Microbes a Tool for the Remediation of Organotin Pollution Determined by Static Headspace Gas Chromatography-Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
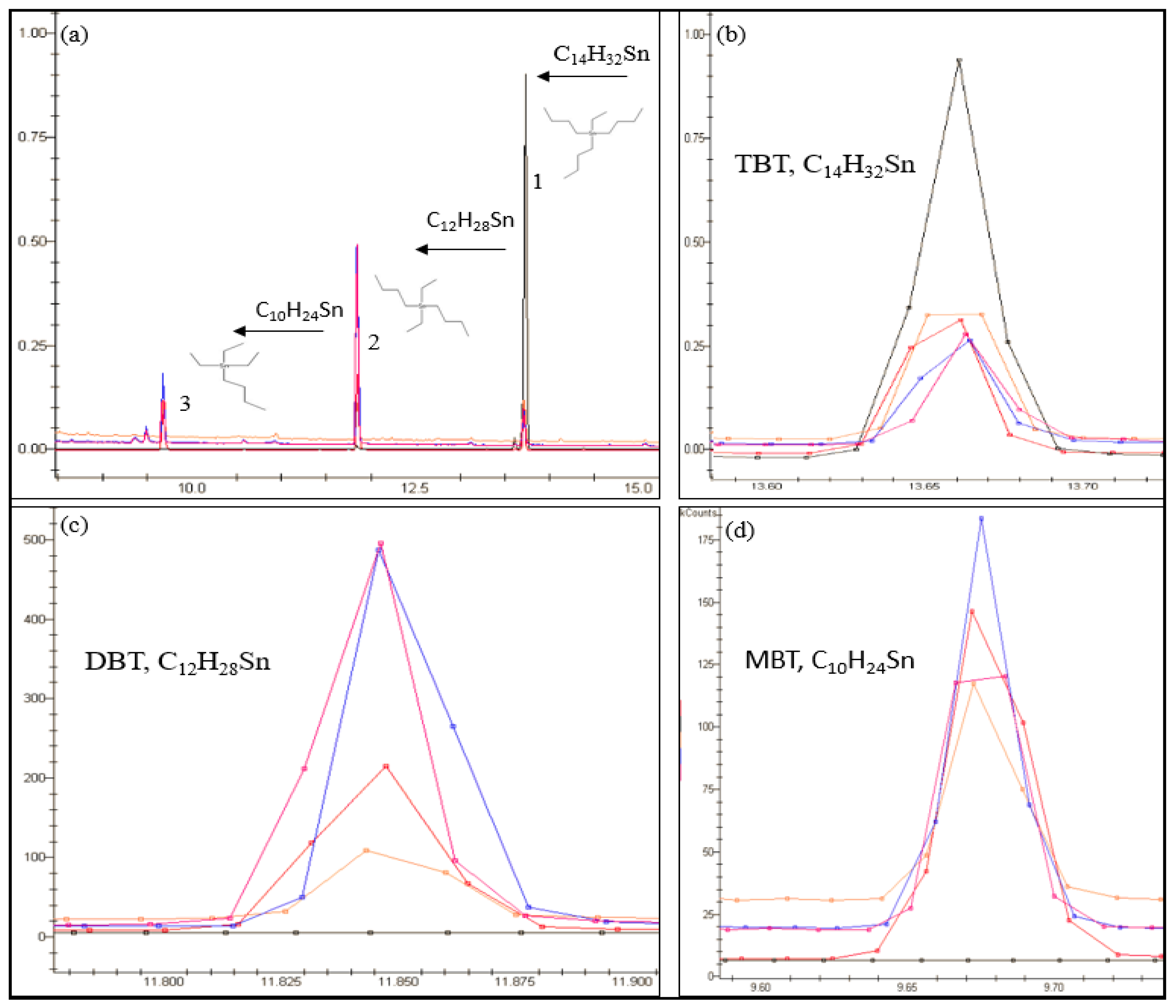
2.1. System Suitability
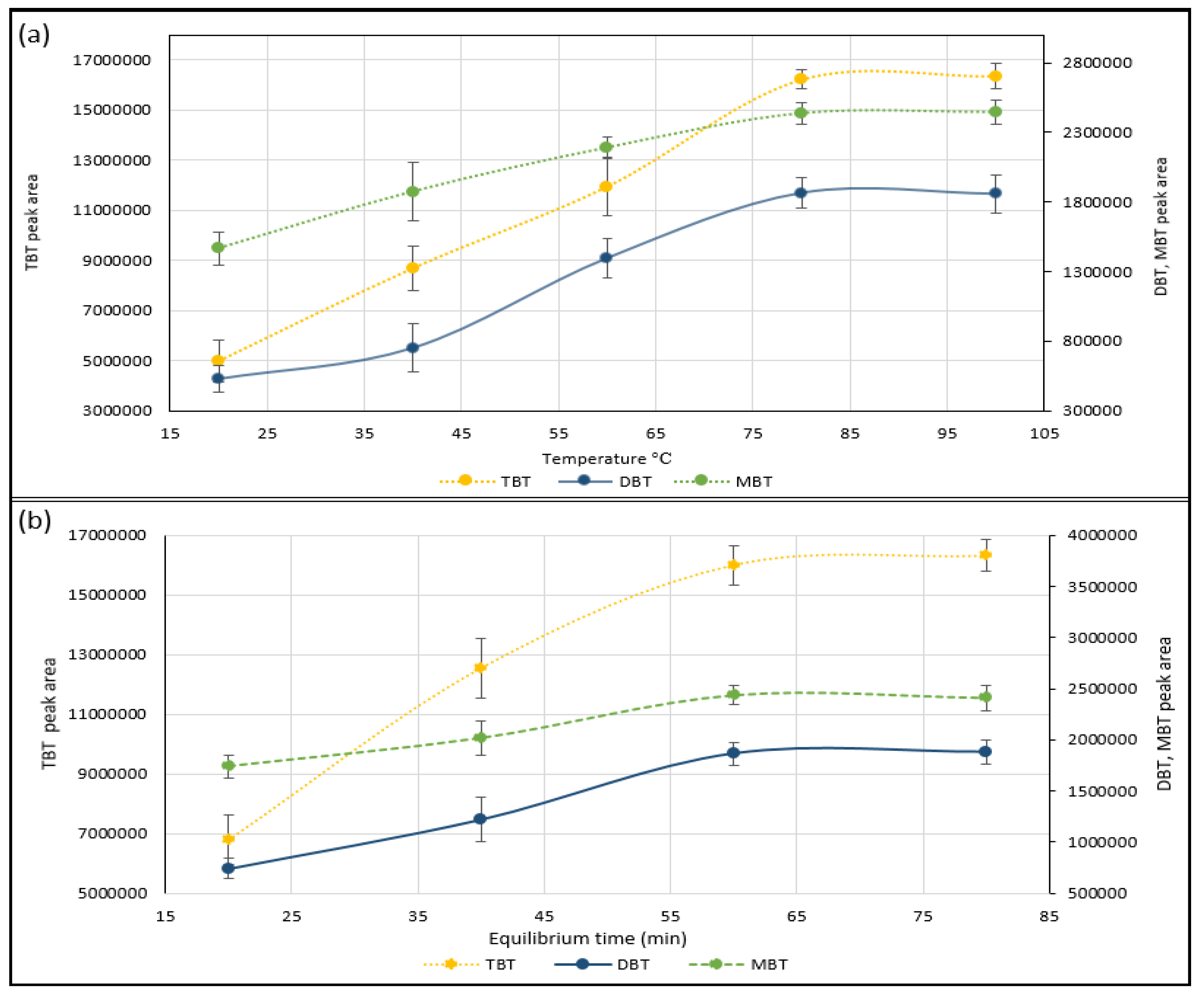
2.2. Optimisation of Static Headspace Conditions
Temperature and Time Effect
2.3. Method Validation
2.3.1. Linearity and Precision
2.3.2. Accuracy & Sensitivity
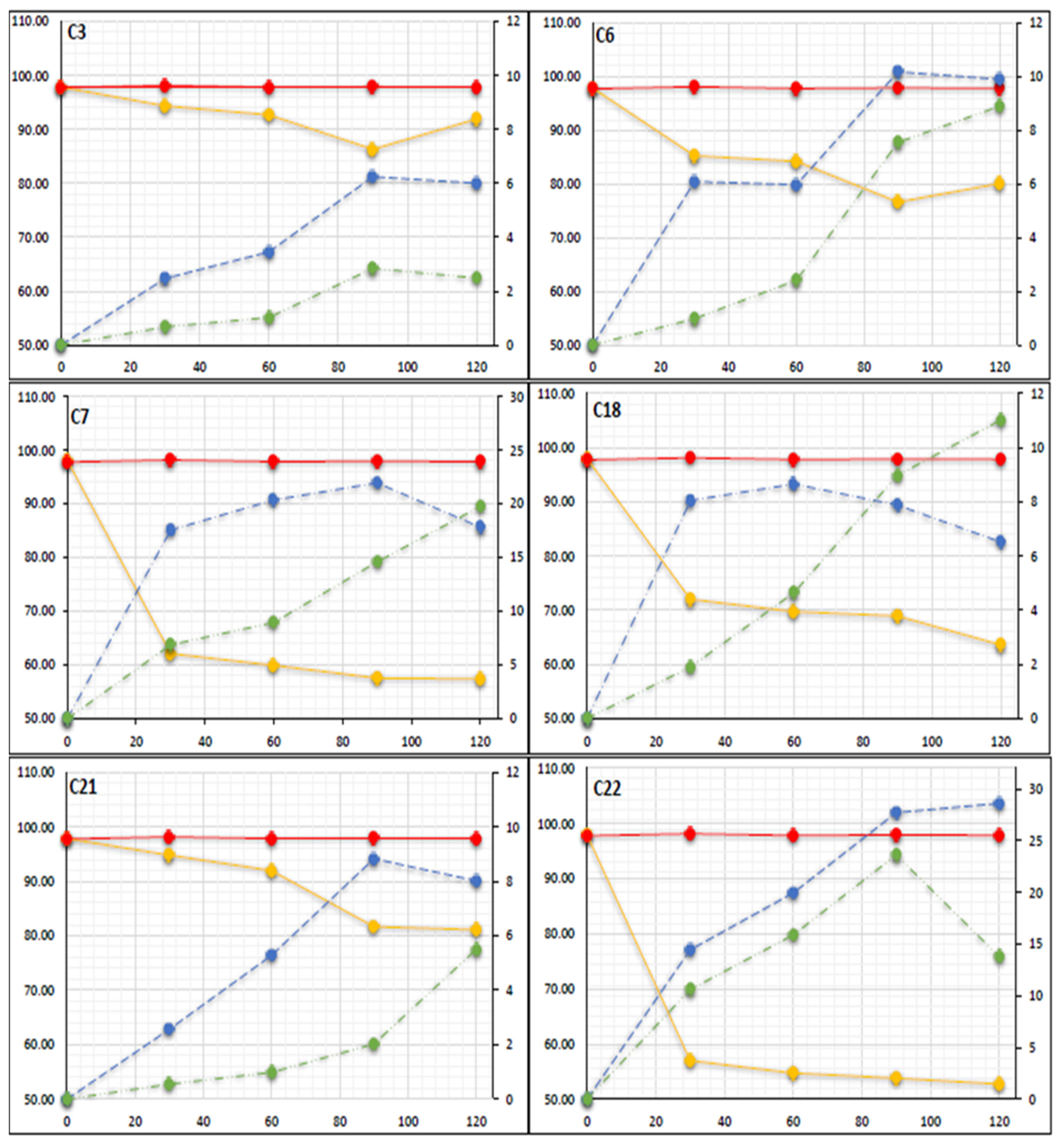
2.4. Microcosm Chemical Analysis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. TBT Degrading Isolates
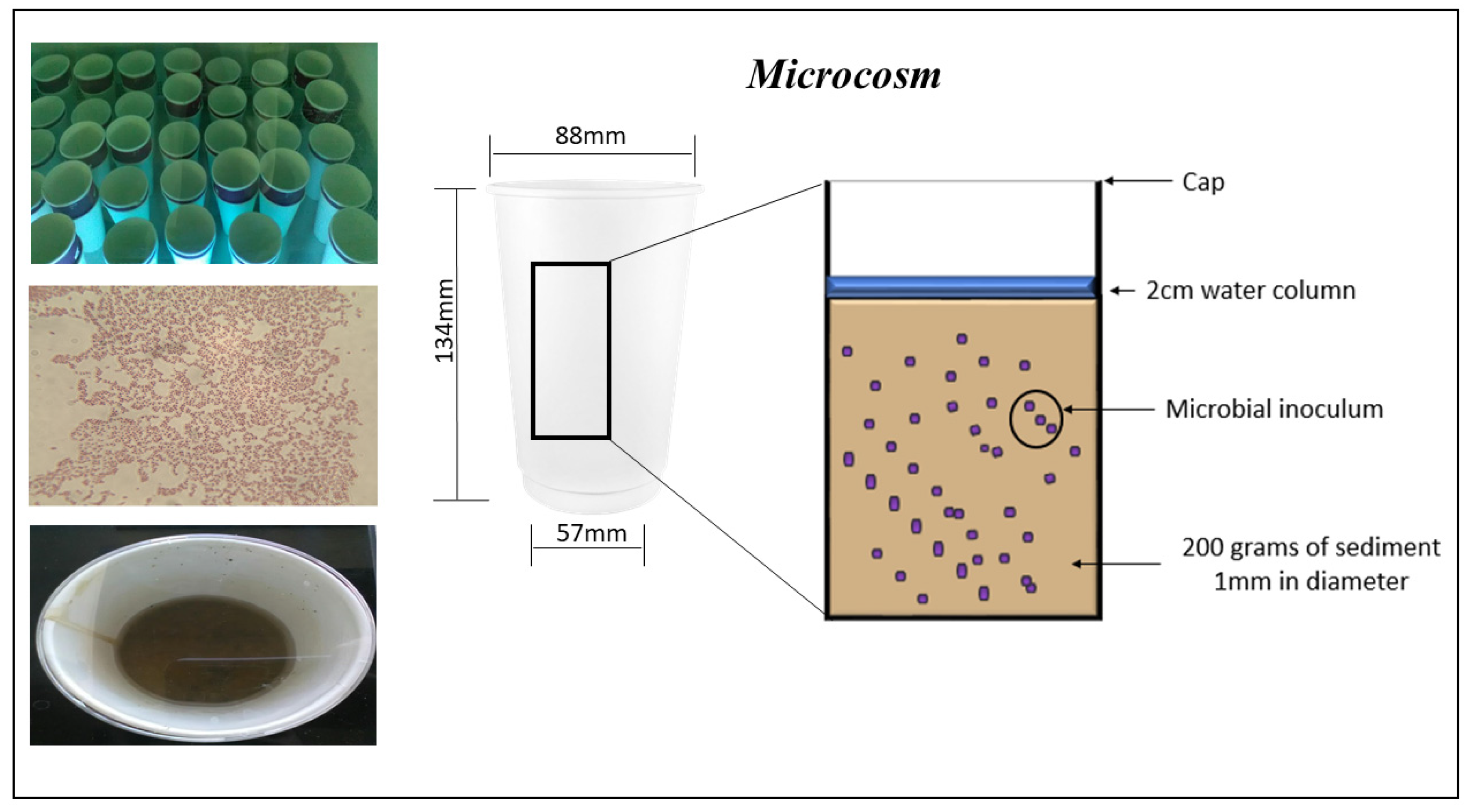
3.3. Microcosm Experiment Setup
3.4. Organotin Chemical Analysis
3.5. GC-MS Operating Conditions
3.6. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoch, M. Organotin compounds in the environment—An overview. Appl. Geochem. 2001, 16, 719–743. [Google Scholar] [CrossRef]
- Sousa, A.C.; Pastorinho, M.R.; Takahashi, S.; Tanabe, S. History on organotin compounds, from snails to humans. Environ. Chem. Lett. 2014, 12, 117–137. [Google Scholar] [CrossRef]
- Cruz, A.; Henriques, I.; Sousa, A.C.; Baptista, I.; Almeida, A.; Takahashi, S.; Tanabe, S.; Correia, A.; Suzuki, S.; Anselmo, A.M.; et al. A microcosm approach to evaluate the degradation of tributyltin (TBT) by Aeromonas molluscorum Av27 in estuarine sediments. Environ. Res. 2014, 132, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Antizar-Ladislao, B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. Environ. Int. 2008, 34, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Roy, U.; Bhosle, S. Microbial transformation of tributyltin chloride by Pseudomonas aeruginosa strain USS25 NCIM-5224. Appl. Organomet. Chem. 2006, 20, 5–11. [Google Scholar] [CrossRef]
