Recent Advances in Zirconium-89 Chelator Development
Abstract
1. Introduction
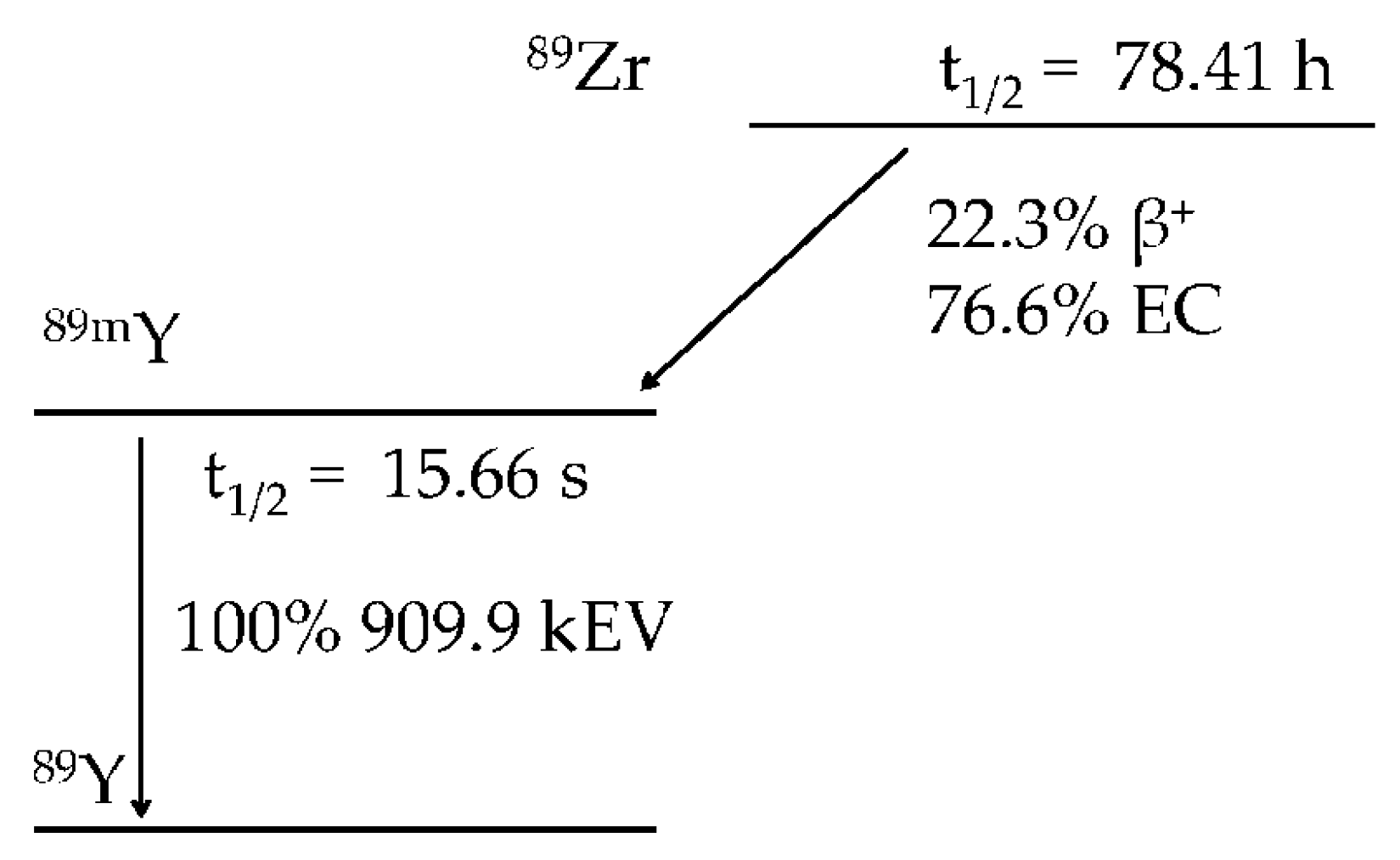
2. Zirconium Chemistry and the Production of Zirconium-89
3. The Rationale for New Zirconium-89 Chelation Strategies
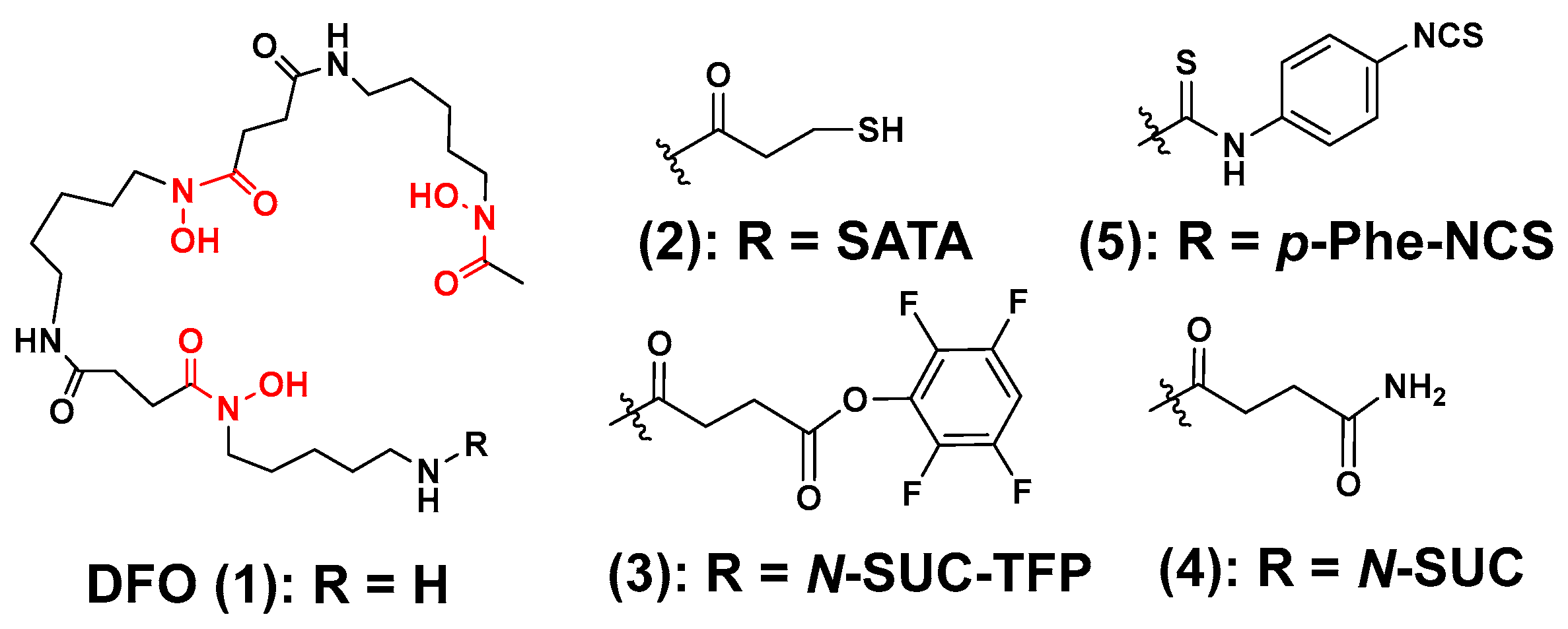
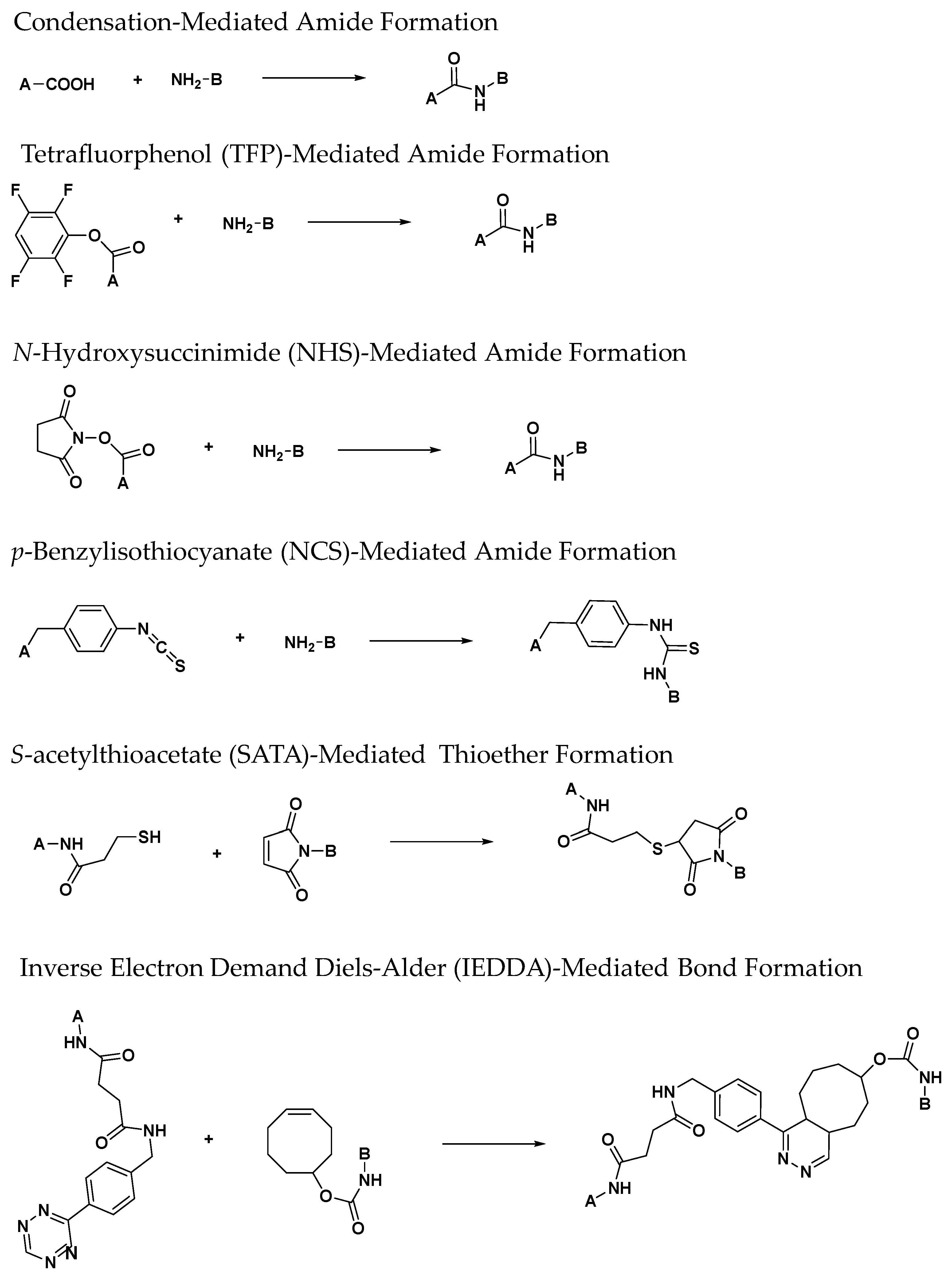
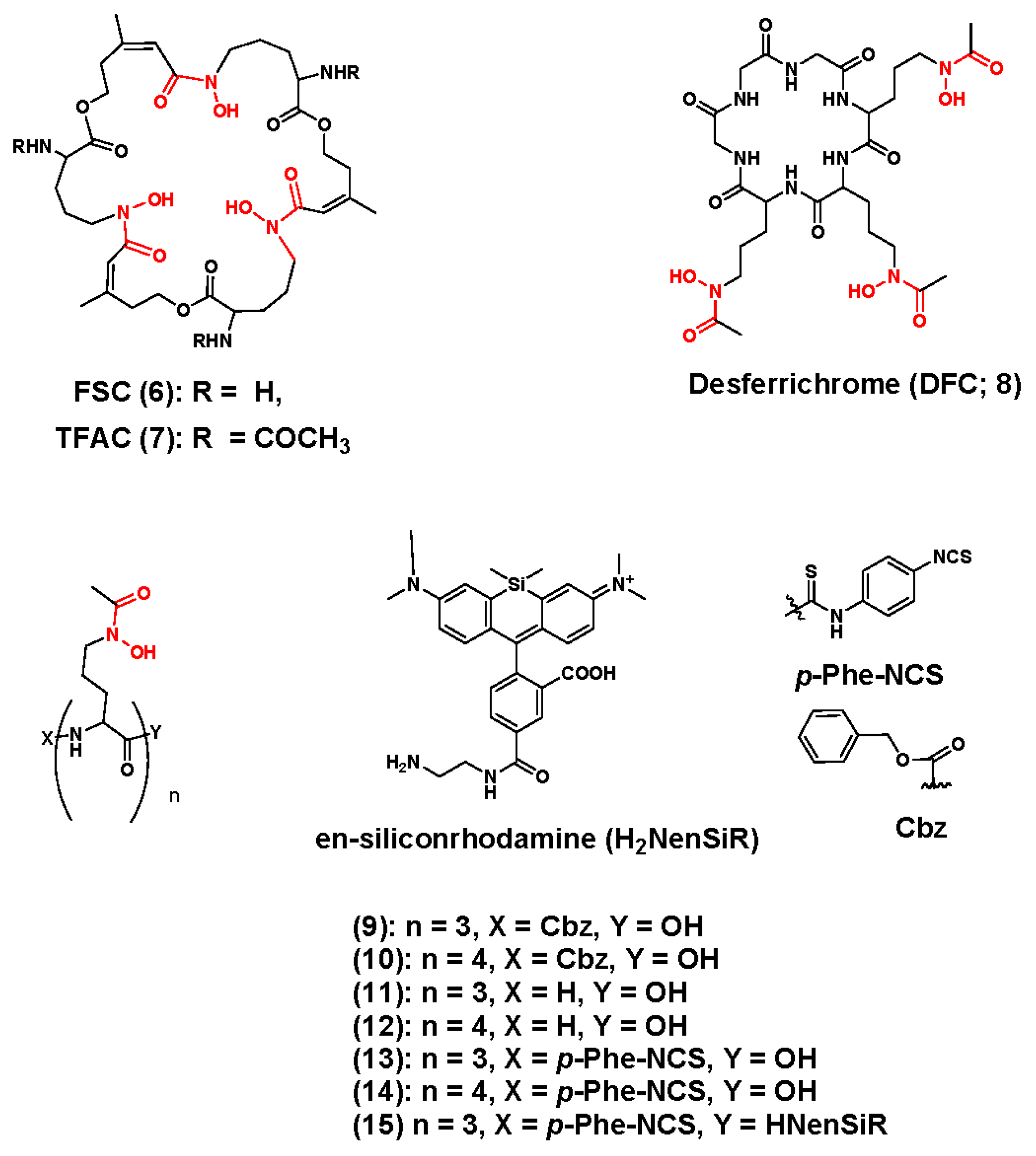
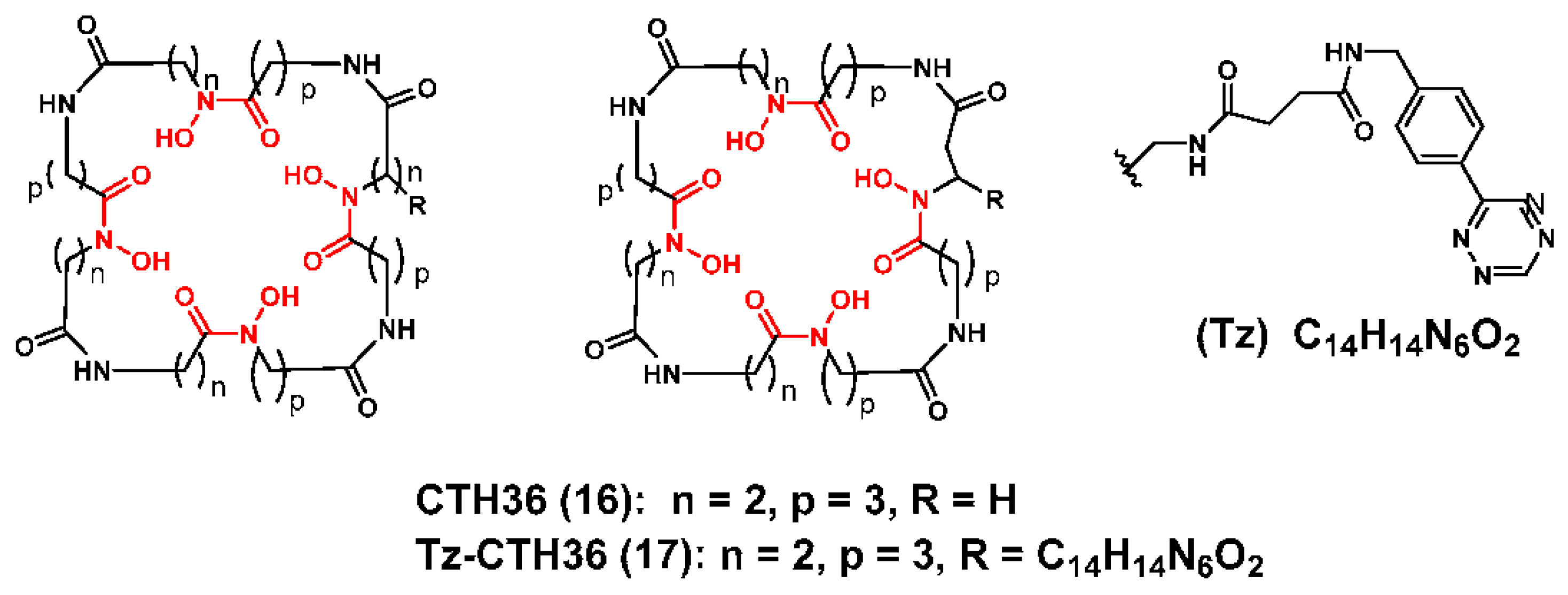
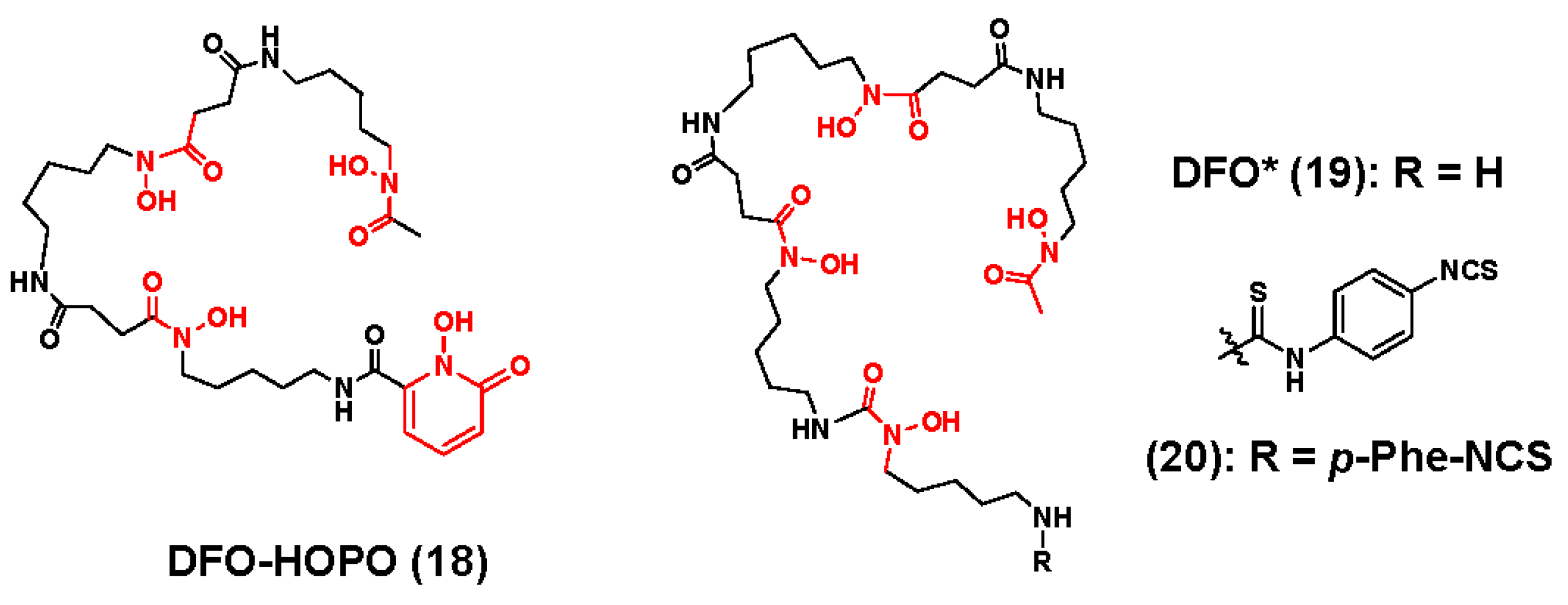
3.1. Zirconium-89 Chelators Containing Hydroxamate Coordinating Units
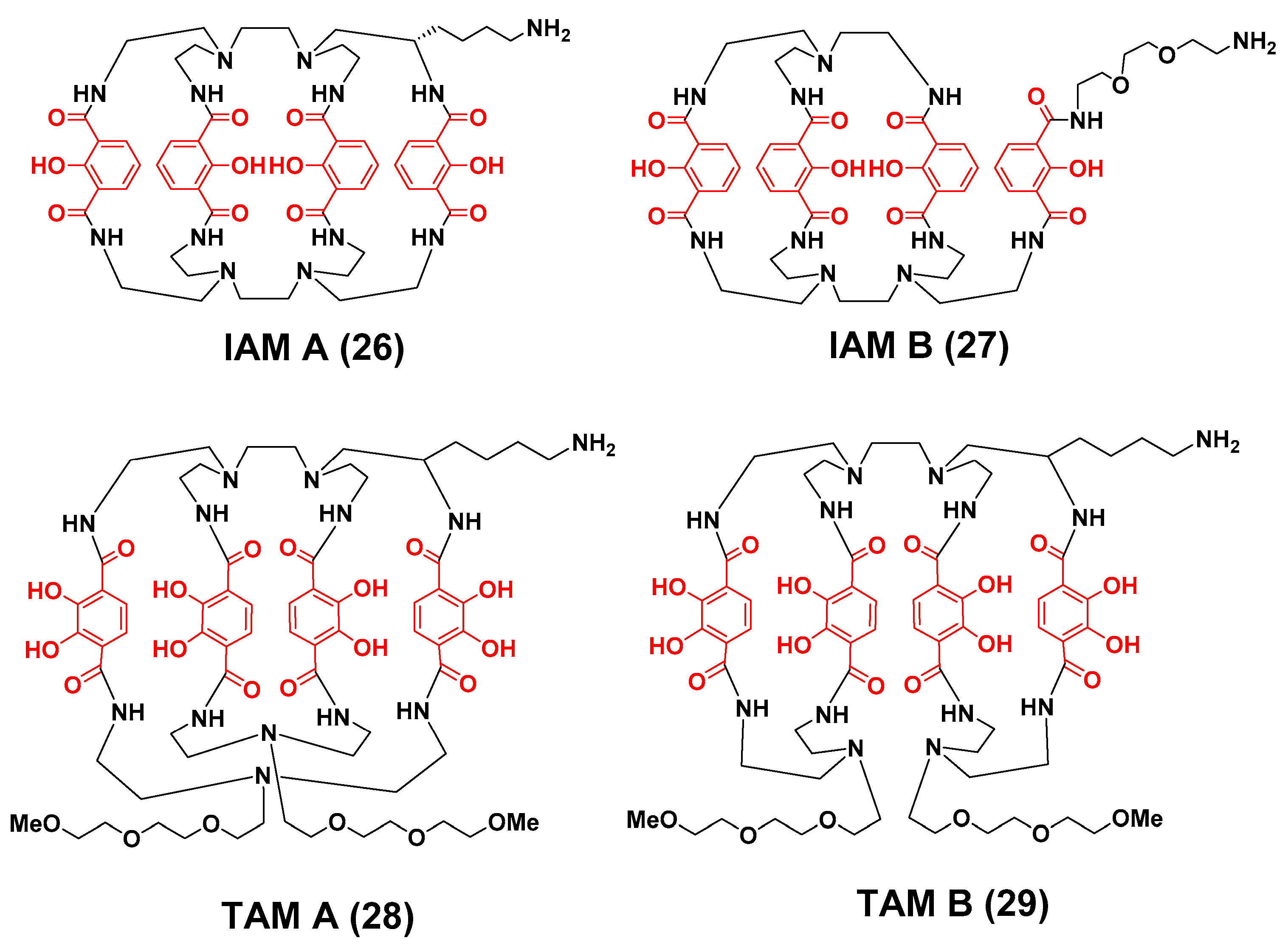
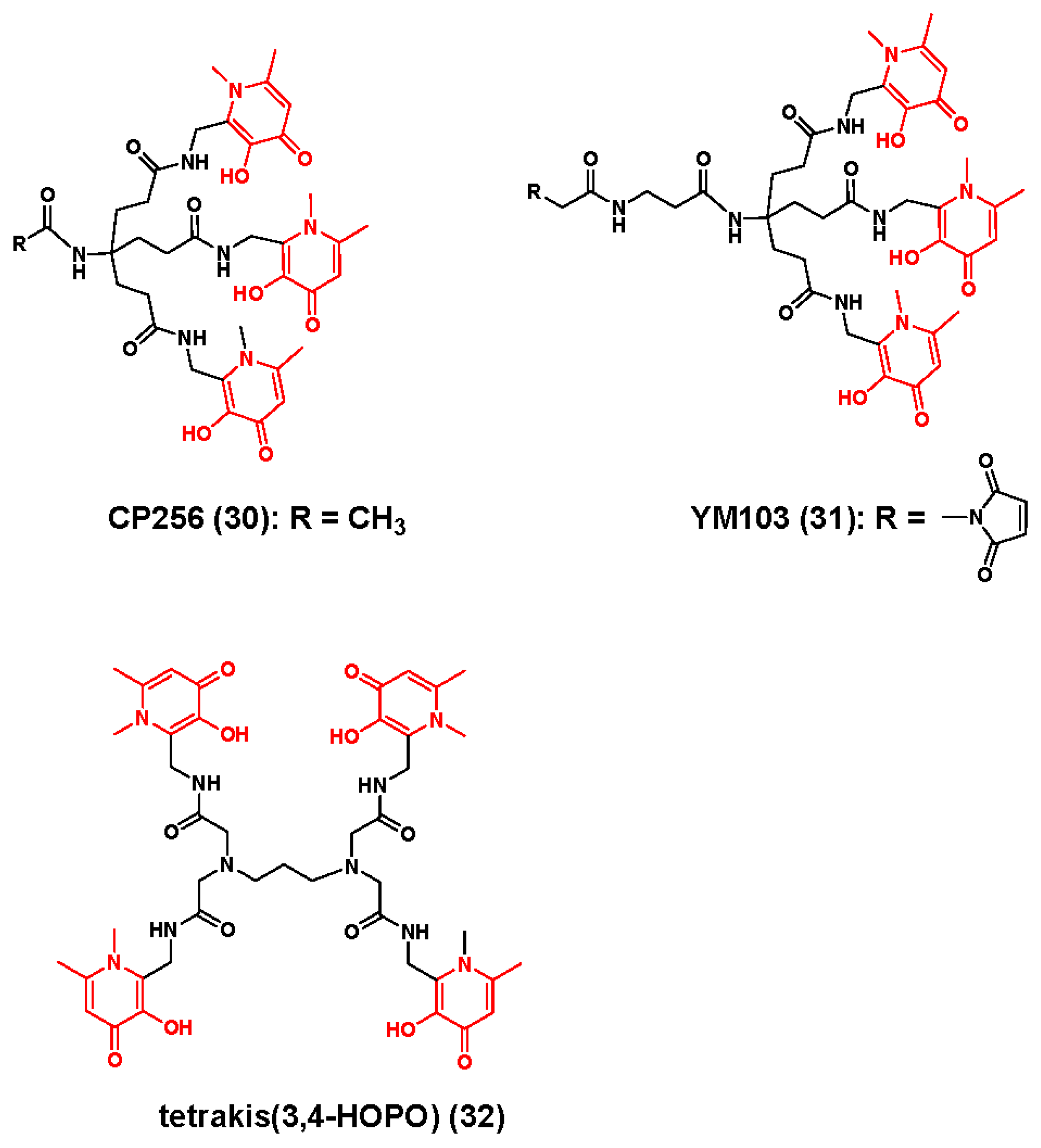
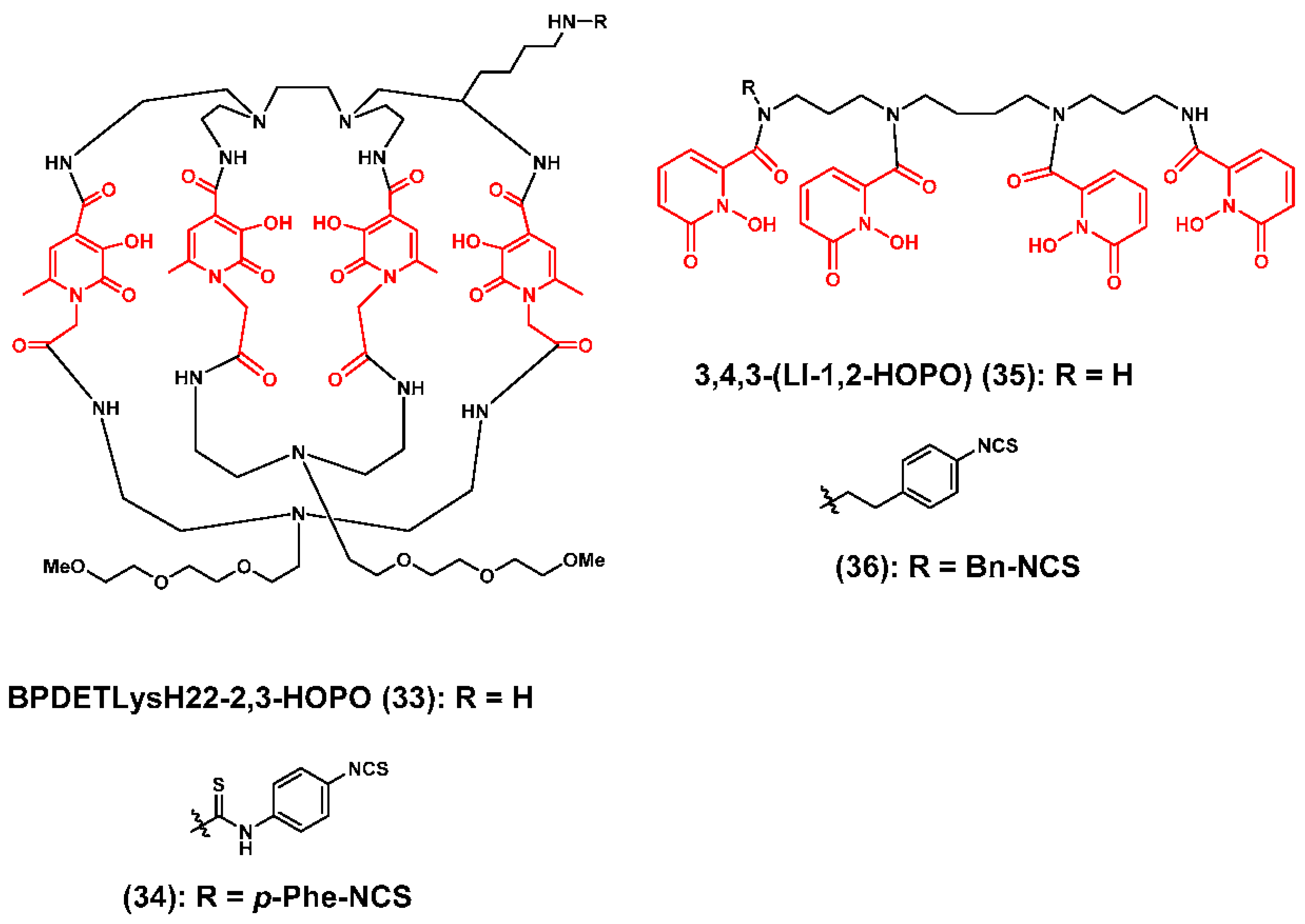
3.2. Zirconium-89 Chelators Containing Hydroxyisopthalamide, Terepthalamide and Hydroxypyridinone Coordinating Units
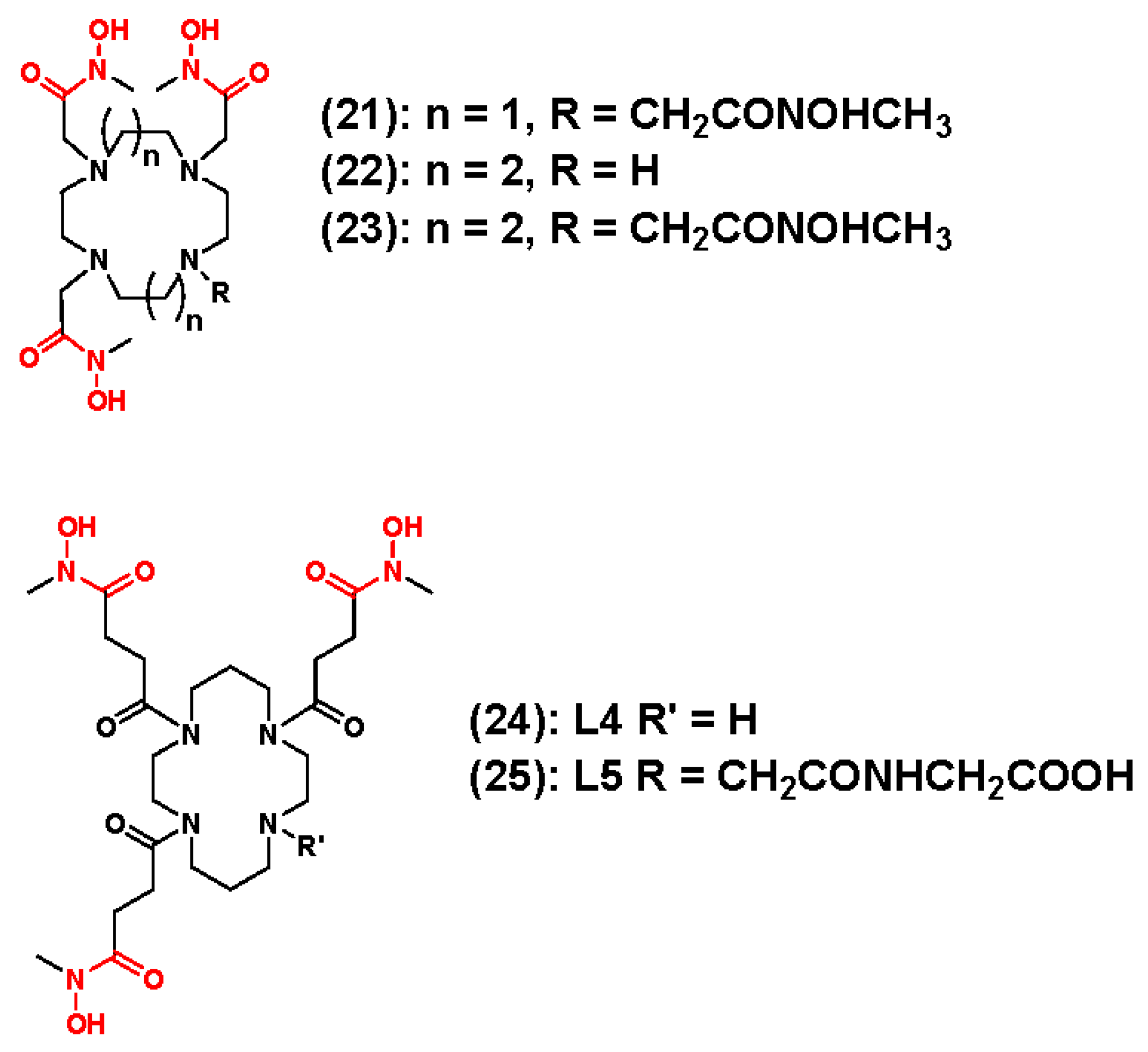
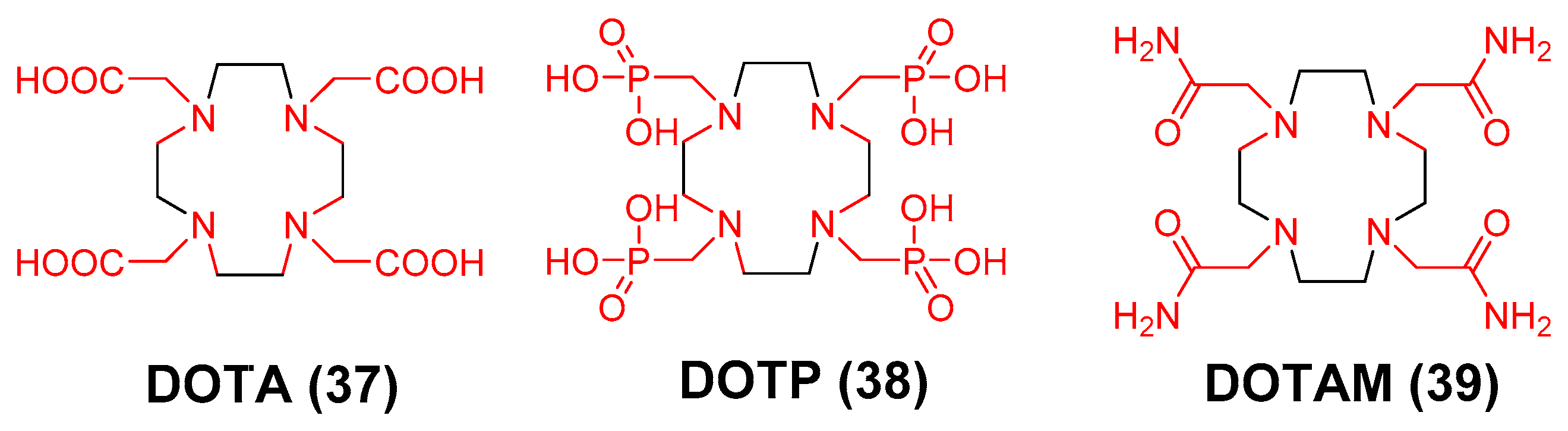
3.3. 89Zr Chelators Containing Tetraazamacrocycles
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BFC | bifunctional chelator |
| Bq | bequerel |
| Ci | curie |
| DFC | desferrichrome |
| DFO | desferrioxamine B |
| DOTA | 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| DTPA | diethylenetriaminepentaacetic acid |
| EDTA | ethylenediaminetetraacetic acid |
| FSC | fusarine C |
| GBq | gigabequerel |
| HOPO | hydroxpyridinoate |
| IAM | hydroxyisopthalamide |
| kBq | kilobequerel |
| keV | kiloelectron volt |
| MBq | Megabequerel |
| mCi | milicurie |
| MeV | megaelectron volt |
| PET | positron emission tomography |
| TAM | terepthalamide |
| TFAC | triacetylfusarine C |
| THPN | 1,3-propanediamine-N,N,N′,N′-tetrakis[(2-(aminoethy)-3-hydroxy-1-6-dimethyl-4(1H)-pyridinone)acetamide] |
| uCi | microcurie |
| α++ | alpha particle |
| β− | beta minus particle |
| β+ | positron |
| γ | gamma photon |
References
- Wang, Y.X.; Choi, Y.; Chen, Z.; Laurent, S.; Gibbs, S.L. Molecular imaging: From bench to clinic. Biomed. Res. Int. 2014, 2014, 357258. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; Prins, N.D.; Pijnenburg, Y.A.; Lemstra, A.W.; van der Flier, W.M.; Adriaanse, S.F.; Windhorst, A.D.; Handels, R.L.; Wolfs, C.A.; Aalten, P.; et al. Impact of molecular imaging on the diagnostic process in a memory clinic. Alzheimers Dement. 2013, 9, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, F.; Nahrendorf, M. Molecular imaging of coronary atherosclerosis and myocardial infarction: Considerations for the bench and perspectives for the clinic. Circ. Res. 2011, 108, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Hruska, C.B.; Boughey, J.C.; Phillips, S.W.; Rhodes, D.J.; Wahner-Roedler, D.L.; Whaley, D.H.; Degnim, A.C.; O‘Connor, M.K. Scientific Impact Recognition Award: Molecular breast imaging: A review of the Mayo Clinic experience. Am. J. Surg. 2008, 196, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Kipper, M.S. Steps in moving molecular imaging to the clinic. J. Nucl. Med. 2008, 49, 58N–60N. [Google Scholar] [PubMed]
- Schafers, M. The future of molecular imaging in the clinic needs a clear strategy and a multidisciplinary effort. Basic Res. Cardiol. 2008, 103, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Glunde, K.; Bhujwalla, Z.M. Will magnetic resonance imaging (MRI)-based contrast agents for molecular receptor imaging make their way into the clinic? J. Cell. Mol. Med. 2008, 12, 187–188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eckelman, W.C.; Rohatagi, S.; Krohn, K.A.; Vera, D.R. Are there lessons to be learned from drug development that will accelerate the use of molecular imaging probes in the clinic? Nucl. Med. Biol. 2005, 32, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Blankenberg, F.G. Molecular imaging with single photon emission computed tomography. How new tracers can be employed in the nuclear medicine clinic. IEEE Eng. Med. Biol. Mag. 2004, 23, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, N.I.; Minoshima, S. FDG-PET and molecular brain imaging in the movement disorders clinic. Neurology 2012, 79, 1306–1307. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, H.; Zhao, S.; Xu, J.; Wu, X.; Cui, J.; Zhang, Y.; Qin, Y.; Liu, Z.; Gao, T.; et al. SPECT and PET radiopharmaceuticals for molecular imaging of apoptosis: From bench to clinic. Oncotarget 2017, 8, 20476–20495. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Gauthier, V.; Collier, T.L.; Liang, S.H.; Vasdev, N. Discovery of PET radiopharmaceuticals at the academia-industry interface. Drug Discov. Today Technol. 2017, 25, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Phelps, M.E. PET Molecular Imaging and Its Biological Implications; Springer: New York, NY, USA, 2004. [Google Scholar]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chem. Rev. 2010, 110, 2858–2902. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.J.; Welch, M.J. Radiometal-labeled agents (non-technetium) for diagnostic imaging. Chem. Rev. 1999, 99, 2219–2234. [Google Scholar] [CrossRef] [PubMed]
