Abstract
A convenient, fast and environmentally benign procedure for the synthesis of a new series of highly functionalized N-alkylated pyridines as privileged medicinal scaffolds was developed via a unique three-component reaction of easily available aromatic as well as heteroaromatic aldehydes, N-alkyl-2-cyanoacetamides and malononitrile in EtOH in the presence of K2CO3 as a base promoter under microwave irradiation. The presented tandem process is presumed to proceed via Knoevenagel condensation, Michael addition, intramolecular cyclization, autoxidation and subsequent aromatization. Particularly valuable features of this protocol, including high product yields, mild conditions, atom-efficiency, simple execution, short reaction times and easy purification make it a highly efficient and promising synthetic strategy to prepare substituted pyridine nuclei. The proposed mechanism of this novel one-pot reaction and structure elucidation of the products are discussed.
1. Introduction
One-pot multi-component reactions (MCRs) in which three or more reactants are combined together in a single synthetic operation to create a highly complex molecule incorporating most atoms present in the starting materials have proven to be a very rapid, powerful and elegant synthetic procedure. The MCRs strategy provides important advantages over conventional multistep synthesis because of its ease of execution, efficiency, simple procedures and equipment, flexibility, atom economic nature, high yields, productivity, convergence, and highly selectivity [1,2,3,4]. In addition, by reducing waste production, the number of operational steps, avoiding the complicated isolation and purification of intermediates, minimization of time, energy consumption, cost, solvents, reagents and expenditure of human labor, MCRs represent eco-friendly processes [5]. These advantages make MCRs well-suited for the easy construction of libraries of ‘drug-like’ molecules [6,7]. In view of the growing interest in the preparation of interesting heterocyclic scaffolds, tremendous scientific efforts are currently being devoted to develop new multi-component procedures for the synthesis of numerous polyfunctionalized heterocyclic scaffolds and discovery of new drugs [6].
Microwave-assisted organic chemistry (MAOC) is one of the high-speed techniques which has attracted a great attention in recent years. The intrinsic advantages of performing various organic transformations under microwave (MW) irradiation conditions are the high yields of relatively pure products and significant acceleration of the rate of the chemical reactions [8,9]. Thus, these are not only environmental friendly but also financially attractive processes [10].
Highly substituted pyridines, known as privileged medicinal scaffolds, are of significant interest as they widely occur as the key constituents in numerous of biologically active natural products and pharmaceuticals [11,12,13,14,15,16,17]. On account of their vast range of eminent pharmacological, physiological, and biological activities, they are considered important structures. Therefore, they have attracted great interest among the all heterocyclic compounds and the interest in their synthesis and chemistry continues undiminished [2,18,19]. Among these pyridine derivatives, 2-aminopyridine-3,5-dicarbonitriles constitute a very important type of heterocyclic compounds in modern medicinal chemistry due to their potential therapeutic applications in the treatment of several diseases and broad spectrum biological activities [20,21,22,23,24,25,26,27,28]. On the other hand, the N-alkylated pyridones are among the most important classes of azaheterocyclic compounds as they widely occur as prevalent core structures in many biologically active natural products, synthetic bioactive substances and active pharmaceuticals [29] that show interesting pharmacological and biological activities such as multiple sclerosis immunomodulators [30], a putative memory-enhancing drug [31,32], and anticancer agents [33]. Accordingly, methods for the efficient synthesis of new derivatives of these compounds have thus attracted the great interest of synthetic and medicinal chemists. However, a literature survey showed that efficient, direct approaches to the selective synthesis of N-alkylated 2-pyridone derivatives are much less well explored, as known methods generally suffer from certain drawbacks such as the lack of generality or selectivity, poor yields, the use of expensive transition-metal catalysts and/or a competitive process between N- and O-alkylation (poor chemoselectivity) [34,35]. Therefore, the development of novel straightforward approaches to densely substituted N-alkylated 2-pyridones still remains as a hot research topic.
In the continuation of our efforts towards performing new synthetic methods for a wide variety of heterocycles under green conditions [36,37,38,39,40,41,42,43,44,45]. We report a general and efficient microwave-assisted one-pot three-component synthesis of a series of dense substituted N-alkylated 2-pyridones, utilizing malononitrile, a wide range of aromatic as well as heteroaromatic aldehydes and variety of N-alkyl-2-cyanoacetamides as building blocks. To the best of our knowledge, there are no reports in the literature on the synthesis of these compounds. Herein, we also report our experimental results using both thermal heating and microwave irradiation methods and we have compared our results, which shows the advantage of the microwave irradiation method. The proposed reaction mechanism is also discussed.
2. Results and Discussion
Initially, N-butyl-2-cyanoacetamide (1a), benzaldehyde (2a) and malononitrile (3) were adopted as simple model substrates for studying the multi-component synthesis of 1-alkyl-6-amino-4-aryl(or het)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitriles. Indeed, after experimentation with different solvents, reaction temperatures and base catalysts, we found that the best result was obtained by stirring the solution of N-butyl-2-cyanoacetamide (1a, 4 mmol), benzaldehyde (2a, 4 mmol), and malononitrile (3, 4 mmol) in ethanol (7 mL) in the presence of K2CO3 (4 mmol) under reflux for one hour, whereupon after cooling and neutralization with HCl, a pale yellow solid was crystallized out. The precipitate was filtered, recrystallized from methanol and identified as the 6-amino-1-butyl-2-oxo-4-phenyl-1,2-dihydropyridine-3,5-dicarbonitrile (4a) (70% yield) (Scheme 1) (Table 1). The structure of the product 4a was elucidated with the help of IR, 1H-NMR, 13C-NMR, mass spectral data, and elemental analyses. Its mass spectrum disclosed a molecular ion peak at m/z = 292 (M+) corresponding to the molecular formula C17H16N4O. The 1H-NMR spectrum of 4a contained a triplet for CH3 (δ = 0.92), a multiplet for CH2 (δ = 1.34), a multiplet for CH2 (δ = 1.51), a triplet for N-CH2 (δ = 4.0), a multiplet for 2 × CHAr (δ = 7.48–7.49), a multiplet for 3 CHAr (δ = 7.54–7.55), and a singlet for NH2 (δ = 8.40). The assignment is supported by the IR absorptions at 3435, 3322, 3286, 3176 cm−1 (NH2), 2965, 2929 cm−1 (aliph. CH), 2212 cm−1 (CN), 1647 cm−1 (amide C=O). The proton-decoupled 13C-NMR spectrum of 4a displayed 15 discreet resonances. Characteristic 13C-NMR signals due to C-5 and C-3 appeared at δ = 75.43 and 87.48 ppm, respectively, those of cyano carbons at δ = 115.90, 116.56 ppm and those of the C-6, C-2 and C-4 atoms at δ = 156.22, 159.35 and 160.36 ppm, respectively. All other aldehydes 2b–f reacted analogously with N-alkyl-2-cyanoacetamides 1a–c and malononitrile (3) under the same reaction conditions, leading to the formation of products 4b–q in 65–77% yields as shown in Table 1 (Scheme 1).