- Landmeyer, J.E.; Tanner, T.L.; Watt, B.E. Biotransformation of tributyltin to tin in freshwater river-bed sediments contaminated by an organotin release. Environ. Sci. Technol. 2004, 38, 4106–4112. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.C.; Oliveira, I.B.; Laranjeiro, F.; Takahashi, S.; Tanabe, S.; Cunha, M.R.; Barroso, C.M. Organotin levels in Nazaré canyon (west Iberian Margin, NE Atlantic) and adjacent coastal area. Mar. Pollut. Bull. 2012, 64, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Kotrikla, A. Environmental management aspects for TBT antifouling wastes from the shipyards. Journal of Environ. Manag. 2009, 90, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.S.; Shim, W.J.; Yim, U.H.; Ha, S.Y.; An, J.G.; Shin, K.H. Three decades of TBT contamination in sediments around a large scale shipyard. J. Hazard. Mater. 2011, 192, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.F.; Mills, G.A.; Parker, R.; Bolam, T.; Birchenough, A.; Kröger, S.; Fones, G.R. Trends in the analysis and monitoring of organotins in the aquatic environment. Trends Environ. Anal. Chem. 2015, 8, 1–11. [Google Scholar] [CrossRef]
- Lam, N.H.; Jeong, H.H.; Kang, S.D.; Kim, D.J.; Ju, M.J.; Horiguchi, T.; Cho, H.S. Organotins and new antifouling biocides in water and sediments from three Korean Special Management Sea Areas following ten years of tributyltin regulation: Contamination profiles and risk assessment. Mar. Pollut. Bull. 2017, 121, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, I.; Bayona, J.M.; Albaiges, J.; Alencastro, L.F.; Tarradellas, J. Organotin speciation in aquatic matrices by CGC/FPD, ECD and MS, and LC/MS. Fresenius’ J. Anal. Chem. 1991, 339, 646–653. [Google Scholar] [CrossRef]