- Deri, M.A.; Zeglis, B.M.; Francesconi, L.C.; Lewis, J.S. PET imaging with (8)(9)Zr: From radiochemistry to the clinic. Nucl. Med. Biol. 2013, 40, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.J.; Redvanly, C.S. Handbook of Radiopharmaceuticals; Wiley: Chichester, UK, 2003; p. 847. [Google Scholar]
- Link, J.M.; Krohn, K.A.; Eary, J.F.; Kishore, R.; Lewellen, T.K.; Johnson, M.W.; Badger, C.C.; Richter, K.Y.; Nelp, W.B. Zr-89 for antibody labeling and positron emission tomography. J. Label. Compd. Radiopharm. 1986, 23, 1297–1298. [Google Scholar]
- Eary, J.F.; Link, J.M.; Kishore, R.; Johnson, M.W.; Badger, C.C.; Richter, K.Y.; Krohn, K.A.; Nelp, W.B. Production of Positron Emitting Zr89 for Antibody Imaging by Pet. J. Nucl. Med. 1986, 27, 983. [Google Scholar]
- Ikotun, O.F.; Lapi, S.E. The rise of metal radionuclides in medical imaging: copper-64, zirconium-89 and yttrium-86. Future Med. Chem. 2011, 3, 599–621. [Google Scholar] [CrossRef] [PubMed]
- Jauw, Y.W.; Menke-van der Houven van Oordt, C.W.; Hoekstra, O.S.; Hendrikse, N.H.; Vugts, D.J.; Zijlstra, J.M.; Huisman, M.C.; van Dongen, G.A. Immuno-Positron Emission Tomography with Zirconium-89-Labeled Monoclonal Antibodies in Oncology: What Can We Learn from Initial Clinical Trials? Front. Pharmacol. 2016, 7, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, G.A.; Huisman, M.C.; Boellaard, R.; Harry Hendrikse, N.; Windhorst, A.D.; Visser, G.W.; Molthoff, C.F.; Vugts, D.J. 89Zr-immuno-PET for imaging of long circulating drugs and disease targets: Why, how and when to be applied? Q. J. Nucl. Med. Mol. Imaging 2015, 59, 18–38. [Google Scholar] [PubMed]
- van Dongen, G.A.; Vosjan, M.J. Immuno-positron emission tomography: Shedding light on clinical antibody therapy. Cancer Biother. Radiopharm. 2010, 25, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Vugts, D.J.; Visser, G.W.; van Dongen, G.A. 89Zr-PET radiochemistry in the development and application of therapeutic monoclonal antibodies and other biologicals. Curr. Top. Med. Chem. 2013, 13, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Price, T.W.; Greenman, J.; Stasiuk, G.J. Current advances in ligand design for inorganic positron emission tomography tracers (68)Ga, (64)Cu, (89)Zr and (44)Sc. Dalton Trans. 2016, 45, 15702–15724. [Google Scholar] [CrossRef] [PubMed]
- Heskamp, S.; Raave, R.; Boerman, O.; Rijpkema, M.; Goncalves, V.; Denat, F. (89)Zr-Immuno-Positron Emission Tomography in Oncology: State-of-the-Art (89)Zr Radiochemistry. Bioconj. Chem. 2017, 28, 2211–2223. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Holland, J.P. Chemical aspects of metal ion chelation in the synthesis and application antibody-based radiotracers. J. Label. Comp. Radiopharm. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Goel, S.; Valdovinos, H.F.; Luo, H.; Hernandez, R.; Barnhart, T.E.; Cai, W. In Vivo Integrity and Biological Fate of Chelator-Free Zirconium-89-Labeled Mesoporous Silica Nanoparticles. ACS Nano 2015, 9, 7950–7959. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Kamkaew, A.; Shen, S.; Valdovinos, H.F.; Sun, H.; Hernandez, R.; Goel, S.; Liu, T.; Thompson, C.R.; Barnhart, T.E.; et al. Facile Preparation of Multifunctional WS2 /WOx Nanodots for Chelator-Free (89) Zr-Labeling and In Vivo PET Imaging. Small 2016, 12, 5750–5758. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Shen, S.; Jiang, D.; Jin, Q.; Ellison, P.A.; Ehlerding, E.B.; Goel, S.; Song, G.; Huang, P.; Barnhart, T.E.; et al. Chelator-Free Labeling of Metal Oxide Nanostructures with Zirconium-89 for Positron Emission Tomography Imaging. ACS Nano 2017, 11, 12193–12201. [Google Scholar] [CrossRef] [PubMed]
- Fairclough, M.; Ellis, B.; Boutin, H.; Jones, A.K.P.; McMahon, A.; Alzabin, S.; Gennari, A.; Prenant, C. Development of a method for the preparation of zirconium-89 radiolabelled chitosan nanoparticles as an application for leukocyte trafficking with positron emission tomography. Appl. Radiat. Isot. 2017, 130, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Chen, F.; Luan, S.; Valdovinos, H.F.; Shi, S.; Graves, S.A.; Ai, F.; Barnhart, T.E.; Theuer, C.P.; Cai, W. Engineering Intrinsically Zirconium-89 Radiolabeled Self-Destructing Mesoporous Silica Nanostructures for In Vivo Biodistribution and Tumor Targeting Studies. Adv. Sci. 2016, 3, 1600122. [Google Scholar] [CrossRef] [PubMed]