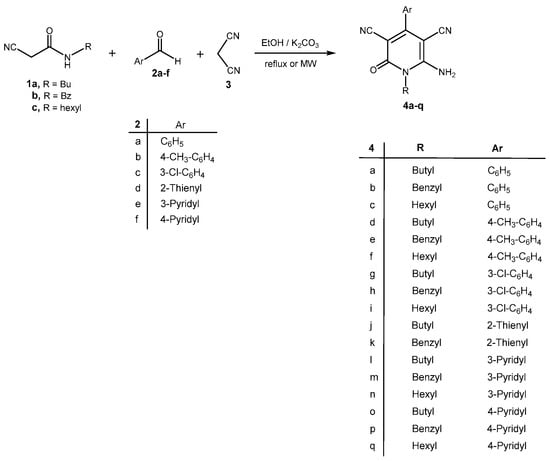
Scheme 1.
One-pot synthesis of 1-alkyl-6-amino-4-aryl(or het)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitriles 4a–q.

Table 1.
Formation of compounds 4a–q under thermal and microwave irradiation.
For the formation of 4, we propose two plausible mechanisms which are shown in Scheme 2. The process expresses a typical cascade reaction in which a Knoevenagel condensation between an aldehyde 2 and malononitrile (3) or N-alkyl-2-cyano-3-phenyl-acrylamide 1 and aldehyde 2 in the presence of K2CO3 as a base catalyst leads to the formation of 2-arylidenemalononitrile (Knoevenagel reagents) 5 and N-alkyl-3-aryl-2-cyano-acrylamide 7, respectively. Then, Michael addition of the active methylene group of 1 to the activated double bond in 5 (or 3 to 7) gives the non-isolable adduct 6, which underwent an in situ cyclization via intramolecular addition of the amide nitrogen atom, as a nucleophile, to the nitrile function to give the intermediate 8. The tautomerisation of the imino (=NH) function to the amino (-NH2) group followed by autoxidation and aromatization afforded the target product 4. Thus, the reaction could proceed via a domino Knoevenagel condensation/Michael addition/intramolecular cyclization/autoxidation reaction sequence.
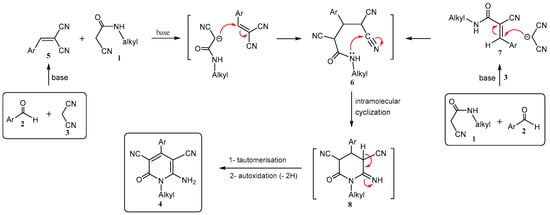
Scheme 2.
Plausible mechanisms for the synthesis of 1-alkyl-6-amino-4-aryl(or het)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitriles 4a–q.
For the investigation of the reaction mechanism, both Knoevenagel reagents 5 and 7 were prepared from the reaction of aldehydes 2 with 3 or 1, respectively, and then these were reacted with active methylene compounds 1 or 3. The products 4 were again formed, but obtained in lower yields compared to our one-pot method, and longer reaction times were also required.
In order to improve the yield and reduce the reaction times, we repeated the reaction of N-butyl-2-cyanoacetamide (1a), benzaldehyde (2a) and malononitrile (3) under microwave irradiation in EtOH in the presence of K2CO3 for 10 min at 90 °C (500 W, 200 rpm), whereupon 4a was isolated in 91% yield. In order to demonstrate the scope of this reaction, a series of substituted aromatic as well as heteroaromatic aldehydes underwent this three-component condensation with different N-alkyl-2-cyanoacetamides and malononitrile by this procedure to give 1-alkyl-6-amino-4-aryl(or het)-2-oxo-1,2-dihydro-pyridine-3,5-dicarbo-nitriles. The results are summarized in Table 1. As is evident from the results shown in Table 1, this method is highly compatible with different aldehydes. Moreover, very good to high yields were also obtained for a heteroaromatic aldehydes when they were employed in this reaction. The microwave method was used in an effort to shorten reaction times and generate high yields. In addition, the analysis of the data in Table 1 indicates that the substituent on the aromatic aldehyde showed slightly different effects on the yields. Reactions of electron rich aromatic aldehydes afforded slightly better yields than electron deficient ones.
3. Experimental
3.1. General Information
All purchased solvents and chemicals were of analytical grade. Melting points were determined on a B-540 melting point apparatus (Büchi, Flawil, Switzerland) and are uncorrected. IR spectra were recorded on a Magna 520 FT-IR spectrophotometer (Nicolet, CA, USA) using potassium bromide disks and signals are reported in cm−1. 1H-NMR and 13C-NMR spectra were recorded on a DPX (850 MHz for 1H-NMR and 213 MHz for 13C-NMR) spectrometer (Bruker, Germany) using DMSO-d6 as a solvent, and TMS as an internal standard; the chemical shifts are given in δ units (ppm). Abbreviations used for NMR signals: s = singlet, d = doublet, t = triplet, and m = multiplet. Mass spectra were recorded on a Shimadzu (Kanagawa, Japan) mass spectrometer at 70 eV. All microwave irradiation experiments were carried out using a Monowave 300 Microwave Synthesis Reactor (MAS) equipped with a MAS 24 autosampler unit (Anton Paar GmbH, Graz, Austria). All experiments were carried out in 10 mL septum-capped microwave vials at 90 °C (500 W maximum power, 200 rpm). Microanalytical data were obtained from the Microanalytical Data Unit at Cairo University (Cairo, Egypt).
3.2. General Procedure for the Synthesis of 1-Alkyl-6-amino-4-aryl(or het)-2-oxo-1,2-dihydro-pyridine-3,5-dicarbonitriles 4a–q
Method I (∆). A mixture of N-alkyl-2-cyanoacetamides 1a–c (4 mmol), aldehydes 2a–f (4 mmol), malononitrile (3) (4 mmol), and K2CO3 (4 mmol) in refluxing EtOH (7 mL) was stirred for 1–4 h. Upon completion as monitored by TLC, the reaction mixture was cooled and poured into H2O. After neutralization with HCl, the resulting solid was filtered off, washed with H2O, dried and recrystallized from MeOH to give pure products 4a–q.
Method II (μω). A mixture of N-alkyl-2-cyano-acetamides 1a–c (2 mmol), aldehydes 2a–f (2 mmol), malononitrile (3) (2 mmol), K2CO3 (2 mmol), and EtOH (2 mL) in a 10 mL septum-capped microwave vials was irradiated under microwave conditions at 90 °C, 500 W, 200 rpm, for 10–15 min. After completion of the reaction, as indicated by TLC, each vial was de-capped and the contents were left to cool to room temperature. Then, the reaction mixture was worked up as described in method I to give compounds 4a–q. Analytical samples were obtained by recrystallization from MeOH.
6-Amino-1-butyl-2-oxo-4-phenyl-1,2-dihydropyridine-3,5-dicarbonitrile (4a). Pale yellow crystals. M.p. 304–305 °C. IR (KBr) 3435, 3322, 3286, 3176 (NH2), 2965, 2929 (aliph. CH), 2212 (CN), 1647 (amide CO) cm−1. 1H-NMR (DMSO-d6) δ 0.92 (t, 3H, J = 6.8 Hz, CH3), 1.34 (m, 2H, CH2), 1.51 (m, 2H, CH2), 4.0 (t, 2H, J = 7.65 Hz, N-CH2), 7.48–7.49 (m, 2Ar-H), 7.54–7.55 (m, 3Ar-H), 8.40 (s, 2H, NH2). 13C-NMR (DMSO-d6) δ 13.74 (CH2), 19.31 (CH2), 28.38 (CH2), 41.87 (N-CH2), 75.43 (C-5), 87.48 (C-3), 115.90 (CN), 116.56 (CN), 127.98 (2Ar-C), 128.63 (2Ar-C), 130.25 (1Ar-C), 134.63 (1Ar-C), 156.22 (C-6), 159.35 (C-2), 160.36 (C-4). MS: m/z (%) = 293 (M+ + 1, 7), 292 (M+, 27), 276 (27), 275 (81), 250 (26), 237 (18), 236 (100), 235 (12), 209 (18), 208 (18), 180 (9), 165 (9), 77 (10); Anal. Calcd. for C17H16N4O (292.34): C, 69.85; H, 5.52; N, 19.17. Found: C, 69.73; H, 5.43; N, 18.99.