- Moscoso-Pérez, C.; Fernández-González, V.; Moreda-Piñeiro, J.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D. Determination of organotin compounds in waters by headspace solid phase microextraction gas chromatography triple quadrupole tandem mass spectrometry under the European Water Framework Directive. J. Chromatogr. A 2015, 1385, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Fettig, I.; Philipp, R.; Jakubowski, N.; Panne, U.; Fisicaro, P.; Alasonati, E. Determination of tributyltin in whole water matrices under the European Water Framework Directive. J. Chromatogr. A 2016, 1459, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, C.; Ryan, D.; Enright, A.M.; Garcia-Cabellos, G. Gas Chromatographic Approach to Evaluate the Efficacy of Organotin Degrading Microbes. Int. J. Environ. Bioremed. Biodegrad. 2017, 5, 18–26. [Google Scholar]
- Takeuchi, M.; Mizuishi, K.; Hobo, T. Determination of organotin compounds in environmental samples. Anal. Sci. 2000, 16, 349–359. [Google Scholar] [CrossRef]
- Tsunoi, S.; Matoba, T.; Shioji, H.; Harino, H.; Tanaka, M. Analysis of organotin compounds by grignard derivatization and gas chromatography–ion trap tandem mass spectrometry. J. Chromatogr. A 2002, 962, 197–206. [Google Scholar] [CrossRef]
- Jiang, G.B.; Liu, J.Y.; Yang, K.W. Speciation analysis of butyltin compounds in Chinese seawater by capillary gas chromatography with flame photometric detection using in-situ hydride derivatization followed by headspace solid-phase microextraction. Anal. Chim. Acta 2000, 421, 67–74. [Google Scholar] [CrossRef]
- Snow, N.H.; Slack, G.C. Head-space analysis in modern gas chromatography. TrAC Trends Anal. Chem. 2002, 21, 608–617. [Google Scholar] [CrossRef]