- Heneweer, C.; Holland, J.P.; Divilov, V.; Carlin, S.; Lewis, J.S. Magnitude of enhanced permeability and retention effect in tumors with different phenotypes: 89Zr-albumin as a model system. J. Nucl. Med. 2011, 52, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Kaittanis, C.; Shaffer, T.M.; Bolaender, A.; Appelbaum, Z.; Appelbaum, J.; Chiosis, G.; Grimm, J. Multifunctional MRI/PET Nanobeacons Derived from the in Situ Self-Assembly of Translational Polymers and Clinical Cargo through Coalescent Intermolecular Forces. Nano Lett. 2015, 15, 8032–8043. [Google Scholar] [CrossRef] [PubMed]
- Karmani, L.; Bouchat, V.; Bouzin, C.; Leveque, P.; Labar, D.; Bol, A.; Deumer, G.; Marega, R.; Bonifazi, D.; Haufroid, V.; et al. (89)Zr-labeled anti-endoglin antibody-targeted gold nanoparticles for imaging cancer: Implications for future cancer therapy. Nanomedicine 2014, 9, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Karmani, L.; Labar, D.; Valembois, V.; Bouchat, V.; Nagaswaran, P.G.; Bol, A.; Gillart, J.; Leveque, P.; Bouzin, C.; Bonifazi, D.; et al. Antibody-functionalized nanoparticles for imaging cancer: Influence of conjugation to gold nanoparticles on the biodistribution of 89Zr-labeled cetuximab in mice. Contrast Media Mol. Imaging 2013, 8, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yu, Z.; Pham, T.T.; Blower, P.J.; Yan, R. A generic (89)Zr labeling method to quantify the in vivo pharmacokinetics of liposomal nanoparticles with positron emission tomography. Int. J. Nanomed. 2017, 12, 3281–3294. [Google Scholar] [CrossRef] [PubMed]
- Majmudar, M.D.; Yoo, J.; Keliher, E.J.; Truelove, J.J.; Iwamoto, Y.; Sena, B.; Dutta, P.; Borodovsky, A.; Fitzgerald, K.; Di Carli, M.F.; et al. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ. Res. 2013, 112, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.; Winter, G.; Baur, B.; Witulla, B.; Solbach, C.; Reske, S.; Linden, M. Synthesis, characterization, and biodistribution of multiple 89Zr-labeled pore-expanded mesoporous silica nanoparticles for PET. Nanoscale 2014, 6, 4928–4935. [Google Scholar] [CrossRef] [PubMed]
- Perez-Medina, C.; Abdel-Atti, D.; Tang, J.; Zhao, Y.; Fayad, Z.A.; Lewis, J.S.; Mulder, W.J.; Reiner, T. Nanoreporter PET predicts the efficacy of anti-cancer nanotherapy. Nat. Commun. 2016, 7, 11838–11840. [Google Scholar] [CrossRef] [PubMed]
- Perez-Medina, C.; Abdel-Atti, D.; Zhang, Y.; Longo, V.A.; Irwin, C.P.; Binderup, T.; Ruiz-Cabello, J.; Fayad, Z.A.; Lewis, J.S.; Mulder, W.J.; et al. A modular labeling strategy for in vivo PET and near-infrared fluorescence imaging of nanoparticle tumor targeting. J. Nucl. Med. 2014, 55, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Perez-Medina, C.; Tang, J.; Abdel-Atti, D.; Hogstad, B.; Merad, M.; Fisher, E.A.; Fayad, Z.A.; Lewis, J.S.; Mulder, W.J.; Reiner, T. PET Imaging of Tumor-Associated Macrophages with 89Zr-Labeled High-Density Lipoprotein Nanoparticles. J. Nucl. Med. 2015, 56, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Polyak, A.; Naszalyi Nagy, L.; Mihaly, J.; Gorres, S.; Wittneben, A.; Leiter, I.; Bankstahl, J.P.; Sajti, L.; Kellermayer, M.; Zrinyi, M.; et al. Preparation and (68)Ga-radiolabeling of porous zirconia nanoparticle platform for PET/CT-imaging guided drug delivery. J. Pharm. Biomed. Anal. 2017, 137, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, T.M.; Harmsen, S.; Khwaja, E.; Kircher, M.F.; Drain, C.M.; Grimm, J. Stable Radiolabeling of Sulfur-Functionalized Silica Nanoparticles with Copper-64. Nano Lett. 2016, 16, 5601–5604. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Fliss, B.C.; Gu, Z.; Zhu, Y.; Hong, H.; Valdovinos, H.F.; Hernandez, R.; Goel, S.; Luo, H.; Chen, F.; et al. Chelator-Free Labeling of Layered Double Hydroxide Nanoparticles for in Vivo PET Imaging. Sci. Rep. 2015, 5, 16930–16940. [Google Scholar] [CrossRef] [PubMed]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 99th ed.; CRC Press: New York, NY, USA, 2007; p. 2560. [Google Scholar]
- Cotton, F.A.; Wilkinson, G.; Gaus, P.L. Basic Inorganic Chemistry, 3th ed.; John Wiley & Sons, Inc.: Singapore, 1995. [Google Scholar]
- Blumenthal, W.B. Zirconium chemsitry in industry. J. Chem. Educ. 1962, 39, 604–610. [Google Scholar] [CrossRef]
- Blumenthal, W.B. Properties of zirconium compounds likely to be of interest to the analytical chemist. Talanta 1968, 15, 877–882. [Google Scholar] [CrossRef]
- Blumenthal, W.B. Zirconium in the ecology. Am. Ind. Hyg. Assoc. J. 1973, 34, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Page, E.M.; Wass, S.A. Zirconium and hafnium 1994. Coord. Chem. Rev. 1996, 152, 411–466. [Google Scholar] [CrossRef]
- Hollink, E.; Stephan, D.W. Zirconium and hafnium. ChemInform 2004, 35, 106–160. [Google Scholar] [CrossRef]
- Cano Sierra, J.; Huerlander, D.; Hill, M.; Kehr, G.; Erker, G.; Frohlich, R. Formation of dinuclear titanium and zirconium complexes by olefin metathesis—Catalytic preparation of organometallic catalyst systems. Chemistry 2003, 9, 3618–3622. [Google Scholar] [CrossRef] [PubMed]
