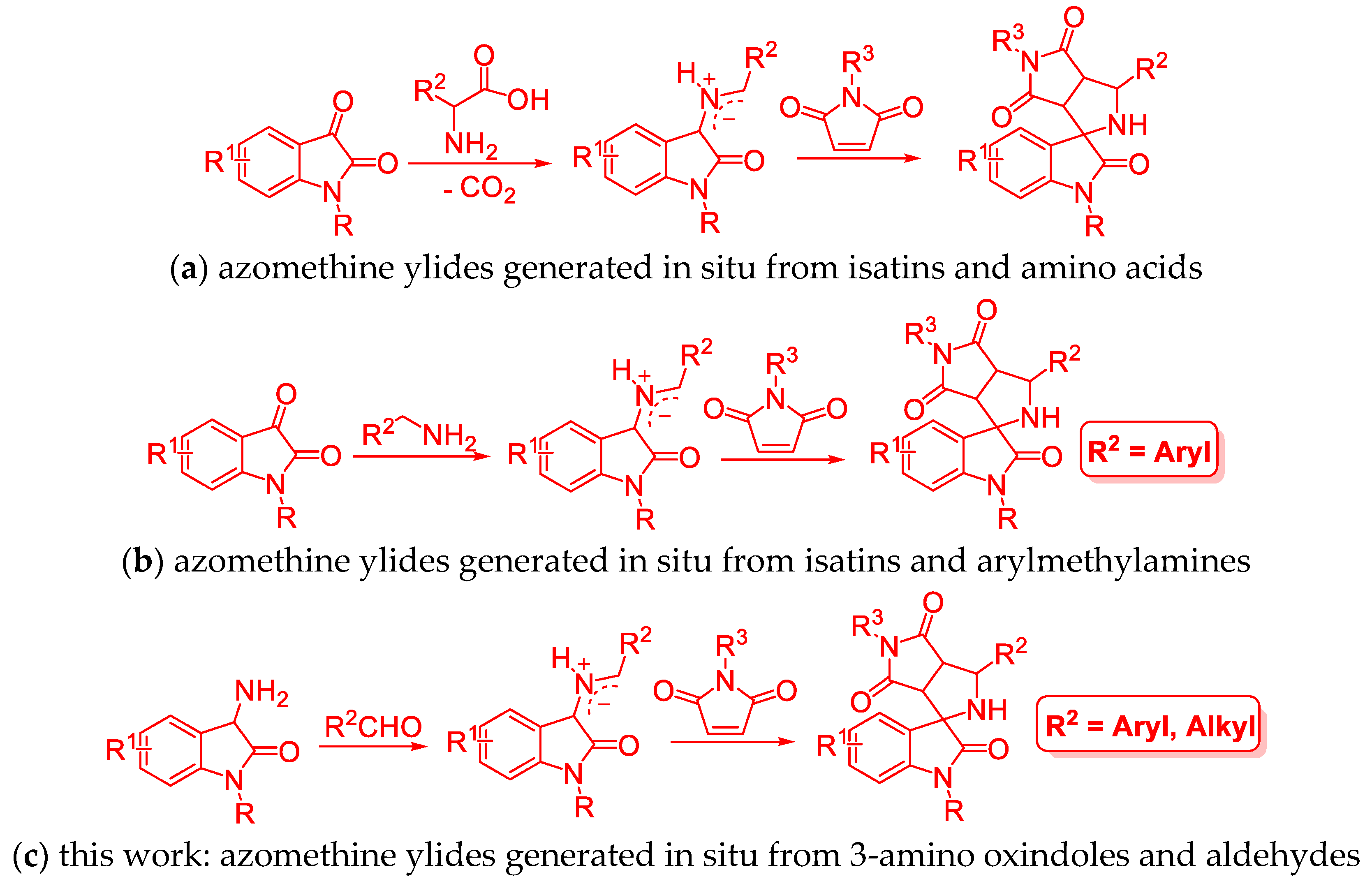
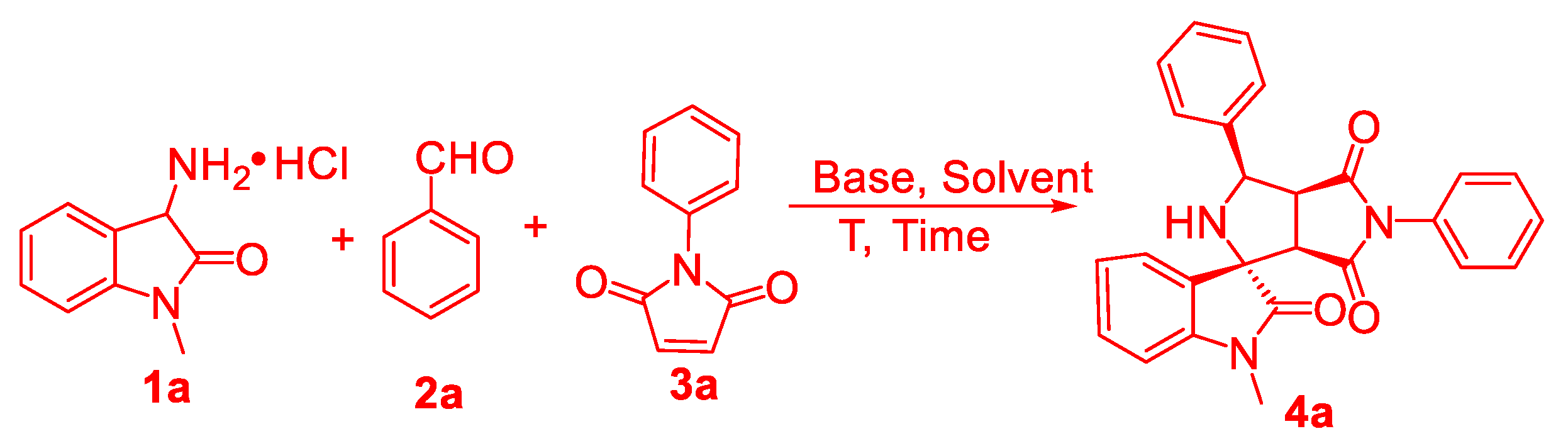
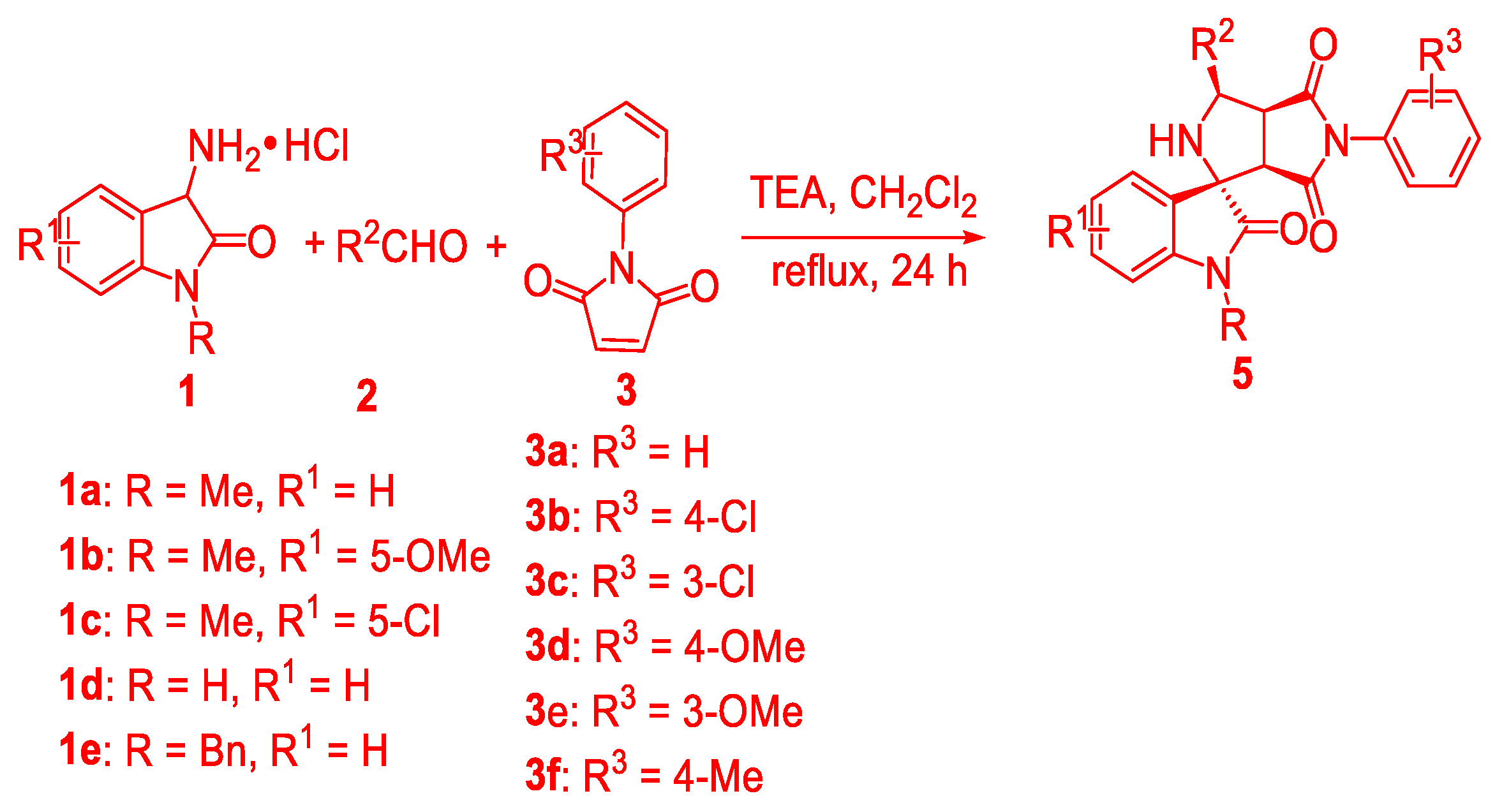
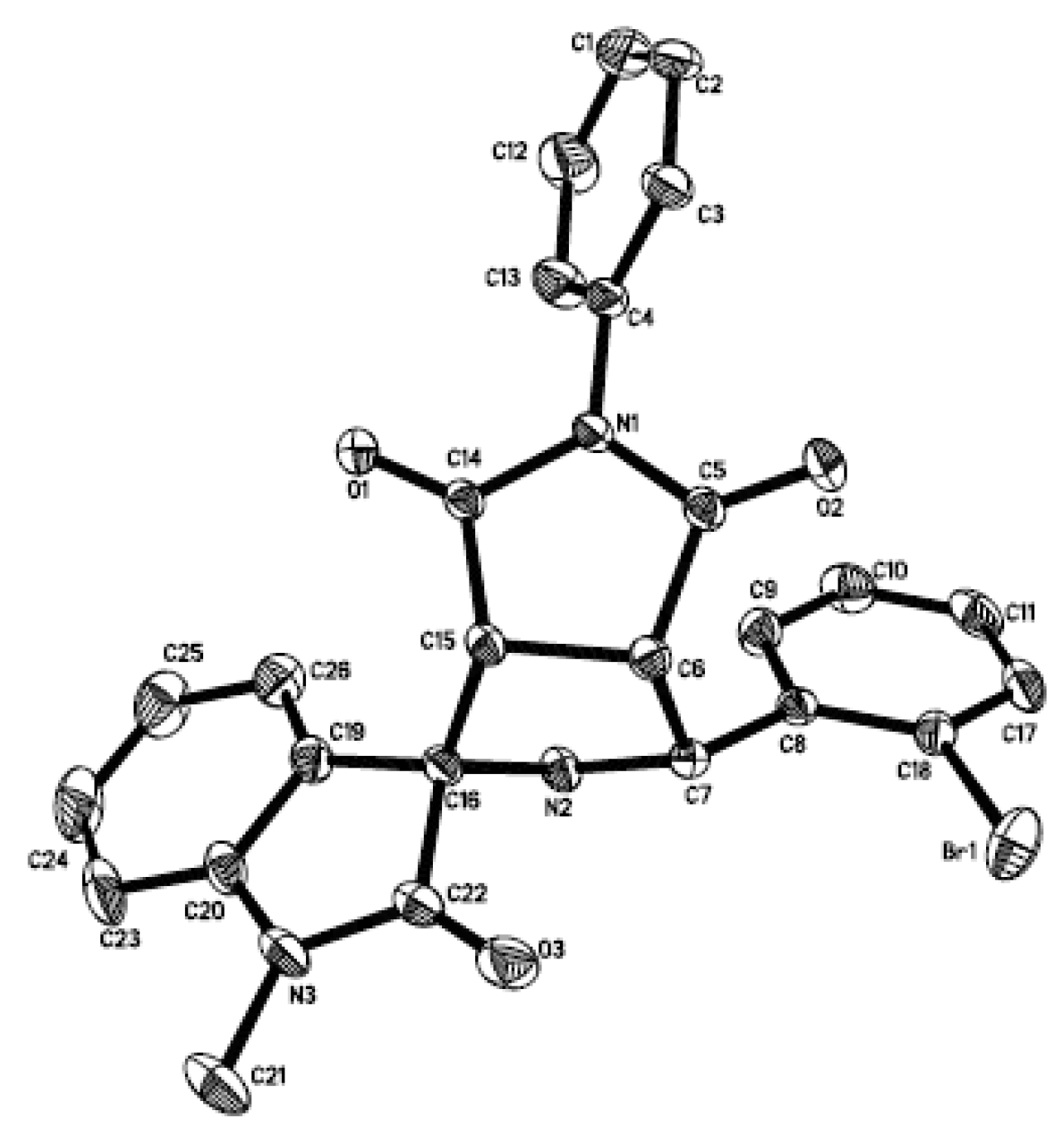
3.2. General Procedure forthe Preparation of Succinimide-Fused Spiro[Pyrrolidine-2,3′-Oxindoles] 4 and 5
3-Amino oxindoles 1 (0.2 mmol), aldehydes 2 (0.2 mmol) and TEA (0.2 mmol) were put into an ordinary test tube equipped with a magnetic stirring bar and then sealed in the air. Then, CH2Cl2 (1 mL) was added. After being stirred at room temperature for 30 min, maleimides 3 (0.22 mmol) and CH2Cl2 (1 mL) were added and the resulting