6-Amino-1-benzyl-2-oxo-4-phenyl-1,2-dihydropyridine-3,5-dicarbonitrile (4b). Pale yellow crystals. M.p. 251–252 °C. IR (KBr) 3439, 3318, 3182 (NH2), 3036 (arom. CH), 2961, 2877 (aliph. CH), 2225, 2215 (CN), 1656 (amide CO) cm−1. 1H-NMR (DMSO-d6) δ 5.35 (s, 2H, CH2), 7.25 (d, 2H, J = 7.65 Hz, Ar-H), 7.31 (t, 1H, J = 7.65 Hz, Ar-H), 7.38 (t, 2H, J = 7.65 Hz, Ar-H), 7.55–7.58 (m, 5Ar-H), 8.45 (s, 2H, NH2). 13C-NMR (DMSO-d6) δ 44.77 (N-CH2), 75.72 (C-5), 87.56 (C-3), 115.79 (CN), 116.48 (CN), 126.54 (2Ar-C), 127.45 (1Ar-C), 128.06 (2Ar-C), 128.58 (2Ar-C), 128.65 (2Ar-C), 130.34 (1Ar-C), 134.45 (1Ar-C), 134.61 (1Ar-C), 156.60 (C-6), 159.51 (C-2), 160.89 (C-4). MS: m/z (%) = 327 (M+ + 1, 6), 326 (M+, 25), 325 (8), 92 (8), 91 (100), 77 (3), 65 (15); Anal. Calcd. for C20H14N4O (326.36): C, 73.61; H, 4.32; N, 17.17. Found: C, 73.76; H, 4.40; N, 17.30.
6-Amino-1-hexyl-2-oxo-4-phenyl-1,2-dihydropyridine-3,5-dicarbonitrile (4c). Colorless crystals. M.p. 252–254 °C. IR (KBr) 3436, 3415, 3328, 3284, 3207 (NH2), 2933, 2869 (aliph. CH), 2209 (CN), 1653 (amide CO) cm−1. 1H-NMR (DMSO-d6) δ 0.88 (t, 3H, J = 6.8 Hz, CH3), 1.28–1.35 (m, 6H, 3CH2), 1.51–1.54 (m, 2H, CH2), 3.99 (t, 2H, J = 6.8 Hz, N-CH2), 7.48–7.49 (m, 2H, Ar-H), 7.54–7.55 (m, 3H, Ar-H), 8.41 (s, 2H, NH2). 13C-NMR (DMSO-d6) δ 13.94 (CH3), 22.03 (CH2), 25.59 (CH2), 26.20 (CH2), 31.0 (CH2), 42.13 (N-CH2), 75.40 (C-5), 87.47 (C-3), 115.89 (CN), 116.54 (CN), 127.99 (2Ar-C), 128.63 (2Ar-C), 130.24 (1Ar-C), 134.62 (1Ar-C), 156.20 (C-6), 159.33 (C-2), 160.35 (C-4). MS: m/z (%) = 321 (M+ + 1, 6), 320 (M+, 25), 305 (6), 304 (33), 303 (100), 263 (6), 261 (5), 250 (18), 237 (14), 236 (66), 235 (5), 220 (5), 209 (8), 208 (8), 165 (7), 77 (6), 69 (8), 57 (6), 56 (10), 55 (34); Anal. Calcd. for C19H20N4O (320.40): C, 71.23; H, 6.29; N, 17.49. Found: C, 71.16; H, 6.44; N, 17.38.
6-Amino-1-butyl-2-oxo-4-(p-tolyl)-1,2-dihydropyridine-3,5-dicarbonitrile (4d). Colorless crystals. M.p. 286–288 °C. IR (KBr) 3416, 3338, 3219 (NH2), 2953, 2932, 2873 (aliph. CH), 2205 (CN), 1653 (amide CO) cm−1. 1H-NMR (DMSO-d6) δ 0.91 (t, 3H, J = 6.8 Hz, CH3), 1.32–1.36 (m, 2H, CH2), 1.49–1.53 (m, 2H, CH2), 2.39 (s, 3H, CH3), 4.0 (t, 2H, J = 7.65 Hz, N-CH2), 7.34 (d, 2H, J = 7.65 Hz, Ar-H), 7.38 (d, 2H, J = 8.5 Hz, Ar-H), 8.38 (s, 2H, NH2). 13C-NMR (DMSO-d6) δ 13.72 (CH3), 20.98 (CH2), 21.50 (CH3), 28.38 (CH2), 41.84 (N-CH2), 75.35 (C-5), 87.37 (C-3), 115.99 (CN), 116.64 (CN), 127.97 (1Ar-C), 129.14 (1Ar-C), 130.20 (1Ar-C), 130.72 (1Ar-C), 131.68 (1Ar-C), 140.08 (1Ar-C), 156.20 (C-6), 159.36 (C-2), 160.37 (C-4). MS: m/z (%) = 307 (M+ + 1, 8), 306 (M+, 33), 290 (31), 289 (88), 264 (27), 251 (19), 250 (100), 249 (15), 236 (7), 235 (6), 234 (12), 233 (24), 223 (7), 222 (11), 221 (7), 207 (7), 206 (6), 205 (6), 194 (8), 180 (7), 179 (11), 140 (7), 91 (9), 77 (4), 65 (7), 57 (6), 56 (8), 55 (16); Anal. Calcd. for C18H18N4O (306.37): C, 70.57; H, 5.92; N, 18.29. Found: C, 70.47; H, 6.06; N, 18.22.
6-Amino-1-benzyl-2-oxo-4-(p-tolyl)-1,2-dihydropyridine-3,5-dicarbonitrile (4e). Colorless crystals. M.p. 303.9–305.9 °C. IR (KBr) 3322, 3143 (NH2), 2930, 2875 (aliph. CH), 2224, 2213 (CN), 1657 (amide CO) cm−1. 1H-NMR (DMSO-d6) δ 2.41 (s, 3H, CH3), 5.34 (s, 2H, N-CH2), 7.24 (d, 2H, J = 6.8 Hz, Ar-H), 7.31 (t, 1H, J = 6.8 Hz, Ar-H), 7.38 (t, 4H, J = 7.65 Hz, Ar-H), 7.44 (d, 2H, J = 8.5 Hz, Ar-H), 8.42 (s, 2H, NH2). 13C-NMR (DMSO-d6) δ 21.0 (CH3), 44.73 (CH2), 75.66 (C-5), 87.48 (C-3), 115.89 (CN), 116.58 (CN), 126.53 (2Ar-C), 127.43 (1Ar-C), 128.05 (2Ar-C), 128.58 (2Ar-C), 129.17 (2Ar-C), 131.67 (1Ar-C), 134.48 (1Ar-C), 140.22 (1Ar-C), 156.59 (C-6), 159.53 (C-2), 160.91 (C-4). MS: m/z (%) = 341 (M+ + 1, 7), 340 (M+, 28), 339 (7), 92 (8), 91 (100), 65 (14); Anal. Calcd. for C21H16N4O (340.39): C, 74.10; H, 4.74; N, 16.46. Found: C, 74.16; H, 4.65; N, 16.59.