- Despagnet-Ayoub, E.; Henling, L.M.; Labinger, J.A.; Bercaw, J.E. Addition of a phosphine ligand switches an N-heterocyclic carbene-zirconium catalyst from oligomerization to polymerization of 1-hexene. Dalton Trans. 2013, 42, 15544–15547. [Google Scholar] [CrossRef] [PubMed]
- Luconi, L.; Giambastiani, G.; Rossin, A.; Bianchini, C.; Lledos, A. Intramolecular sigma-bond metathesis/protonolysis on zirconium(IV) and hafnium(IV) pyridylamido olefin polymerization catalyst precursors: Exploring unexpected reactivity paths. Inorg. Chem. 2010, 49, 6811–6813. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.J.; Davis, M.E. Synthesis of terephthalic acid via Diels-Alder reactions with ethylene and oxidized variants of 5-hydroxymethylfurfural. Proc. Natl. Acad. Sci. USA 2014, 111, 8363–8367. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, R.L.; Krska, S.W.; Bergman, R.G. Zirconium-mediated metathesis of imines: A study of the scope, longevity, and mechanism of a complicated catalytic system. J. Am. Chem. Soc. 2000, 122, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Kehr, G.; Frohlich, R.; Wibbeling, B.; Erker, G. (N-pyrrolyl)B(C6F5)2—A new organometallic Lewis acid for the generation of group 4 metallocene cation complexes. Chemistry 2000, 6, 258–266. [Google Scholar] [CrossRef]
- Millward, D.B.; Sammis, G.; Waymouth, R.M. Ring-opening reactions of oxabicyclic alkene compounds: Enantioselective hydride and ethyl additions catalyzed by group 4 metals. J. Org. Chem. 2000, 65, 3902–3909. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Ishitani, H. Novel binuclear chiral zirconium catalysts used in enantioselective strecker reactions. Chirality 2000, 12, 540–543. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kusakabe, K.; Ishitani, H. Chiral catalyst optimization using both solid-phase and liquid-phase methods in asymmetric aza Diels-Alder reactions. Org. Lett. 2000, 2, 1225–1227. [Google Scholar] [CrossRef] [PubMed]
- Illemassene, M.; Perrone, J. Chemical Thermodynamics of Compounds and Complexes of U, Np, Pu, Am, Tc, Se, Ni and Zr with Selected Organic Liquids; Elsevier Science: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Ekberg, C.; Kallvenius, G.; Albinsson, Y.; Brown, P.L. Studies on the hydrolytic behavior of zirconium(IV). J. Solut. Chem. 2004, 33, 47–79. [Google Scholar] [CrossRef]
- Singhal, A.; Toth, L.M.; Lin, J.S.; Affholter, K. Zirconium(IV) tetramer/octamer hydrolysis equilibrium in aqueous hydrochloric acid solution. J. Am. Chem. Soc. 1996, 118, 11529–11534. [Google Scholar] [CrossRef]
- Saha, G.B.; Porile, N.T.; Yaffe, L. (P,Xn) and (P,Pxn) Reactions of Yttrium-89 with 5-85-Mev Protons. Phys. Rev. 1966, 144, 962–982. [Google Scholar] [CrossRef]
- Kasbollah, A.; Eu, P.; Cowell, S.; Deb, P. Review on production of 89Zr in a medical cyclotron for PET radiopharmaceuticals. J. Nucl. Med. Technol. 2013, 41, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Alzimami, K.S.; Ma, A.K. Effective dose to staff members in a positron emission tomography/CT facility using zirconium-89. Br. J. Radiol. 2013, 86, 20130318. [Google Scholar] [CrossRef] [PubMed]
- Makris, N.E.; Boellaard, R.; Visser, E.P.; de Jong, J.R.; Vanderlinden, B.; Wierts, R.; van der Veen, B.J.; Greuter, H.J.; Vugts, D.J.; van Dongen, G.A.; et al. Multicenter harmonization of 89Zr PET/CT performance. J. Nucl. Med. 2014, 55, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J.L.; Zeglis, B.M.; Abdel-Atti, D.; Sawada, R.; Scholz, W.W.; Lewis, J.S. Pretargeted Immuno-PET of Pancreatic Cancer: Overcoming Circulating Antigen and Internalized Antibody to Reduce Radiation Doses. J. Nucl. Med. 2016, 57, 453–459. [Google Scholar] [CrossRef] [PubMed]
- DeJesus, O.T.; Nickles, R.J. Pretargeted immuno-PET of pancreatic cancer: Overcoming circulating antigen and internalized antibody to reduce radiation doses. Appl. Radiat. Isot. 1990, 42, 789–795. [Google Scholar] [CrossRef]
- Zweit, J.; Downey, S.; Sharma, H.L. Production of No-Carrier-Added Zirconium-89 for Positron Emission Tomography. Appl. Radiat. Isot. 1991, 42, 199–215. [Google Scholar] [CrossRef]
- Meijs, W.E.; Herscheid, J.D.M.; Haisma, H.J.; van Leuffen, P.J.; Mooy, R.; Pinedo, H.M. Production of Highly Pure No-Carrier Added Zr-89 for the Labeling of Antibodies with a Positron Emitter. Appl. Radiat. Isot. 1994, 45, 1143–1149. [Google Scholar] [CrossRef]
- Verel, I.; Visser, G.W.; Boellaard, R.; Stigter-van Walsum, M.; Snow, G.B.; van Dongen, G.A. 89Zr immuno-PET: Comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J. Nucl. Med. 2003, 44, 1271–1281. [Google Scholar] [PubMed]