mixture was stirred at reflux for 24 h. The crude reaction mixture was directly purified by flash column chromatography on silica gel (petroleum ether/ethyl acetate = 7:1–3:1) to give the correspondingsuccinimide-fused spiro[pyrrolidine-2,3′-oxindole] products 4 or 5. All the products were confirmed by 1H-NMR, 13C-NMR and HRMS spectroscopic analysis. The diastereomeric ratio was determined by crude NMR analysis.
1-Methyl-3′,5′-diphenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4a). White solid, 71.9 mg, 85% yield. 88:12 dr. 1H-NMR (400 MHz, CDCl3): δ 7.56 (d, J = 7.2 Hz, 2H), 7.48–7.43 (m, 2H), 7.41–7.36 (m, 4H), 7.36–7.31 (m, 2H), 7.24 (d, J = 7.4 Hz, 2H), 7.14 (t, J = 7.6 Hz, 1H), 6.90 (d, J = 7.8 Hz, 1H), 5.83 (d, J = 8.8 Hz, 1H), 4.03 (t, J = 8.4 Hz, 1H), 3.55 (d, J = 7.9 Hz, 1H), 3.25 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.8, 174.1, 173.5, 143.7, 138.0, 130.2, 129.1, 128.5, 128.4, 128.3, 127.3, 127.0, 126.2, 125.4, 122.8, 108.4, 68.0, 60.8, 50.9, 49.5, 26.2; HRMS (ESI): m/z calcd for C26H21NaN3O3+ [M + Na]+ 446.1481, found 446.1493.
3′-(4-Fluorophenyl)-1-methyl-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4b). White solid, 70.6 mg, 80% yield. 81:19 dr. 1H-NMR (400 MHz, CDCl3): δ 7.51 (dd, J = 7.7, 5.8 Hz, 2H), 7.45 (t, J = 7.6 Hz, 2H), 7.38 (dd, J = 13.7, 7.0 Hz, 2H), 7.32 (d, J = 7.5 Hz, 1H), 7.22 (d, J = 7.6 Hz, 2H), 7.12 (t, J = 7.5 Hz, 1H), 7.06 (t, J = 8.4 Hz, 2H), 6.89 (d, J = 7.8 Hz, 1H), 5.80 (d, J = 8.7 Hz, 1H), 3.97 (t, J = 8.3 Hz, 1H), 3.53 (d, J = 7.9 Hz, 1H), 3.23 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.7, 174.2, 173.5, 143.7, 130.3, 129.2, 128.9, 128.8, 128.6, 126.9, 126.1, 125.3, 122.8, 115.4, 115.2, 108.5, 67.9, 60.0, 50.8, 49.4, 26.2; HRMS (ESI): m/z calcd for C26H20FN3NaO3+ [M + Na]+ 442.1567, found 442.1579.