6-Amino-1-hexyl-2-oxo-4-(p-tolyl)-1,2-dihydropyridine-3,5-dicarbonitrile (4f). Colorless crystals. M.p. 260.4–261.7 °C. IR (KBr) 3416, 3284, 3204 (NH2), 2965, 2927, 2857 (aliph. CH), 2210 (CN), 1652 (amide CO) cm−1. 1H-NMR (DMSO-d6) δ 0.88 (t, 3H, J = 6.8 Hz, CH3), 1.29–1.34 (m, 6H, 3CH2), 1.50–1.53 (m, 2H, CH2), 2.39 (s, 3H, CH3), 3.98 (t, 2H, J = 7.65 Hz, CH2), 7.34 (d, 2H, J = 8.5 Hz, Ar-H), 7.38 (d, 2H, J = 7.65 Hz, Ar-H), 8.37 (br s, 2H, NH2). 13C-NMR (DMSO-d6) δ 13.94 (CH3), 20.98 (CH3), 22.01 (CH2), 25.57 (CH2), 26.19 (CH2), 30.97 (CH2), 42.06 (N-CH2), 75.36 (C-5), 87.32 (C-3), 116.00 (CN), 116.66 (CN), 127.97 (2Ar-C), 129.14 (2Ar-C), 131.69 (1Ar-C), 140.08 (1Ar-C), 156.19 (C-6), 159.36 (C-2), 160.36 (C-4). MS: m/z (%) = 335 (M+ + 1, 8), 334 (M+, 25), 319 (5), 318 (27), 317 (78), 277 (6), 275 (5), 264 (26), 251 (22), 250 (100), 246 (10), 234 (9), 233 (15), 222 (7), 179 (6), 69 (7), 56 (9), 55 (33); Anal. Calcd. for C20H22N4O (334.42): C, 71.83; H, 6.63; N, 16.75. Found: C, 71.90; H, 6.57; N, 16.91.
6-Amino-1-butyl-4-(3-chlorophenyl)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile (4g). Colorless crystals. M.p. 249.6–251.6 °C. IR (KBr) 3415, 3340, 3201 (NH2), 2959, 2872 (aliph. CH), 2213 (CN), 1655 (amide CO) cm−1. 1H-NMR (DMSO-d6) δ 0.92 (t, 3H, J = 6.8 Hz, CH3), 1.32–1.37 (m, 2H, CH2), 1.49–1.53 (m, 2H, CH2), 4.01 (t, 2H, J = 7.65 Hz, N-CH2), 7.46 (d, 1H, J = 6.8 Hz, Ar-H), 7.59 (t, 1H, J = 8.5 Hz, Ar-H), 7.60 (s, 1Ar-H), 7.63 (d, 1H, J = 8.5 Hz, Ar-H), 8.47 (s, 2H, NH2). 13C-NMR (DMSO-d6) δ 13.72 (CH3), 19.29 (CH2), 28.35 (CH2), 41.91 (N-CH2), 75.45 (C-5), 87.54 (C-3), 115.68 (CN), 116.32 (CN), 126.81 (1Ar-C), 127.78 (1Ar-C), 130.14 (1Ar-C), 130.71 (1Ar-C), 133.21 (1Ar-C), 136.61 (1Ar-C), 156.15 (C-6), 158.76 (C-2), 159.18 (C-4). MS: m/z (%) = 328 (M+ + 2, 11), 326 (M+, 33), 312 (11), 311 (37), 310 (34), 309 (100), 286 (8), 284 (23), 272 (29), 271 (18), 270 (87), 269 (8), 243 (16), 242 (10), 207 (13), 199 (6), 180 (15), 165 (7), 68 (5), 57 (10), 56 (15), 55 (24); Anal. Calcd. for C17H15ClN4O (326.78): C, 62.48; H, 4.63; Cl, 10.85; N, 17.15. Found: C, 62.40; H, 4.59; Cl, 10.96; N, 17.21.
6-Amino-1-benzyl-4-(3-chlorophenyl)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile (4h). Pale yellow crystals. M.p. 231.8–233.3 °C. IR (KBr) 3643, 3471, 3331, 3193 (NH2), 3062 (arom. CH), 2987 (aliph. CH), 2225, 2212 (CN), 1661 (amide CO) cm−1. 1H-NMR (DMSO-d6) δ 5.34 (s, 2H, CH2), 7.23 (d, 2H, J = 7.65 Hz, Ar-H), 7.31 (t, 1H, J = 7.65 Hz, Ar-H), 7.38 (t, 2H, J = 7.65 Hz, Ar-H), 7.53 (d, 1H, J = 7.65 Hz, Ar-H), 7.61 (t, 1H, J = 7.65 Hz, Ar-H), 7.64–7.65 (m, 1Ar-H), 7.68 (s, 1Ar-H), 8.50 (br s, 2H, NH2). 13C-NMR (DMSO-d6) δ 44.75 (CH2), 75.77 (C-5), 87.65 (C-3), 115.59 (CN), 116.27 (CN), 126.53 (2Ar-C), 126.84 (1Ar-C), 127.46 (1Ar-C), 127.86 (1Ar-C), 128.56 (2Ar-C), 130.20 (1Ar-C), 130.72 (1Ar-C), 133.21 (1Ar-C), 134.33 (1Ar-C), 136.62 (1Ar-C), 156.52 (C-6), 159.29 (C-2), 159.33 (C-4). MS: m/z (%) = 362 (M+ + 2, 4), 360 (M+ + 12), 92 (8), 91 (100), 65 (13); Anal. Calcd. for C20H13ClN4O (360.80): C, 66.58; H, 3.63; Cl, 9.83; N, 15.53. Found: C, 66.67; H, 3.76; Cl, 9.67; N, 15.46.
6-Amino-4-(3-chlorophenyl)-1-hexyl-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile (4i). Colorless crystals. M.p. 235.9–236.6 °C. IR (KBr) 3423, 3292, 3180 (NH2), 3079 (arom. CH), 2954, 2934, 2869 (aliph. CH), 2214 (CN), 1645 (amide CO) cm−1. 1H-NMR (DMSO-d6) δ 0.88 (t, 3H, J = 6.8 Hz, CH3), 1.28–1.35 (m, 6H, 3CH2), 1.50–1.53 (m, 2H, CH2), 3.99 (t, 2H, J = 7.65 Hz, N-CH2), 7.46 (d, 1H, J = 7.6 Hz, Ar-H), 7.58 (d, 1H, J = 7.65 Hz, Ar-H), 7.60 (s, 1Ar-H), 7.62–7.63 (m, 1Ar-H), 8.46 (br s, 2H, NH2). 13C-NMR (DMSO-d6) δ 13.95 (CH3), 22.02 (CH2), 25.55 (CH2), 26.16 (CH2), 30.98 (CH2), 42.13 (N-CH2), 75.44 (C-5), 87.53 (C-3), 115.67 (CN), 116.32 (CN), 126.8 (1Ar-C), 127.76 (1Ar-C), 130.14 (1Ar-C), 130.71 (1Ar-C), 133.20 (1Ar-C), 136.61 (1Ar-C), 156.13 (C-6), 158.75 (C-2), 159.17 (C-4). MS: m/z (%) = 356 (M+ + 2, 9), 354 (M+, 26), 340 (11), 339 (35), 338 (32), 337 (94), 297 (8), 286 (8), 284 (24), 273 (7), 272 (34), 271 (23), 270 (100), 269 (7), 243 (11), 242 (7), 180 (8), 69 (12), 56 (19), 55 (51); Anal. Calcd. for C19H19ClN4O (354.84): C, 64.31; H, 5.40; Cl, 9.99; N, 15.79. Found: C, 64.42; H, 5.47; Cl, 10.14; N, 15.71.