- Wardencki, W.; Curyło, J.; Namieśnik, J. Trends in solventless sample preparation techniques for environmental analysis. J. Biochem. Biophys. Methods 2007, 70, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Kolb, B. Headspace sampling with capillary columns. J. Chromatogr. A 1999, 842, 163–205. [Google Scholar] [CrossRef]
- Ettre, L.S. Headspace Analysis of Food and Flavors, Theory and Practice; Rouseff, R., Cadwsliader, K., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2001; p. 9. [Google Scholar]
- Yilmazcan, O.; OZER, E.T.; Izgi, B.; Gucer, S. Optimization of static head-space gas chromatography-mass spectrometry-conditions for the determination of benzene, toluene, ethyl benzene, xylene, and styrene in model solutions. Ekoloji 2013, 22, 76–83. [Google Scholar] [CrossRef]
- Dubey, S.K.; Roy, U. Biodegradation of tributyltins (organotins) by marine bacteria. Appl. Organomet. Chem. 2003, 17, 3–8. [Google Scholar] [CrossRef]
- Sakultantimetha, A.; Keenan, H.; Dyer, M.; Beattie, T.; Bangkedphol, S.; Songsasen, A. Isolation of tributyltin-degrading bacteria Citrobacter braakii and enterobacter cloacae from butyltin-polluted sediment. In Contaminated Sediments: Sustainable Management and Remediation; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Jeong, B.G.; Hong, S.W.; Choi, Y.S.; Kumaran, R.S.; Kim, M.A.; Kim, S.H.; Kim, H.J. Isolation of tributyltin chloride resistance bacteria and rapid electrochemical determination of bacterial organotin degradation activity. Bull. Korean Chem. Soc. 2011, 32, 356–358. [Google Scholar] [CrossRef]