3′-(3-Fluorophenyl)-1-methyl-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4c). White solid, 61.8 mg, 70% yield. 62:38 dr. 1H-NMR (400 MHz, CDCl3): δ 7.45 (t, J = 7.6 Hz, 2H), 7.39 (dd, J = 15.4, 7.8 Hz, 3H), 7.32 (t, J = 9.0 Hz, 3H), 7.22 (d, J = 7.6 Hz, 2H), 7.13 (t, J = 7.5 Hz, 1H), 7.00 (dd, J = 10.4, 5.5 Hz, 1H), 6.89 (d, J = 7.8 Hz, 1H), 5.81 (d, J = 8.8 Hz, 1H), 4.01 (t, J = 8.4 Hz, 1H), 3.54 (d, J = 7.9 Hz, 1H), 3.24 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.7, 174.0, 173.4, 143.7, 130.3, 129.9, 129.2, 128.7, 127.0, 126.2, 123.2, 122.9, 115.1, 114.1, 113.9, 108.5, 67.9, 60.1, 50.8, 49.4, 26.2; HRMS (ESI): m/z calcd for C26H20FN3NaO3+[M + Na]+ 442.1567, found 442.1576.
3′-(2-Fluorophenyl)-1-methyl-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4d). White solid, 79.4 mg, 90% yield. 92:8 dr. 1H-NMR (400 MHz, CDCl3): δ 7.57 (t, J = 7.1 Hz, 1H), 7.43–7.38 (m, 4H), 7.30 (d, J = 5.9 Hz, 1H), 7.20 (d, J = 7.6 Hz, 2H), 7.16–7.08 (m, 4H), 6.89 (d, J = 7.8 Hz, 1H), 6.01 (d, J = 8.4 Hz, 1H), 4.13 (t, J = 8.2 Hz, 1H), 3.61 (d, J = 8.0 Hz, 1H), 3.24 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.6, 174.0, 173.5, 143.8, 130.3, 129.5, 128.6, 127.0, 126.9, 126.7, 126.2, 122.9, 115.2, 115.0, 108.5, 67.8, 54.7, 51.1, 48.0, 26.2; HRMS (ESI): m/z calcd for C26H20FN3NaO3+ [M + Na]+ 442.1567, found 442.1573.
3′-(4-Chlorophenyl)-1-methyl-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4e). White solid, 72.2 mg, 79% yield. 81:19 dr. 1H-NMR (400 MHz, CDCl3): δ 7.47 (t, J = 8.4 Hz, 5H), 7.38–7.30 (m, 4H), 7.24 (d, J = 7.5 Hz, 2H), 7.14 (t, J = 7.5 Hz, 1H), 6.90 (d, J = 7.8 Hz, 1H), 5.80 (d, J = 8.7 Hz, 1H), 4.00 (t, J = 8.3 Hz, 1H), 3.56 (d, J = 7.9 Hz, 1H), 3.25 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.7, 174.0, 173.4, 143.7, 136.5, 133.9, 131.7, 130.3, 129.2, 128.7, 128.6, 126.9, 126.1, 125.3, 122.9, 108.5, 67.9, 60.1, 50.8, 49.4, 26.2; HRMS (ESI): m/z calcd for C26H20ClN3NaO3+ [M + Na]+ 480.1091, found 480.1090.
3′-(3-Chlorophenyl)-1-methyl-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4f). White solid, 64.9 mg, 71% yield. 76:24 dr. 1H-NMR (400 MHz, CDCl3): δ 7.58 (s, 1H), 7.47 (t, J = 7.5 Hz, 2H), 7.41 (t, J = 7.5 Hz, 3H), 7.34 (dd, J = 14.0, 7.1 Hz, 3H), 7.24 (d, J = 7.6 Hz, 2H), 7.14 (t, J = 7.5 Hz, 1H), 6.90 (d, J = 7.8 Hz, 1H), 5.80 (d, J = 8.8 Hz, 1H), 4.03 (t, J = 8.4 Hz, 1H), 3.54 (d, J = 7.9 Hz, 1H), 3.25 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.6, 174.0, 173.3, 143.7, 140.3, 134.4, 130.3, 129.7, 129.2, 128.7, 128.4, 127.1, 126.7, 126.2, 125.9, 122.9, 108.4, 68.0, 60.1, 50.7, 49.4, 26.2; HRMS (ESI): m/z calcd for C26H20ClN3NaO3+ [M + Na]+ 480.1091, found 480.1109.
