costa, C.A.R.; Murray, P.M.; Deneke, C.; Galembeck, F.; Scott, J.L.; Sharma, R.I. Predicting Ligand-Free Cell Attachment on Next-Generation Cellulose–Chitosan Hydrogels. ACS Omega 2018, 3, 937–945. [Google Scholar] [CrossRef]
- Filion, T.M.; Kutikov, A.; Song, J. Chemically modified cellulose fibrous meshes for use as tissue engineering scaffolds. Bioorg. Med. Chem. Lett. 2011, 21, 5067–5070. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Si, J.; Cui, Z.; Ye, J.; Wang, X.; Wang, Q.; Peng, K.; Chen, W.; Chen, S.C. Biomimetic composite scaffolds based on surface modification of polydopamine on electrospun poly(lactic acid)/cellulose nanofibrils. Carbohydr. Polym. 2017, 174, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Si, J.; Cui, Z.; Wang, Q.; Peng, K.; Chen, W.; Peng, X.; Chen, S.-C. Surface Modification of Electrospun TPU Nanofiber Scaffold with CNF Particles by Ultrasound-Assisted Technique for Tissue Engineering. Macromol. Mater. Eng. 2017, 302, 1–9. [Google Scholar] [CrossRef]
- Qi, A.; Hoo, S.P.; Friend, J.; Yeo, L.; Yue, Z.; Chan, P.P.Y. Hydroxypropyl cellulose methacrylate as a photo-patternable and biodegradable hybrid paper substrate for cell culture and other bioapplications. Adv. Healthc. Mater. 2014, 3, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Wali, A.; Zhang, Y.; Sengupta, P.; Higaki, Y.; Takahara, A.; Badiger, M.V. Electrospinning of non-ionic cellulose ethers/polyvinyl alcohol nanofibers: Characterization and applications. Carbohydr. Polym. 2018, 181, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Hoo, S.P.; Loh, Q.L.; Yue, Z.; Fu, J.; Tan, T.T.Y.; Choong, C.; Chan, P.P.Y. Preparation of a soft and interconnected macroporous hydroxypropyl cellulose methacrylate scaffold for adipose tissue engineering. J. Mater. Chem. B 2013, 1, 3107. [Google Scholar] [CrossRef]
- Kageyama, T.; Osaki, T.; Enomoto, J.; Myasnikova, D.; Nittami, T.; Hozumi, T.; Ito, T.; Fukuda, J. In Situ Cross-Linkable Gelatin-CMC Hydrogels Designed for Rapid Engineering of Perfusable Vasculatures. ACS Biomater. Sci. Eng. 2016, 2, 1059–1066. [Google Scholar] [CrossRef]
- Ninan, N.; Muthiah, M.; Park, I.K.; Elain, A.; Thomas, S.; Grohens, Y. Pectin/carboxymethyl cellulose/microfibrillated cellulose composite scaffolds for tissue engineering. Carbohydr. Polym. 2013, 98, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Kolewe, K.W.; Dobosz, K.M.; Rieger, K.A.; Chang, C.C.; Emrick, T.; Schiffman, J.D. Antifouling Electrospun Nanofiber Mats Functionalized with Polymer Zwitterions. ACS Appl. Mater. Interfaces 2016, 8, 27585–27593. [Google Scholar] [CrossRef] [PubMed]
- Ramphul, H.; Bhaw-Luximon, A.; Jhurry, D. Sugar-cane bagasse derived cellulose enhances performance of polylactide and polydioxanone electrospun scaffold for tissue engineering. Carbohydr. Polym. 2017, 178, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Gouma, P.I. Electrospun bioscaffolds that mimic the topology of extracellular matrix. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Chau, M.; De France, K.J.; Kopera, B.; Machado, V.R.; Rosenfeldt, S.; Reyes, L.; Chan, K.J.W.; Förster, S.; Cranston, E.D.; Hoare, T.; et al. Composite Hydrogels with Tunable Anisotropic Morphologies and Mechanical Properties. Chem. Mater. 2016, 28, 3406–3415. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Synthesis of mechanically stiff and bioactive hybrid hydrogels for bone tissue engineering applications. Chem. Eng. J. 2017, 317, 119–131. [Google Scholar] [CrossRef]
- Stumpf, T.R.; Yang, X.; Zhang, J.; Cao, X. In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mater. Sci. Eng. C 2016, 82, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Saska, S.; Barud, H.S.; Gaspar, A.M.M.; Marchetto, R.; Ribeiro, S.J.L.; Messaddeq, Y. Bacterial cellulose-hydroxyapatite nanocomposites for bone regeneration. Int. J. Biomater. 2011, 2011, 175362. [Google Scholar] [CrossRef] [PubMed]