- Holland, J.P.; Sheh, Y.; Lewis, J.S. Standardized methods for the production of high specific-activity zirconium-89. Nucl. Med. Biol. 2009, 36, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Infantino, A.; Cicoria, G.; Pancaldi, D.; Ciarmatori, A.; Boschi, S.; Fanti, S.; Marengo, M.; Mostacci, D. Prediction of (89)Zr production using the Monte Carlo code FLUKA. Appl. Radiat. Isot. 2011, 69, 1134–1137. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Mukhopadhyay, U.; Waligorski, G.J.; Balatoni, J.A.; Gonzalez-Lepera, C. Semi-automated production of (8)(9)Zr-oxalate/(8)(9)Zr-chloride and the potential of (8)(9)Zr-chloride in radiopharmaceutical compounding. Appl. Radiat. Isot. 2016, 107, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Queern, S.L.; Aweda, T.A.; Massicano, A.V.F.; Clanton, N.A.; El Sayed, R.; Sader, J.A.; Zyuzin, A.; Lapi, S.E. Production of Zr-89 using sputtered yttrium coin targets (89)Zr using sputtered yttrium coin targets. Nucl. Med. Biol. 2017, 50, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Sharifian, M.; Sadeghi, M.; Alirezapour, B.; Yarmohammadi, M.; Ardaneh, K. Modeling and experimental data of zirconium-89 production yield. Appl. Radiat. Isot. 2017, 130, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Siikanen, J.; Tran, T.A.; Olsson, T.G.; Strand, S.E.; Sandell, A. A solid target system with remote handling of irradiated targets for PET cyclotrons. Appl. Radiat. Isot. 2014, 94, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Wooten, A.L.; Madrid, E.; Schweitzer, G.D.; Lawrence, L.A.; Mebrahtu, E.; Lewis, B.C.; Lapi, S.E. Routine production of 89Zr using an automated module. Appl. Sci. 2013, 3, 593–613. [Google Scholar] [CrossRef]
- O‘Hara, M.J.; Murray, N.J.; Carter, J.C.; Kellogg, C.M.; Link, J.M. Hydroxamate column-based purification of zirconium-89 ((89)Zr) using an automated fluidic platform. Appl. Radiat. Isot. 2018, 132, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Petrik, M.; Zhai, C.; Novy, Z.; Urbanek, L.; Haas, H.; Decristoforo, C. In Vitro and In Vivo Comparison of Selected Ga-68 and Zr-89 Labelled Siderophores. Mol. Imaging Biol. 2016, 18, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. Int. 2016, 23, 3984–3999. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Saha, N.; Donofrio, R.S.; Bestervelt, L.L. Microbial siderophores: A mini review. J. Basic Microbiol. 2013, 53, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Gorden, A.E.; Xu, J.; Raymond, K.N.; Durbin, P. Rational design of sequestering agents for plutonium and other actinides. Chem. Rev. 2003, 103, 4207–4282. [Google Scholar] [CrossRef] [PubMed]
- Meijs, W.E.; Herscheid, J.D.M.; Haisma, H.J.; Pinedo, H.M. Evaluation of desferal as a bifunctional chelating agent for labeling antibodies with Zr-89. Appl. Radiat. Isot. 1992, 43, 1443–1447. [Google Scholar] [CrossRef]
- Rudd, S.E.; Roselt, P.; Cullinane, C.; Hicks, R.J.; Donnelly, P.S. A desferrioxamine B squaramide ester for the incorporation of zirconium-89 into antibodies. Chem. Commun. 2016, 52, 11889–11892. [Google Scholar] [CrossRef] [PubMed]
- Hermanoson, G.T. Bioconjugate Techniques, 2th ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Meijs, W.E.; Haisma, H.J.; Van der Schors, R.; Wijbrandts, R.; Van den Oever, K.; Klok, R.P.; Pinedo, H.M.; Herscheid, J.D. A facile method for the labeling of proteins with zirconium isotopes. Nucl. Med. Biol. 1996, 23, 439–448. [Google Scholar] [CrossRef]
- Perk, L.R.; Vosjan, M.J.; Visser, G.W.; Budde, M.; Jurek, P.; Kiefer, G.E.; van Dongen, G.A. p-Isothiocyanatobenzyl-desferrioxamine: A new bifunctional chelate for facile radiolabeling of monoclonal antibodies with zirconium-89 for immuno-PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.P.; Divilov, V.; Bander, N.H.; Smith-Jones, P.M.; Larson, S.M.; Lewis, J.S. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J. Nucl. Med. 2010, 51, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Tinianow, J.N.; Pandya, D.N.; Pailloux, S.L.; Ogasawara, A.; Vanderbilt, A.N.; Gill, H.S.; Williams, S.P.; Wadas, T.J.; Magda, D.; Marik, J. Evaluation of a 3-hydroxypyridin-2-one (2,3-HOPO) Based Macrocyclic Chelator for (89)Zr(4+) and Its Use for ImmunoPET Imaging of HER2 Positive Model of Ovarian Carcinoma in Mice. Theranostics 2016, 6, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Pandit-Taskar, N.; O’Donoghue, J.A.; Beylergil, V.; Lyashchenko, S.; Ruan, S.; Solomon, S.B.; Durack, J.C.; Carrasquillo, J.A.; Lefkowitz, R.A.; Gonen, M.; et al. (8)(9)Zr-huJ591 immuno-PET imaging in patients with advanced metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.P.; Vasdev, N. Charting the mechanism and reactivity of zirconium oxalate with hydroxamate ligands using density functional theory: Implications in new chelate design. Dalton Trans. 2014, 43, 9872–9884. [Google Scholar] [CrossRef] [PubMed]