6-Amino-1-butyl-2-oxo-4-(thiophen-2-yl)-1,2-dihydropyridine-3,5-dicarbonitrile (4j). Yellow crystals. M.p. 264.4–265.9 °C. IR (KBr) 3408, 3327, 3285, 3223 (NH2), 2959, 2940, 2874 (aliph. CH), 2207 (CN), 1634 (amide CO) cm−1. 1H-NMR (DMSO-d6) δ 0.91 (t, 3H, J = 6.8 Hz, CH3), 1.33 (m, 2H, CH2), 1.51 (m, 2H, CH2), 3.99 (t, 2H, J = 7.65, N-CH2), 7.26 (dd, 1H, J = 3.4, 3.4 Hz, thiophene-H), 7.51 (dd, J = 1.7, 0.85 Hz, thiophene-H), 7.91 (dd, J = 1.7, 1.7 Hz, thiophene-H), 8.41 (br s, 2H, NH2). 13C-NMR (DMSO-d6) δ 13.73 (CH3), 19.3 (CH2), 28.31 (CH2), 41.95 (N-CH2), 75.37 (C-5), 87.42 (C-3), 116.04 (CN), 116.64 (CN), 127.72 (thiophene-C), 130.32 (thiophene-C), 130.79 (thiophene-C), 133.37 (thiophene-C), 152.45 (C-6), 156.31 (C-2), 159.30 (C-4). MS: m/z (%) = 299 (M+ + 1, 8), 298 (M+, 34), 283 (7), 282 (27), 281 (81), 269 (7), 256 (24), 244 (6), 243 (18), 242 (100), 241 (7), 228 (7), 215 (13), 214 (19), 213 (6), 208 (12), 198 (5), 185 (7), 182 (9), 176 (9), 171 (8), 160 (7), 159 (6), 69 (11), 58 (8), 57 (11), 56 (10), 55 (21); Anal. Calcd. for C15H14N4OS (298.36): C, 60.38; H, 4.73; N, 18.78; S, 10.75. Found: C, 60.30; H, 4.68; N, 18.89; S, 10.89.
6-Amino-1-benzyl-2-oxo-4-(thiophen-2-yl)-1,2-dihydropyridine-3,5-dicarbonitrile (4k). Pale yellow crystals. M.p. 236.8–238.8 °C. IR (KBr) 3444, 3303, 3215 (NH2), 3102 (arom. CH), 2209 (CN), 1660 (amide CO) cm−1. 1H-NMR (DMSO-d6) δ 5.32 (s, 2H, CH2), 7.22 (d, 2H, J = 6.8 Hz, Ar-H), 7.28 (dd, J = 3.4, 3.4 Hz, thiophene-H), 7.31 (t, 1H, J = 7.65 Hz, Ar-H), 7.37 (t, 2H, J = 7.65 Hz, Ar-H), 7.56 (dd, 1H, J = 1.7, 0.85 Hz, thiophene-H), 7.94 (dd, 1H, J = 1.7, 1.7 Hz, thiophene-H), 8.45 (br s, 2H, NH2). 13C-NMR (DMSO-d6) δ 44.85 (CH2), 75.63 (C-5), 87.52 (C-3), 115.94 (CN), 116.57 (CN), 126.48 (2Ar-C), 127.44 (1Ar-C), 127.75 (thiophene-C), 128.59 (2Ar-C), 130.51 (thiophene-C), 131.0 (thiophene-C), 133.32 (thiophene-C), 134.37 (1Ar-C), 153.0 (C-6), 156.68 (C-2), 159.44 (C-4). MS: m/z (%) = 333 (M+ + 1, 7), 332 (M+, 31), 92 (8), 91 (100), 65 (17); Anal. Calcd. for C18H12N4OS (332.38): C, 65.05; H, 3.64; N, 16.86; S, 9.65. Found: C, 65.15; H, 3.70; N, 16.98; S, 9.4.
6-Amino-1-butyl-2-oxo-4-(pyridin-3-yl)-1,2-dihydropyridine-3,5-dicarbonitrile (4l). Colorless powder. M.p. 236.6–238.5 °C. IR (KBr) 3509, 3382, 3336 (NH2), 3068 (arom. CH), 2958, 2866 (aliph. CH), 2214, 2193 (CN), 1640 (amide CO) cm−1. 1H-NMR (DMSO-d6) δ 0.92 (t, 3H, J = 7.65, CH3), 1.32–1.37 (m, 2H, CH2), 1.50–1.53 (m, 2H, CH2), 4.01 (t, 2H, J = 7.65, CH2), 7.60 (ddd, 1H, J = 6, 6, 0.85 Hz, pyridine-H), 7.97–7.98 (m, 1H, pyridine-H), 8.50 (br s, 2H, NH2), 8.70 (dd, J = 3.40, 0.85 Hz, pyridine-H), 8.75 (dd, J = 6, 0.85 Hz, pyridine-H). MS: m/z (%) = 294 (M+ + 1, 8), 293 (M+, 28), 277 (31), 276 (100), 251 (14), 238 (11), 237 (46), 221 (5), 210 (5), 209 (21), 182 (5), 155 (5), 79 (5), 78 (5), 57 (12), 56 (11), 55 (18); Anal. Calcd. for C16H15N5O (293.33): C, 65.52; H, 5.15; N, 23.88. Found: C, 65.44; H, 5.20; N, 24.05.
6-Amino-1-benzyl-2-oxo-4-(pyridin-3-yl)-1,2-dihydropyridine-3,5-dicarbonitrile (4m). Colorless powder. M.p. 164.4–166.5 °C. IR (KBr) 3527, 3380, 3267, 3114 (NH2), 2219 (CN), 1635 (amide CO) cm−1. 1H-NMR (DMSO-d6) δ 5.34 (s, 2H, CH2), 7.24 (d, 2H, J = 6.8 Hz, Ar-H), 7.31 (t, 1H, J = 6.8 Hz, Ar-H), 7.38 (t, 2H, J = 7.65 Hz, Ar-H), 7.62 (dd, 1H, J = 7.65, 5.1 Hz, pyridine-H), 8.03 (d, 1H, J = 8.5 Hz, pyridine-H), 8.54 (br s, 2H, NH2), 8.76 (m, 2H, pyridine-H). 13C-NMR (DMSO-d6) δ 44.81 (CH2), 75.92 (C-5), 87.86 (C-3), 115.65 (CN), 116.34 (CN), 123.62 (1Ar-C), 126.56 (1Ar-C), 127.50 (1Ar-C), 128.59 (2Ar-C), 130.85 (1Ar-C), 134.32 (pyridine-C), 136.09 (pyridine-C), 148.14 (pyridine-C), 151.28 (pyridine-C), 156.58 (C-6),157.68 (C-2), 159.32 (C-4). MS: m/z (%) = 328 (M+ + 1, 6), 327 (M+, 25), 92 (8), 91 (100), 65 (15); Anal. Calcd. for C19H13N5O (327.35): C, 69.71; H, 4.00; N, 21.39. Found: C, 69.82; H, 3.94; N, 21.35.