- Orelma, H.; Filpponen, I.; Johansson, L.S.; Laine, J.; Rojas, O.J. Modification of cellulose films by adsorption of cmc and chitosan for controlled attachment of biomolecules. Biomacromolecules 2011, 12, 4311–4318. [Google Scholar] [CrossRef] [PubMed]
- Tomihata, K.; Ikada, Y. In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials 1997, 18, 567–575. [Google Scholar] [CrossRef]
- Entcheva, E.; Bien, H.; Yin, L.; Chung, C.-Y.; Farrell, M.; Kostov, Y. Functional cardiac cell constructs on cellulose-based scaffolding. Biomaterials 2004, 25, 5753–5762. [Google Scholar] [CrossRef] [PubMed]
- Laus, G.; Bentivoglio, G.; Schottenberger, H.; Kahlenberg, V.; Kopacha, H.; Röder, T.; Sixta, H. Ionic liquids: Current developments, potential and drawbacks for industrial applications. Lenzing. Ber. 2005, 84, 71–85. [Google Scholar] [CrossRef]
- Coombs Obrien, J.; Torrente-Murciano, L.; Mattia, D.; Scott, J.L. Continuous Production of Cellulose Microbeads via Membrane Emulsification. ACS Sustain. Chem. Eng. 2017, 5, 5931–5939. [Google Scholar] [CrossRef]
- Gale, E.; Wirawan, R.H.; Silveira, R.L.; Pereira, C.S.; Johns, M.A.; Skaf, M.S.; Scott, J.L. Directed Discovery of Greener Cosolvents: New Cosolvents for Use in Ionic Liquid Based Organic Electrolyte Solutions for Cellulose Dissolution. ACS Sustain. Chem. Eng. 2016, 4, 6200–6207. [Google Scholar] [CrossRef]
- Gale, E.M.; Johns, M.A.; Wirawan, R.H.; Scott, J.L. Combining random walk and regression models to understand solvation in multi-component solvent systems. Phys. Chem. Chem. Phys. 2017, 19, 17805–17815. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.A.; Bernardes, A.; De Azevêdo, E.R.; Guimarães, F.E.G.; Lowe, J.P.; Gale, E.M.; Polikarpov, I.; Scott, J.L.; Sharma, R.I. On the subtle tuneability of cellulose hydrogels: Implications for binding of biomolecules demonstrated for CBM 1. J. Mater. Chem. B 2017, 5, 3879–3887. [Google Scholar] [CrossRef]
- Zou, H.; Luo, Q.; Zhou, D. Affinity membrane chromatography for the analysis and purification of proteins. J. Biochem. Biophys. Methods 2001, 49, 199–240. [Google Scholar] [CrossRef]
- Pelton, R. Bioactive paper provides a low-cost platform for diagnostics. Trends Anal. Chem. 2009, 28, 925–942. [Google Scholar] [CrossRef]
- Brash, J.L.; Ten Hove, P. Protein adsorption studies on “standard” polymeric materials. J. Biomater. Sci. Polym. Ed. 1993, 4, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Eto, Y.; Takano, S.; Nakamori, S.; Shibai, H.; Yamanaka, S. A new bacterial cellulose substrate for mammalian cell culture—A new bacterial cellulose substrate. Cytotechnology 1993, 13, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Perets, A.; Baruch, Y.; Weisbuch, F.; Shoshany, G.; Neufeld, G.; Cohen, S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J. Biomed. Mater. Res. 2003, 65, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Merle, C.; Perret, S.; Lacour, T.; Jonval, V.; Hudaverdian, S.; Garrone, R.; Ruggiero, F.; Theisen, M. Hydroxylated human homotrimeric collagen I in Agrobacterium tumefaciens-mediated transient expression and in transgenic tobacco plant. FEBS Lett. 2002, 515, 114–118. [Google Scholar] [CrossRef]
- Granja, P.L.; De Jéso, B.; Bareille, R.; Rouais, F.; Baquey, C.; Barbosa, M.A. Cellulose phosphates as biomaterials. In vitro biocompatibility studies. React. Funct. Polym. 2006, 66, 728–739. [Google Scholar] [CrossRef]