- Bramhachari, P.V.; Kumar, B.A.; Deepika, K.V.; Gnanender, S. Alcaligenes sp. Strain VBAK101: A Potent Tributyltin Chloride (TBTCL) Resistant Bacteria Isolated from Vishakaptanam Shipping Harbour Sediments. Res. J. Microbiol. 2014, 9, 82. [Google Scholar] [CrossRef][Green Version]
- Khanolkar, D.S.; Dubey, S.K.; Naik, M.M. Biotransformation of tributyltin chloride to less toxic dibutyltin dichloride and monobutyltin trichloride by Klebsiella pneumoniae strain SD9. Int. Biodeterior. Biodegrad. 2015, 104, 212–218. [Google Scholar] [CrossRef]
- Dai, S.; Huang, G.; Chen, C. Fate of 14C-labeled tributyltin in an estuarine microcosm. Appl. Organomet. Chem. 1998, 12, 585–590. [Google Scholar] [CrossRef]
- Suehiro, F.; Kobayashi, T.; Nonaka, L.; Tuyen, B.C.; Suzuki, S. Degradation of tributyltin in microcosm using Mekong River sediment. Microb. Ecol. 2006, 55, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Tessier, E.; Amouroux, D.; Morin, A.; Christian, L.; Thybaud, E.; Vindimian, E.; Donard, O.F. (Tri) Butyltin biotic degradation rates and pathways in different compartments of a freshwater model ecosystem. Sci. Total Environ. 2007, 388, 214–233. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.; Mustafa, M.B.; Johari, W.L.W.; Zulkifli, S.Z.; Yusuff, F.B.M. Tributyltin (TBT) tolerance of indigenous and non-indigenous bacterial species. Water Air Soil Pollut. 2016, 227, 258. [Google Scholar] [CrossRef]
- Scott, D.R. The NIST/EPA/MSDC Mass Spectral Database, Personal Computer Versions 1.0 and 2.0; Chemometrics and Intelligent Laboratory Systems, 1990; 16, 193–202. [CrossRef]
- Belkin, M.A.; Olson, L.K.; Caruso, J.A. Radiofrequency glow discharge as an ion source for gas chromatography with mass spectrometric detection. J. Anal. At. Spectrom. 1997, 12, 1255–1261. [Google Scholar] [CrossRef]