- Abou, D.S.; Ku, T.; Smith-Jones, P.M. In vivo biodistribution and accumulation of 89Zr in mice. Nucl. Med. Biol. 2011, 38, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Hyman, D.M.; Ross, D.S.; Corben, A.; Chandarlapaty, S.; Goldfarb, S.; McArthur, H.; Erinjeri, J.P.; Solomon, S.B.; Kolb, H.; et al. Detection of HER2-Positive Metastases in Patients with HER2-Negative Primary Breast Cancer Using 89Zr-Trastuzumab PET/CT. J. Nucl. Med. 2016, 57, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Guerard, F.; Lee, Y.S.; Tripier, R.; Szajek, L.P.; Deschamps, J.R.; Brechbiel, M.W. Investigation of Zr(IV) and 89Zr(IV) complexation with hydroxamates: Progress towards designing a better chelator than desferrioxamine B for immuno-PET imaging. Chem. Commun. 2013, 49, 1002–1004. [Google Scholar] [CrossRef] [PubMed]
- Bailey, G.A.; Price, E.W.; Zeglis, B.M.; Ferreira, C.L.; Boros, E.; Lacasse, M.J.; Patrick, B.O.; Lewis, J.S.; Adam, M.J.; Orvig, C. H(2)azapa: A versatile acyclic multifunctional chelator for (67)Ga, (64)Cu, (111)In, and (177)Lu. Inorg. Chem. 2012, 51, 12575–12589. [Google Scholar] [CrossRef] [PubMed]
- Price, E.W.; Zeglis, B.M.; Lewis, J.S.; Adam, M.J.; Orvig, C. H6phospa-trastuzumab: Bifunctional methylenephosphonate-based chelator with 89Zr, 111In and 177Lu. Dalton Trans. 2014, 43, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Summer, D.; Rangger, C.; Franssen, G.M.; Laverman, P.; Haas, H.; Petrik, M.; Haubner, R.; Decristoforo, C. Novel Bifunctional Cyclic Chelator for (89)Zr Labeling-Radiolabeling and Targeting Properties of RGD Conjugates. Mol. Pharm. 2015, 12, 2142–2150. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, O.; Zhu, L.; Niu, G.; Weiss, I.D.; Szajek, L.P.; Ma, Y.; Sun, X.; Yan, Y.; Kiesewetter, D.O.; Liu, S.; et al. MicroPET imaging of integrin alphavbeta3 expressing tumors using 89Zr-RGD peptides. Mol. Imaging Biol. 2011, 13, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Summer, D.; Garousi, J.; Oroujeni, M.; Mitran, B.; Andersson, K.G.; Vorobyeva, A.; Lofblom, J.; Orlova, A.; Tolmachev, V.; Decristoforo, C. Cyclic versus Noncyclic Chelating Scaffold for (89)Zr-Labeled ZEGFR:2377 Affibody Bioconjugates Targeting Epidermal Growth Factor Receptor Overexpression. Mol. Pharm. 2018, 15, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.J.; Wilson, J.J.; Boros, E. Multifunctional Desferrichrome Analogues as Versatile (89)Zr(IV) Chelators for ImmunoPET Probe Development. Mol. Pharm. 2017, 14, 2831–2842. [Google Scholar] [CrossRef] [PubMed]
- Seibold, U.; Wangler, B.; Wangler, C. Rational Design, Development, and Stability Assessment of a Macrocyclic Four-Hydroxamate-Bearing Bifunctional Chelating Agent for (89) Zr. ChemMedChem 2017, 12, 1555–1571. [Google Scholar] [CrossRef] [PubMed]
- Allott, L.; Da Pieve, C.; Meyers, J.; Spinks, T.; Ciobota, D.M.; Kramer-Marek, G.; Smith, G. Evaluation of DFO-HOPO as an octadentate chelator for zirconium-89. Chem. Commun. 2017, 53, 8529–8532. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Bauman, A.; Mari, C.; Fischer, C.A.; Blacque, O.; Haussinger, D.; Gasser, G.; Mindt, T.L. An octadentate bifunctional chelating agent for the development of stable zirconium-89 based molecular imaging probes. Chem. Commun. 2014, 50, 11523–11525. [Google Scholar] [CrossRef] [PubMed]
- Vugts, D.J.; Klaver, C.; Sewing, C.; Poot, A.J.; Adamzek, K.; Huegli, S.; Mari, C.; Visser, G.W.; Valverde, I.E.; Gasser, G.; et al. Comparison of the octadentate bifunctional chelator DFO*-pPhe-NCS and the clinically used hexadentate bifunctional chelator DFO-pPhe-NCS for (89)Zr-immuno-PET. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Briand, M.; Aulsebrook, M.L.; Mindt, T.L.; Gasser, G. A solid phase-assisted approach for the facile synthesis of a highly water-soluble zirconium-89 chelator for radiopharmaceutical development. Dalton Trans. 2017, 46, 16387–16389. [Google Scholar] [CrossRef] [PubMed]
- Richardson-Sanchez, T.; Tieu, W.; Gotsbacher, M.P.; Telfer, T.J.; Codd, R. Exploiting the biosynthetic machinery of Streptomyces pilosus to engineer a water-soluble zirconium(IV) chelator. Org. Biomol. Chem. 2017, 15, 5719–5730. [Google Scholar] [CrossRef] [PubMed]
- Gotsbacher, M.P.; Telfer, T.J.; Witting, P.K.; Double, K.L.; Finkelstein, D.I.; Codd, R. Analogues of desferrioxamine B designed to attenuate iron-mediated neurodegeneration: Synthesis, characterisation and activity in the MPTP-mouse model of Parkinson’s disease. Metallomics 2017, 9, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Holland, J.P.; Kenton, N.; Rotile, N.; Caravan, P. Macrocycle-Based Hydroxamate Ligands for Complexation and Immunoconjugation of (89)Zirconium for Positron Emission Tomography (PET) Imaging. Chempluschem 2016, 81, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, N.B.; Pandya, D.N.; Xu, J.; Tatum, D.; Magda, D.; Wadas, T.J. Evaluation of macrocyclic hydroxyisophthalamide ligands as chelators for zirconium-89. PLoS ONE 2017, 12, e0178767–e0178777. [Google Scholar] [CrossRef] [PubMed]