- Quero, F.; Nogi, M.; Lee, K.Y.; Vanden Poel, G.; Bismarck, A.; Mantalaris, A.; Yano, H.; Eichhorn, S.J. Cross-linked bacterial cellulose networks using glyoxalization. ACS Appl. Mater. Interfaces 2011, 3, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Sanchavanakit, N.; Sangrungraungroj, W.; Kaomongkolgit, R.; Banaprasert, T.; Pavasant, P.; Phisalaphong, M. Growth of human keratinocytes and fibroblasts on bacterial cellulose film. Biotechnol. Prog. 2006, 22, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Märtson, M.; Viljanto, J.; Hurme, T.; Laippala, P.; Saukko, P. Is cellulose sponge degradable or stable as implantation material? An in vivo subcutaneous study in the rat. Biomaterials 1999, 20, 1989–1995. [Google Scholar] [CrossRef]
- Ashton, W.H.; Moser, C.E. Oxidized Cellulose Product and Method for Preparing the Same. U.S. Patent No. 3364200A, 16 January 1968. [Google Scholar]
- Petty, J.D.; Huckins, J.N.; David, A.U.S. Preparation Method of Bioresorbable Oxidized Cellulose. U.S. Patent No. 2009/0306363 A1, 28 February 2007. [Google Scholar]
- Linsky, C.B.; Cunningham, T.J. Methods and Materials for Prevention of Surgical Adhesions. U.S. Patent No. 5002551A, 22 August 1985. [Google Scholar]
- Broadnax, C.H., Jr. Surgical Hemostat Comprising Oxidized Cellulose. U.S. Patent No. 4626253A, 5 October 1984. [Google Scholar]
- Domingues, R.M.A.; Gomes, M.E.; Reis, R.L. The Potential of Cellulose Nanocrystals in Tissue Engineering Strategies. Biomacromolecules 2014, 15, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Pappadà, S.; Madaghiele, M.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Crosslinking of cellulose derivatives and hyaluronic acid with water-soluble carbodiimide. Polymer 2005, 46, 11206–11212. [Google Scholar] [CrossRef]
- Nakajima, N.; Ikada, Y. Mechanism of Amide Formation by Carbodiimide for Bioconjugation in Aqueous Media. Bioconjug. Chem. 1995, 6, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Olde Damink, L.H.H.; Dijkstra, P.J.; Van Luyn, M.J.A.; Van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials 1996, 17, 765–773. [Google Scholar] [CrossRef]
| Type of Nanocellulose | Selected References and Synonyms | Typical Sources | Formation and Average Size |
|---|---|---|---|
| Microfibrillated cellulose (MFC) | Microfibrillated cellulose [48], nanofibrils and microfibrils [35], nanofibrillated cellulose [49] | Wood, sugar beet, potato tuber, hemp, flax delamination | Delamination of wood pulp by mechanical pressure before and/or after chemical or enzymatic treatment Diameter: 5–60 nm Length: several micrometres |
| Nanocrystalline cellulose (NCC) | Cellulose nanocrystals, crystallites [50], whiskers [51], rod-like cellulose microcrystals [52] | Wood, cotton, hemp, flax, wheat straw, mulberry bark, ramie, Avicel, tunicin, cellulose from algae and bacteria | Acid hydrolysis of cellulose from many sources Diameter: 5–70 nm Length: 100–250 nm (from plant celluloses); 100 nm to several micrometres (from celluloses of tunicates, algae, bacteria) |
| Bacterial nanocellulose (BNC) | Bacterial cellulose [40], microbial cellulose [53], biocellulose [54] | Low-molecular-weight sugars and alcohols | Bacterial synthesis Diameter: 20–100 nm; different types of nanofiber networks |
| Cellulose Type | Modification | Scaffold Form | Tissue Culture Application |
|---|---|---|---|
| Bacterial Cellulose | Mannosylated | Membranes | Enhanced fibroblast growth [76] |
| Cationisation and oxidation | Membranes | Protein free cell attachment [76] | |
| Silanisation | Lyophilised membranes | Wound dressing [77] | |
| TEMPO-mediated oxidation | Hydrogel with hydroxyapatite and crosslinked by glutaraldehyde | Bone tissue [78] | |
| RGD and xyloglucan-peptide grafting | Membranes | Engineering blood vessels [79] | |
| Modified with heparin | 3D porous scaffold loaded with vascular endothelial growth factor (VEGF) | Tissue regeneration [80] | |
| Peptides fused to a carbohydrate-binding module (CBM3) | Membranes | Promoting neuronal and mesenchymal stem cell (MSC) adhesion [81] | |