- Segovia-Martinez, L.; Bouzas-Blanco, A.; Campíns-Falcó, P.; Seco-Torrecillas, A. Improving detection limits for organotin compounds in several matrix water samples by derivatization-headspace-solid-phase microextraction and GC–MS. Talanta 2010, 80, 1888–1893. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Snow, N.H.; Bullock, G.P. Novel techniques for enhancing sensitivity in static headspace extraction-gas chromatography. J. Chromatogr. A 2010, 1217, 2726–2735. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Moon, H.B.; An, Y.R.; Choi, S.G.; Choi, H.G. Accumulation of butyltin compounds in cetaceans from Korean coastal waters. Mar. Pollut. Bull. 2011, 62, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Sakultantimetha, A.; Keenan, H.E.; Beattie, T.K.; Aspray, T.J.; Bangkedphol, S.; Songsasen, A. Acceleration of tributyltin biodegradation by sediment microorganisms under optimized environmental conditions. Int. Biodeterior. Biodegrad. 2010, 64, 467–473. [Google Scholar] [CrossRef]
- Jin, J.; Yang, L.; Chan, S.M.; Luan, T.; Li, Y.; Tam, N.F. Effect of nutrients on the biodegradation of tributyltin (TBT) by alginate immobilized microalga, Chlorella vulgaris, in natural river water. J. Hazard. Mater. 2011, 185, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Instrument Conditions | |
|---|---|
| Carrier gas | Helium (99.999%) |
| Flow rate | 1 mL/min |
| Injector port temperature | 280 °C |
| Column | 5% biphenyl and 95% dimethylpolysiloxane 30 m length × 0.25 mm internal diameter × 0.25 μm film thickness. |
| Temperature program | |
| Initial temperature | 50 °C for 1 min |
| Ramp | 10°/min |
| Final temperature | 300 °C held for 4 min |
| Split ratio | Splitless |