- Pandya, D.N.; Pailloux, S.; Tatum, D.; Magda, D.; Wadas, T.J. Di-macrocyclic terephthalamide ligands as chelators for the PET radionuclide zirconium-89. Chem. Commun. 2015, 51, 2301–2303. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.T.; Meszaros, L.K.; Paterson, B.M.; Berry, D.J.; Cooper, M.S.; Ma, Y.; Hider, R.C.; Blower, P.J. Tripodal tris(hydroxypyridinone) ligands for immunoconjugate PET imaging with (89)Zr(4+): Comparison with desferrioxamine-B. Dalton Trans. 2015, 44, 4884–4900. [Google Scholar] [CrossRef] [PubMed]
- Buchwalder, C.; Rodriguez-Rodriguez, C.; Schaffer, P.; Karagiozov, S.K.; Saatchi, K.; Hafeli, U.O. A new tetrapodal 3-hydroxy-4-pyridinone ligand for complexation of (89)zirconium for positron emission tomography (PET) imaging. Dalton Trans. 2017, 46, 9654–9663. [Google Scholar] [CrossRef] [PubMed]
- Deri, M.A.; Ponnala, S.; Zeglis, B.M.; Pohl, G.; Dannenberg, J.J.; Lewis, J.S.; Francesconi, L.C. Alternative chelator for (8)(9)Zr radiopharmaceuticals: Radiolabeling and evaluation of 3,4,3-(LI-1,2-HOPO). J. Med. Chem. 2014, 57, 4849–4860. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Goldman, D.A.; Gonen, M.; Pham, H.; Castillo, R.; Lyashchenko, S.K.; Lewis, J.S.; Dang, C. Initial Results of a Prospective Clinical Trial of 18F-Fluciclovine PET/CT in Newly Diagnosed Invasive Ductal and Invasive Lobular Breast Cancers. J. Nucl. Med. 2016, 57, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Pandya, D.N.; Bhatt, N.; Yuan, H.; Day, C.S.; Ehrmann, B.M.; Wright, M.; Bierbach, U.; Wadas, T.J. Zirconium tetraazamacrocycle complexes display extraordinary stability and provide a new strategy for zirconium-89-based radiopharmaceutical development. Chem. Sci. 2017, 8, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.G.; Hild, F.; Munha, R.F.; Veiros, L.F.; Dagorne, S.; Martins, A.M. Synthesis and structural characterization of novel cyclam-based zirconium complexes and their use in the controlled ROP of rac-lactide: Access to cyclam-functionalized polylactide materials. Dalton Trans. 2012, 41, 14288–14298. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.G.; Madeira, F.; Munha, R.F.; Barroso, S.; Veiros, L.F.; Martins, A.M. Reactions of heteroallenes with cyclam-based Zr(IV) complexes. Dalton Trans. 2015, 44, 1441–1455. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.; Solari, E.; Floriani, C.; Chiesi-Villa, A.; Rizzoli, C. New directions in amido-transition metal chemistry: The preparation and reaction of mixed amino-amido macrocyclic ligands. J. Chem Soc. Dalton Trans. 1997, 2385–2386. [Google Scholar] [CrossRef]
- Munha, R.F.; Veiros, L.F.; Duarte, M.T.; Fryzuk, M.D.; Martins, A.M. Synthesis and structural studies of amido, hydrazido and imido zirconium(IV) complexes incorporating a diamido/diamine cyclam-based ligand. Dalton Trans. 2009, 7494–7508. [Google Scholar] [CrossRef] [PubMed]
- Angelis, S.; Solari, E.; Floriani, C.; Chiesi-Villa, A.; Rizzoli, C. Mono- and bis(dibenzotetramethyltetraaza[14]annulene) complexes of Group IV metals including the structure of the lithium derivative of the macrocyclic ligand. Inorg. Chem. 1992, 31, 2520–2527. [Google Scholar] [CrossRef]
- Jewula, P.; Berthet, J.-C.; Chambron, Y.; Rousselin, P.T.; Meyer, M. Synthesis and Structural Study of Tetravalent (Zr4+, Hf4+, Ce4+, Th4+, U4+) Metal Complexes with Cyclic Hydroxamic Acids. Eur. J. Inorg. Chem. 2015, 1529–1541. [Google Scholar] [CrossRef]
- Li, A.; Ma, H.; Huang, J. Highly Thermally Stable Eight-Coordinate Dichloride Zirconium Complexes Supported by Tridentate [ONN] Ligands: Syntheses, Characterization, and Ethylene Polymerization Behavior. Organometallics 2013, 32, 7460–7469. [Google Scholar] [CrossRef]
- Solari, E.; Maltese, C.; Franceschi, F.; Floriani, C.; Chiesi-Villa, A.; Rizzoli, C. Geometrical isomerism and redox behaviour in zirconium-Schiff base complexes: The formation of C-C bonds functioning as two-electron reservoirs. J. Chem. Soc. Dalton Trans. 1997, 2903–2910. [Google Scholar] [CrossRef]