6-Amino-1-hexyl-2-oxo-4-(pyridin-3-yl)-1,2-dihydropyridine-3,5-dicarbonitrile (4n). Colorless powder. M.p. 222.9–224.3 °C. IR (KBr) 3364, 3338, 3214 (NH2), 2957, 2938, 2859 (aliph. CH), 2220, 2207 (CN), 1648 (amide CO) cm−1. 1H-NMR (DMSO-d6) δ 0.88 (t, 3H, J = 6.8 Hz, CH3), 1.32 (m, 6H, 3CH2), 1.52 (m, 2H, CH2), 3.99 (t, 2H, J = 7.65 Hz, N-CH2),7.60 (dd, 1H, J = 7.65, 5.1 Hz, pyridine-H), 7.98 (m, 1H, pyridine-H), 8.50 (br s, 2H, NH2), 8.70 (d, J = 1.7 Hz, pyridine-H), 8.75 (dd, J = 5.1, 1.7 Hz, pyridine-H). 13C-NMR (DMSO-d6) δ 13.97 (CH3), 22.03 (CH2), 25.58 (CH2), 26.16 (CH2), 30.99 (CH2), 42.18 (N-CH2), 75.62 (C-5), 87.75 (C-3), 115.75 (CN), 116.41 (CN), 123.62 (pyridine-C), 130.85 (pyridine-C), 136.03 (pyridine-C), 148.09 (pyridine-C), 151.21 (pyridine-C), 156.20 (C-6), 157.13 (C-2), 159.17 (C-4). MS: m/z (%) = 322 (M+ + 1, 8), 321 (M+, 22), 306 (5), 305 (32), 304 (99), 264 (9), 262 (7), 251 (25), 238 (31), 237 (100), 236 (7), 223 (8), 222 (8), 221 (9), 210 (9), 209 (34), 195 (7), 194 (6), 182 (6), 181 (6), 167 (6), 166 (6), 155 (6), 78 (6), 69 (12), 67 (5), 57 (5), 56 (23), 55 (54); Anal. Calcd. for C18H19N5O (321.38): C, 67.27; H, 5.96; N, 21.79. Found: C, 67.36; H, 5.91; N, 21.93.
6-Amino-1-butyl-2-oxo-4-(pyridin-4-yl)-1,2-dihydropyridine-3,5-dicarbonitrile (4o). Brownish powder. M.p. 313.4–315 °C. IR (KBr) 3358, 3282 (NH2), 3090 (arom. CH), 2973, 2958, 2939, 2864 (aliph. CH), 2230, 2209 (CN), 1663 (amide CO) cm−1. 1H-NMR (DMSO-d6) δ 0.91 (t, 3H, J = 7.65 Hz, CH3), 1.34 (m, 2H, CH2), 1.51 (m, 2H, CH2), 4.0 (t, 2H, J = 7.65 Hz, N-CH2), 7.52 (dd, 2H, J = 6, 1.7 Hz, pyridine-H), 8.53 (br s, 2H, NH2), 8.79 (dd, 2H, J = 6, 1.7 Hz, pyridine-H). 13C-NMR (DMSO-d6) δ 13.73 (CH3), 19.29 (CH2), 28.30 (CH2), 41.96 (N-CH2), 74.92 (C-5), 87.06 (C-3), 115.43 (CN), 116.08 (CN), 122.50 (2 pyridine-C), 142.43 (pyridine-C), 150.15 (pyridine-C), 150.18 (pyridine-C), 156.23 (C-6), 157.77 (C-2), 159.10 (C-4). MS: m/z (%) = 294 (M+ + 1, 9), 293 (M+, 21), 277 (26), 276 (84), 264 (5), 251 (26), 238 (21), 237 (100), 236 (7), 223 (7), 221 (6), 210 (21), 209 (18), 182 (6), 181 (5), 155 (5), 57 (9), 56 (14), 55 (16); Anal. Calcd. for C16H15N5O (293.33): C, 65.52; H, 5.15; N, 23.88. Found: C, 65.69; H, 5.08; N, 23.99.
6-Amino-1-benzyl-2-oxo-4-(pyridin-4-yl)-1,2-dihydropyridine-3,5-dicarbonitrile (4p). Brownish powder. M.p. 276.3–278.4 °C. IR (KBr) 3357, 3269 (NH2), 3088 (arom. CH), 2234, 2208 (CN), 1653 (amide CO) cm−1. 1H-NMR (DMSO-d6) δ 5.32 (s, 2H, CH2), 7.25 (d, 2H, J = 7.65 Hz, Ar-H), 7.31 (t, 1H, J = 7.65 Hz, Ar-H), 7.38 (t, 2H, J = 7.65 Hz, Ar-H), 7.57 (d, 2H, J = 5.1 Hz, pyridine-H), 8.79 (d, J = Hz, 2 pyridine-H). 13C-NMR (DMSO-d6) δ 44.71 (CH2), 75.56 (C-5), 86.38 (C-3), 115.65 (CN), 11629 (CN), 122.57 (2 pyridine-C), 126.64 (2 Ar-C), 127.41 (1 Ar-C), 128.54 (2 Ar-C), 134.64 (1 Ar-C), 142.61 (pyridine-C), 150.16 (2 pyridine-C), 156.71 (C-6), 158.0 (C-2), 159.50 (C-4). MS: m/z (%) = 328 (M+ + 1, 6), 327 (M+, 25), 92 (8), 91 (100), 65 (15); Anal. Calcd. for C19H13N5O (327.35): C, 69.71; H, 4.00; N, 21.39. Found: C, 69.59; H, 4.07; N, 21.21.
6-Amino-1-hexyl-2-oxo-4-(pyridin-4-yl)-1,2-dihydropyridine-3,5-dicarbonitrile (4q). Brownish powder. M.p. 327.5–328.7 °C. IR (KBr) 3352, 3280 (NH2), 2956, 2930, 2854 (aliph. CH), 2227, 2208 (CN), 1656 (amide CO) cm−1. 1H-NMR (DMSO-d6) δ 0.88 (t, 3H, J = 7.65 Hz, CH3), 1.30 (m, 6H, 3 CH2), 1.52 (m, 2H, CH2), 3.99 (t, 2H, J = 7.65 Hz, N-CH2), 7.52 (dd, 2H, J = 6, 1.7 Hz, pyridine-H), 8.53 (br s, 2H, NH2), 8.78 (dd, 2H, J = 6, 1.7 Hz, pyridine-H). 13C-NMR (DMSO-d6) δ 13.96 (CH3), 22.02 (CH2), 25.56 (CH2), 26.12 (CH2), 30.97 (CH2), 42.2 (N-CH2), 74.91 (C-5), 87.07 (C-3), 115.43 (CN), 116.08 (CN), 122.50 (2 pyridine-C), 142.43 (pyridine-C), 150.16 (1 pyridine-C), 150.18 (1 pyridine-C), 156.22 (C-6), 157.77 (C-2), 159.09 (C-4). MS: m/z (%) = 322 (M+ + 1, 6), 321 (M+, 16), 305 (23), 304 (75), 264 (7), 262 (6), 251 (25), 238 (27), 237 (100), 210 (12), 209 (12), 69 (10), 56 (20), 55 (41); Anal. Calcd. for C18H19N5O (321.38): C, 67.27; H, 5.96; N, 21.79. Found: C, 67.21; H, 5.92; N, 21.69.