| Detector temperature | 280 °C |
| Injection conditions | Headspace |
| Thermosatatting temperature | 90 °C |
| Thermosatatting time | 65 min |
| Agitation speed | 350 rpm |
| Agitation on time | 10 s |
| Agitation off time | 2 s |
| Syringe temperature | 95 °C |
| Syringe volume | 2.5 mL |
| Injection volume | 1.00 mL |
| Injection fill speed | 100 µL/s |
| Injection speed | 200 µL/s |
| Syringe flushing time | 4 min at 1 bar pressure |
| Mass spectrometer conditions | |
| Mode | Electron ionisation (70 Ev) |
| Acquisition mode | Selected ion monitoring |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finnegan, C.; Ryan, D.; Enright, A.-M.; Garcia-Cabellos, G. Microbes a Tool for the Remediation of Organotin Pollution Determined by Static Headspace Gas Chromatography-Mass Spectrometry. Molecules 2018, 23, 627. https://doi.org/10.3390/molecules23030627
Finnegan C, Ryan D, Enright A-M, Garcia-Cabellos G. Microbes a Tool for the Remediation of Organotin Pollution Determined by Static Headspace Gas Chromatography-Mass Spectrometry. Molecules. 2018; 23(3):627. https://doi.org/10.3390/molecules23030627
Chicago/Turabian StyleFinnegan, Christopher, David Ryan, Anne-Marie Enright, and Guiomar Garcia-Cabellos. 2018. "Microbes a Tool for the Remediation of Organotin Pollution Determined by Static Headspace Gas Chromatography-Mass Spectrometry" Molecules 23, no. 3: 627. https://doi.org/10.3390/molecules23030627
APA StyleFinnegan, C., Ryan, D., Enright, A.-M., & Garcia-Cabellos, G. (2018). Microbes a Tool for the Remediation of Organotin Pollution Determined by Static Headspace Gas Chromatography-Mass Spectrometry. Molecules, 23(3), 627. https://doi.org/10.3390/molecules23030627