- Kato, C.; Shinohara, A.; Hayashi, K.; Nomiya, K. Syntheses and X-ray crystal structures of zirconium(IV) and hafnium(IV) complexes containing monovacant Wells-Dawson and Keggin polyoxotungstates. Inorg. Chem. 2006, 45, 8108–8119. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Strosberg, J. Radionuclide Therapy for Neuroendocrine Tumors. Curr. Oncol. Rep. 2017, 19, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Pandya, D.N.; Hantgan, R.; Budzevich, M.M.; Kock, N.D.; Morse, D.L.; Batista, I.; Mintz, A.; Li, K.C.; Wadas, T.J. Preliminary Therapy Evaluation of (225)Ac-DOTA-c(RGDyK) Demonstrates that Cerenkov Radiation Derived from (225)Ac Daughter Decay Can Be Detected by Optical Imaging for In Vivo Tumor Visualization. Theranostics 2016, 6, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Virgolini, I.; Traub, T.; Novotny, C.; Leimer, M.; Fuger, B.; Li, S.R.; Patri, P.; Pangerl, T.; Angelberger, P.; Raderer, M.; et al. Experience with indium-111 and yttrium-90-labeled somatostatin analogs. Curr. Pharm. Des. 2002, 8, 1781–1807. [Google Scholar] [CrossRef] [PubMed]
- Virgolini, I.; Britton, K.; Buscombe, J.; Moncayo, R.; Paganelli, G.; Riva, P. In- and Y-DOTA-lanreotide: Results and implications of the MAURITIUS trial. Semin. Nucl. Med. 2002, 32, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Fiebiger, W.C.; Scheithauer, W.; Traub, T.; Kurtaran, A.; Gedlicka, C.; Kornek, G.V.; Virgolini, I.; Raderer, M. Absence of therapeutic efficacy of the somatostatin analogue lanreotide in advanced primary hepatic cholangiocellular cancer and adenocarcinoma of the gallbladder despite in vivo somatostatin-receptor expression. Scand. J. Gastroenterol. 2002, 37, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Davis, C.B.; Abdel-Atti, D.; Carlin, S.D.; Chen, A.; Aggeler, R.; Agnew, B.J.; Lewis, J.S. Chemoenzymatic strategy for the synthesis of site-specifically labeled immunoconjugates for multimodal PET and optical imaging. Bioconj. Chem. 2014, 25, 2123–2128. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Emmetiere, F.; Pillarsetty, N.; Weissleder, R.; Lewis, J.S.; Reiner, T. Building Blocks for the Construction of Bioorthogonally Reactive Peptides via Solid-Phase Peptide Synthesis. ChemistryOpen 2014, 3, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Lewis, J.S. The bioconjugation and radiosynthesis of 89Zr-DFO-labeled antibodies. J. Vis. Exp. 2015, 96, 354–366. [Google Scholar]
- Laforest, R.; Lapi, S.E.; Oyama, R.; Bose, R.; Tabchy, A.; Marquez-Nostra, B.V.; Burkemper, J.; Wright, B.D.; Frye, J.; Frye, S.; et al. [(89)Zr]Trastuzumab: Evaluation of Radiation Dosimetry, Safety, and Optimal Imaging Parameters in Women with HER2-Positive Breast Cancer. Mol. Imaging Biol. 2016, 18, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Makris, N.E.; Boellaard, R.; van Lingen, A.; Lammertsma, A.A.; van Dongen, G.A.; Verheul, H.M.; Menke, C.W.; Huisman, M.C. PET/CT-derived whole-body and bone marrow dosimetry of 89Zr-cetuximab. J. Nucl. Med. 2015, 56, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Makris, N.E.; van Velden, F.H.; Huisman, M.C.; Menke, C.W.; Lammertsma, A.A.; Boellaard, R. Validation of simplified dosimetry approaches in (8)(9)Zr-PET/CT: The use of manual versus semi-automatic delineation methods to estimate organ absorbed doses. Med. Phys. 2014, 41, 102503–102533. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Gambhir, S.S. Radiation Dosimetry Study of [(89)Zr]rituximab Tracer for Clinical Translation of B cell NHL Imaging using Positron Emission Tomography. Mol. Imaging Biol. 2015, 17, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Perk, L.R.; Visser, O.J.; Stigter-van Walsum, M.; Vosjan, M.J.; Visser, G.W.; Zijlstra, J.M.; Huijgens, P.C.; van Dongen, G.A. Preparation and evaluation of (89)Zr-Zevalin for monitoring of (90)Y-Zevalin biodistribution with positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.N.; Visser, O.J.; Vosjan, M.J.; van Lingen, A.; Hoekstra, O.S.; Zijlstra, J.M.; Huijgens, P.C.; van Dongen, G.A.; Lubberink, M. Biodistribution, radiation dosimetry and scouting of 90Y-ibritumomab tiuxetan therapy in patients with relapsed B-cell non-Hodgkin’s lymphoma using 89Zr-ibritumomab tiuxetan and PET. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 512–520. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatt, N.B.; Pandya, D.N.; Wadas, T.J. Recent Advances in Zirconium-89 Chelator Development. Molecules 2018, 23, 638. https://doi.org/10.3390/molecules23030638
Bhatt NB, Pandya DN, Wadas TJ. Recent Advances in Zirconium-89 Chelator Development. Molecules. 2018; 23(3):638. https://doi.org/10.3390/molecules23030638
Chicago/Turabian StyleBhatt, Nikunj B., Darpan N. Pandya, and Thaddeus J. Wadas. 2018. "Recent Advances in Zirconium-89 Chelator Development" Molecules 23, no. 3: 638. https://doi.org/10.3390/molecules23030638
APA StyleBhatt, N. B., Pandya, D. N., & Wadas, T. J. (2018). Recent Advances in Zirconium-89 Chelator Development. Molecules, 23(3), 638. https://doi.org/10.3390/molecules23030638