4. Conclusions
In summary, we have developed a novel, facile, efficient, rapid, and environmentally friendly approach for the one-pot multicomponent synthesis of new diversely substituted 6-amino-2-oxo-pyridine-3,5-dicarbonitrile derivatives from simple and readily available diverse aldehydes, various N-alkyl-2-cyanoacetamides and malononitrile in the presence of K2CO3 under heating or under microwave activation. The ease of work-up, rapid access, general applicability, greenness of procedure and high isolated yields of products make this new strategy a very useful addition to modern synthetic methods and attractive for academic research and potential applications. Further exploration of the reaction scope and synthetic applications of this methodology are currently under studying in our laboratory.
Author Contributions
Ramadan Ahmed Mekheimer wrote the manuscript. Najla Hosain Hassan Alsofyani carried out all experiments and helped in edited the manuscript. Mariam Abdullah Al-Sheikh and Hanadi Yousef Medrasi discussed the IR, NMR and MS data. They also provided conceptual guidance, supervised the project, and helped in edited the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bayat, M.; Nasri, S.; Notash, B. Synthesis of new 3-cyanoacetamide pyrrole and 3-acetonitrile pyrrole derivatives. Tetrahedron 2017, 73, 1522–1527. [Google Scholar] [CrossRef]
- Xin, X.; Wang, Y.; Kumar, S.; Liu, X.; Lin, Y.; Dong, D. Efficient one-pot synthesis of substituted pyridines through multicomponent reaction. Org. Biomol. Chem. 2010, 8, 3078–3082. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Kundu, A.; Pramanik, A. Regioselective synthesis of two types of highly substituted 2-pyridones through similar multicomponent reactions. Tetrahedron Lett. 2012, 53, 3030–3034. [Google Scholar] [CrossRef]
- Dӧmling, A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.A.; Amini-Ghalandarabad, S.; Mesbah, F.; Tasmimi, M.; Izadyar, M.; Fakhari, A.R.; Salimi, A.R. Efficient synthesis of novel spiro[indole-3,6′-pyrano[2,3-d][1,3]-thiazolo[3,2-a]pyrimidine derivatives through an organobase-catalyzed, three-component reaction. Tetrahedron 2015, 71, 2458–2462. [Google Scholar] [CrossRef]
- Hadjebi, M.; Hashtroudi, M.S.; Bijanzadeh, H.R.; Balalaie, S. Novel four-component approach for the synthesis of polyfunctionalized 1,4-dihydropyridines in aqueous media. Helv. Chim. Acta 2011, 94, 382–388. [Google Scholar] [CrossRef]
- Azzam, S.H.S.; Pasha, M.A. Microwave-assisted, mild, facile, and rapid one-pot three-component synthesis of some novel pyrano[2,3-d]pyrimidine-2,4,7-triones. Tetrahedron Lett. 2012, 53, 7056–7059. [Google Scholar] [CrossRef][Green Version]
- Kappe, C.O.; Dallinger, D. Controlled microwave heating in modern organic synthesis: Highlights from the 2004–2008 literature. Mol. Divers. 2009, 13, 71–193. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Shi, F.; Tu, S.J. Microwave-assisted multicomponent reactions in the heterocyclic chemistry. Curr. Org. Chem. 2010, 14, 357–378. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Gil, S.; Parra, M. New synthetic methods to 2-pyridone rings. Curr. Org. Chem. 2005, 9, 1757–1779. [Google Scholar] [CrossRef]
- Henry, G.D. De novo synthesis of substituted pyridines. Tetrahedron 2004, 60, 6043–6061. [Google Scholar] [CrossRef]
- Khaksar, S.; Yaghoobi, M. A concise and versatile synthesis of 2-amino-3-cyanopyridine derivatives in 2,2,2-trifluoroethanol. J. Fluorine Chem. 2012, 142, 41–44. [Google Scholar] [CrossRef]
- Movassaghi, M.; Hill, M.D.; Ahmad, O.K. Direct synthesis of pyridine derivatives. J. Am. Chem. Soc. 2007, 129, 10096–10097. [Google Scholar] [CrossRef] [PubMed]
- Teague, S.J. Synthesis of heavily substituted 2-aminopyridines by displacement of a 6-methylsulfinyl group. J. Org. Chem. 2008, 73, 9765–9766. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, Q.; Yuan, H.; Liu, Q. Temperature-controlled synthesis of substituted pyridine derivatives via the [5C + 1N] annulation of 1,1-bisalkylthio-1,4-pentanedienes and ammonium acetate. J. Org. Chem. 2008, 73, 2442–2445. [Google Scholar] [CrossRef] [PubMed]
- Mobinikhaledi, A.; Asadbegi, S.; Bodaghifard, M.A. Convenient, multicomponent, one-pot synthesis of highly substituted pyridines under solvent-free conditions. Synth. Commun. 2016, 19, 1605–1611. [Google Scholar] [CrossRef]
- Reddy, T.R.; Mutter, R.; Heal, W.; Guo, K.; Gillet, V.J.; Pratt, S.; Chen, B. Library design, synthesis, and screening: Pyridine dicarbonitriles as potential prion disease therapeutics. J. Med. Chem. 2006, 49, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Cocco, M.T.; Congiu, C.; Lilliu, V.; Onnis, V. Synthesis and antiproliferative activity of 2,6-dibenzylamino-3,5-dicyanopyridines on human cancer cell lines. Eur. J. Med. Chem. 2005, 40, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- May, B.C.H.; Zorn, J.A.; Witkop, J.; Sherrill, J.; Wallace, A.C.; Legname, G.; Prusiner, S.B.; Cohen, F.E. Structure-activity relationship study of prion inhibition by 2-amino-pyridine-3,5-dicarbonitrile-based compounds: Parallel synthesis, bioactivity, and in vitro Pharmacokinetics. J. Med. Chem. 2007, 50, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Beukers, M.W.; Chang, L.C.W.; Von Frijtag Drabbe Künzel, J.K.; Mulder-Krieger, T.; Spanjersberg, R.F.; Brussee, J.; Ijzerman, A.P. New, non-adenosine, high-potency agonists for the human adenosine A2B receptor with an improved selectivity profile compared to the reference agonist N-ethylcarboxamidoadenosine. J. Med. Chem. 2004, 47, 3707–3709. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.C.W.; Von Frijtag Drabbe Künzel, J.K.; Mulder-Krieger, T.; Spanjersberg, R.F.; Roerink, S.F.; van den Hout, G.; Beukers, M.W.; Brussee, J.; Ijzerman, A.P. A Series of ligands displaying a remarkable agonistic-antagonistic profile at the adenosine A1 receptor. J. Med. Chem. 2005, 48, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Chioua, M.; Alvarez Perez, M.; Soriano Santamaria, E.; Valderas Cortina, C.; Marco Contelles, J.L.; De Los Rios, S.C.; Romero Martinez, A.; Villarroya Sanchez, M.; Garcia Lopez, M.; et al. 2-Amino-3,5-dicyanopyridine and 2-Chloro-3,5-dicyanopyridine Derivatives as Cholinesterase Enzymes Inhibitors and with Neuroprotective Capacity. Spanish Patent ES 2,365,233 A1, 27 September 2011. [Google Scholar]