| Tri-calcium phosphate and hydroxyapatite blend | Hydrogel | Bone tissue implants [82] | |
| Collagen and hydroxyapatite blend | Hydrogel crosslinked by procyanidins | Bone tissue [83] | |
| Hydroxyapatite and glycosaminoglycan blends | Layered scaffolds | Repair of osteochondral defects [84] | |
| Alginate blend | Porous scaffold crosslinked with Ca2+ | Biocompatibility and porous [85] | |
| Nanocrystalline Cellulose | Dialdehyde cellulose crosslinked with collagen | 3D porous scaffold | Dielectric behaviour relevant to neural tissue engineering [86] |
| Acetate esterification | Interconnected highly porous scaffold | Hydrophobic and lipophilic scaffolds [87] | |
| Phosphorylation | Thin films | In vitro cell culture and in vivo tissue regeneration [88] | |
| Oxidised cellulose grafted with soybean protein isolate | Scaffold soaked in doubly concentrated simulated body fluid | Biomimetic calcium phosphate mineralisation [89] | |
| Copolymer dispersed with cellulose nanocrystals | 3D nanocomposites | Biomedical and tissue engineering applications [90] | |
| CNC and reduced graphene oxide blended in PLA matrix | Nanocomposite film | Antibacterial activity [91] | |
| Nanocellulose blended with nanochitin | CAD generated porous structure | Biomimetic tissue engineering [64] | |
| Microfibrillated Cellulose | Cationisation and glyoxalation | Regenerated modified cellulose films | Tailoring scaffold properties to regulate cell response [92] |
| Cellulose-chitosan infusions | Hydrogels | Cell attachment [93] | |
| Oxidation followed by sulfonation | Electrospun fibre meshes | Bone tissue [94] | |
| Decellularisation followed by glutaraldehyde crosslinking | 3D cellulose scaffolds | In vitro culture of mammalian cells in a 3D environment [66] | |
| Dopamine coated | Electrospun PLA/CNF composite nanofibres | Enhance cell biocompatibility [95] | |
| Polyurethane coated in a CNF dispersion | Electrospun nanofibres | Tissue engineering [96] | |
| Cellulose Derivatives | Hydroxypropyl cellulose (HPC) crosslinked by methyl acrylate | Biocompatible and hydrolytically degradable scaffold | Long term cell culture [97] |
| Ethyl hydroxyethyl cellulose (EHEC) crosslinked with citric acid | Electrospun nanofibres | Drug delivery and as scaffolds in tissue engineering [98] | |
| HPC modified with methacrylic anhydride | 3D hydrogel constructed with interconnecting pores | Adipose tissue [99] | |
| Crosslinked gelatin/carboxymethyl cellulose (CMC) blend | Hydrogel with perfusable vascular networks | Engineering vascularised and cell-dense 3D tissues and organs [100] | |
| CMC/MFC/pectin blend | Lyophilised hydrogels | Biocompatible composite scaffolds [101] | |
| Cellulose acetate with polymer graft and polydopamine (PDA) coating | Electrospun nanofibre mats | Antifouling surface [102] | |
| Cellulose acetate blended with PLA or PDO | Electrospun nanofibre mats | Biomineralisation [103] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Courtenay, J.C.; Sharma, R.I.; Scott, J.L. Recent Advances in Modified Cellulose for Tissue Culture Applications. Molecules 2018, 23, 654. https://doi.org/10.3390/molecules23030654
Courtenay JC, Sharma RI, Scott JL. Recent Advances in Modified Cellulose for Tissue Culture Applications. Molecules. 2018; 23(3):654. https://doi.org/10.3390/molecules23030654
Chicago/Turabian StyleCourtenay, James C., Ram I. Sharma, and Janet L. Scott. 2018. "Recent Advances in Modified Cellulose for Tissue Culture Applications" Molecules 23, no. 3: 654. https://doi.org/10.3390/molecules23030654
APA StyleCourtenay, J. C., Sharma, R. I., & Scott, J. L. (2018). Recent Advances in Modified Cellulose for Tissue Culture Applications. Molecules, 23(3), 654. https://doi.org/10.3390/molecules23030654