- Chen, H.; Zhang, W.; Tam, R.; Raney, A.K. Novel Heterocyclic Compounds as ikk2 Inhibitors with Anti-Hbv Activity. Patent No. WO 2005,058,315 A1, 30 June 2005. [Google Scholar]
- Levy, S.B.; Alekshun, M.N.; Podlogar, B.L.; Ohemeng, K.; Verma, A.K.; Warchol, T.; Bhatia, B.; Bowser, T.; Grier, M. Transcription Factor Modulating Compounds and Methods of Use Thereof. US Patent 20,050,124,678 A1, 4 May 2005. [Google Scholar]
- Harada, H.; Watanuki, S.; Takuwa, T.; Kawaguchi, K.; Okazaki, T.; Hirano, Y.; Saitoh, C. Medicin Comprising Dicyanopyridine Derivative. PCT Int. Appl. WO 2,002,006,237 A1, 24 January 2002. [Google Scholar]
- Perrier, V.; Wallace, A.C.; Kaneko, K.; Safar, J.; Prusiner, S.B.; Cohen, F.E. Mimicking dominant negative inhibition of prion replication through structure-based drug design. Proc. Nat. Acad. Sci. USA 2000, 97, 6073–6078. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Mutter, R.; Heal, W.; Reddy, T.R.K.; Cope, H.; Pratt, S.; Thompson, M.J.; Chen, B. Synthesis and evaluation of a focused library of pyridine dicarbonitriles against prion disease. Eur. J. Med. Chem. 2008, 43, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xue, S.; Yang, Y.; Feng, J.; Liu, P.; Zhang, Y.; Zhu, J.; Xu, Z.; Hall, A.; Zhao, B.; et al. Regioselectivity and mechanism of synthesizing N-substituted 2-pyridones and 2-substituted pyridines via metal-free C-O and C-N bond-cleaving of oxazoline[3,2-a]pyridiniums. Sci. Rep. 2017, 7, 41287. [Google Scholar] [CrossRef] [PubMed]
- Sorbera, L.A.; Castaner, J. Laquinimod. Treatment of multiple sclerosis, Immuno-modulator. Drugs Future 2003, 28, 1059–1063. [Google Scholar] [CrossRef]
- Ferraz, A.C.; Angelucci, M.E.M.; Da Costa, M.L.; Batista, I.R.; De Olivera, B.H.; Da Cunha, C. Pharmacological evaluation of ricinine, a central nervous system stimulant isolated from Ricinus communis. Pharmacol. Biochem. Behav. 1999, 63, 367–375. [Google Scholar] [CrossRef]
- Tkachova, V.P.; Gorobets, N.Y.; Tkachov, R.P.; Musatov, V.I.; Dyachenko, V.D. Competing transformations of 2-cyanoacetanilides in the reactions with derivatives of ethoxymethylenemalonic acid. Arkivoc 2012, 6, 398–411. [Google Scholar]
- Ando, M.; Wada, T.; Sato, N. Facile one-pot synthesis of N-difluoromethyl-2-pyridone derivatives. Org. Lett. 2006, 8, 3805–3808. [Google Scholar] [CrossRef] [PubMed]
- Conreaux, D.; Bossharth, E.; Monteiro, N.; Desbordes, P.; Balme, G. A practical procedure for the selective N-alkylation of 4-alkoxy-2-pyridones and its use in a sulfone-mediated synthesis of N-methyl-4-methoxy-2-pyridone. Tetrahedron Lett. 2005, 46, 7917–7920. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.-P.; Huang, L.; You, S.-L. Highly regio- and enantioselective synthesis of N-substituted 2-pyridones: Iridium-catalyzed intermolecular asymmetric allylic amination. Angew. Chem. Int. Ed. 2015, 54, 1873–1876. [Google Scholar] [CrossRef] [PubMed]
- Mekheimer, R.A.; Abdel Hameed, A.M.; Sadek, K.U. Solar thermochemical reactions: Four-component synthesis of polyhydroquinoline derivatives induced by solar thermal energy. Green Chem. 2008, 10, 592–593. [Google Scholar] [CrossRef]
- Mekheimer, R.A.; Ameen, M.A.; Sadek, K.U. Solar thermochemical reactions II: Synthesis of 2-aminothiophenes via Gewald reaction induced by solar thermal energy. Chin. Chem. Lett. 2008, 19, 788–790. [Google Scholar] [CrossRef]
- Mekheimer, R.A.; Sadek, K.U. Microwave-assisted reactions: Three component process for the synthesis of 2-amino-2-chromenes under microwave heating. J. Heterocycl. Chem. 2009, 46, 149–151. [Google Scholar] [CrossRef]
- Al-Zaydi, K.M.; Borik, R.M.; Mekheimer, R.A.; Elnagdi, M.H. Green chemistry: A facile synthesis of polyfunctionally substituted thieno[3,4-c]pyridinones and thieno[3,4-d]pyridazinones under neat reaction conditions. Ultrason. Sonochem. 2010, 17, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Mekheimer, R.A.; Abdel Hameed, A.M.; Mohamed, S.M.; Sadek, K.U. Green, three component highly efficient synthesis of 2-amino-5,6,7,8-tetrahydro-4H-chromen-3-carbonitriles in water at ambient temperature. Green Chem. Lett. Rev. 2010, 3, 161–163. [Google Scholar] [CrossRef]
- Mekheimer, R.A.; Hilmy, N.M.; Abdel Hameed, A.; Dacrory, S.; Sadek, K.U. Simple, three component, highly efficient green synthesis of thiazolo[3,2-a]pyridine derivatives under neat conditions. Synth. Commun. 2011, 41, 2511–2516. [Google Scholar] [CrossRef]
- Mekheimer, R.A.; Al-Zaydi, K.M.; Al-Shamary, A.; Sadek, K.U. Solar thermochemical reactions IV: Unusual reaction of nitrones with acetonitrile derivatives induced by solar thermal energy. Green Sustain. Chem. 2011, 1, 176–181. [Google Scholar] [CrossRef][Green Version]
- Sadek, K.U.; Mekheimer, R.A.; Mohamed, T.M.; Moustafa, M.S.; Elnagdi, M.H. Regioselectivity in the multicomponent reaction of 5-aminopyrazoles, cyclic 1,3-diketones and dimethylformamide dimethylacetal under controlled microwave heating. Beilstein J. Org. Chem. 2012, 8, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.U.; Mekheimer, R.A.; Abdel Hameed, A.M.; Elnahas, F.; Elnagdi, M.H. Green and highly efficient synthesis of 2-arylbenzothiazoles using glycerol without catalyst at ambient temperature. Molecules 2012, 17, 6011–6019. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.U.; Abdel Hameed, A.M.; Mekheimer, R.A.; abd Elmonem, M.; Elnagdi, M.H. Zn (L-proline)2: An efficient and recyclable catalytic system for the asymmetric multicomponent synthesis of 2-amino-4H-chromenes in water under controlled microwave heating. Curr. Microw. Chem. 2016, 3, 227–232. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 4a–q are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).