3′-(2-Chlorophenyl)-1-methyl-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4g). White solid, 75.9 mg, 83% yield. 81:19 dr. 1H-NMR (400 MHz, CDCl3): δ 7.69 (dd, J = 5.6, 3.8 Hz, 1H), 7.48–7.44 (m, 2H), 7.42 (d, J = 7.6 Hz, 3H), 7.40–7.34 (m, 2H), 7.33 (d, J = 7.6 Hz, 1H), 7.20 (d, J = 7.5 Hz, 2H), 7.14 (t, J = 7.5 Hz, 1H), 6.91 (d, J = 7.8 Hz, 1H), 6.11 (d, J = 8.4 Hz, 1H), 4.31 (t, J = 8.1 Hz, 1H), 3.62 (d, J = 8.0 Hz, 1H), 3.26 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.6, 173.8, 173.5, 143.8, 136.0, 134.0, 130.3, 129.4, 129.1, 129.0, 128.5, 127.0, 126.8, 126.7, 126.2, 122.9, 108.5, 67.6, 57.6, 51.0, 47.0, 26.2; HRMS (ESI): m/z calcd for C26H20ClN3NaO3+ [M + Na]+ 480.1091, found 480.1116.
3′-(3,4-Dichlorophenyl)-1-methyl-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4h). White solid, 83.5 mg, 85% yield. 86:14 dr. 1H-NMR (400 MHz, CDCl3): δ 7.64 (s, 1H), 7.46 (dd, J = 15.9, 7.9 Hz, 3H), 7.42–7.35 (m, 3H), 7.35–7.29 (m, 1H), 7.22 (d, J = 7.6 Hz, 2H), 7.13 (t, J = 7.5 Hz, 1H), 6.89 (d, J = 7.8 Hz, 1H), 5.76 (dd, J = 8.6, 3.2 Hz, 1H), 3.99 (t, J = 8.3 Hz, 1H), 3.53 (d, J = 7.9 Hz, 1H), 3.23 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.5, 173.9, 173.2, 143.7, 138.5, 132.1, 130.4, 129.3, 128.9, 128.8, 127.0, 126.2, 124.9, 122.9, 108.5, 67.9, 59.6, 50.7, 49.3, 26.2; HRMS (ESI): m/z calcd for C26H20Cl2N3O3+ [M + H]+ 492.0882, found 492.0905.
3′-(4-Bromophenyl)-1-methyl-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4i). White solid, 81.2 mg, 81% yield. >95:5 dr. 1H-NMR (400 MHz, CDCl3): δ 7.47 (dd, J = 16.4, 8.1 Hz, 4H), 7.39 (dd, J = 12.5, 5.4 Hz, 4H), 7.31 (d, J = 7.3 Hz, 1H), 7.22 (d, J = 7.6 Hz, 2H), 7.12 (t, J = 7.5 Hz, 1H), 6.89 (d, J = 7.8 Hz, 1H), 5.77 (d, J = 7.4 Hz, 1H), 3.98 (t, J = 8.3 Hz, 1H), 3.55 (d, J = 7.9 Hz, 1H), 3.23 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.7, 173.9, 173.3, 143.7, 137.0, 131.5, 130.3, 129.2, 129.0, 128.6, 126.9, 126.1, 125.3, 122.9, 122.1, 108.4, 67.9, 60.2, 50.9, 49.3, 26.2; HRMS (ESI): m/z calcd for C26H20BrN3NaO3+ [M + Na]+ 524.0586, found 524.0567.
3′-(3-Bromophenyl)-1-methyl-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4j).White solid, 65.1 mg, 65% yield. 67:33 dr. 1H-NMR (400 MHz, CDCl3): δ 7.72 (s, 1H), 7.46 (t, J = 7.7 Hz, 4H), 7.40 (t, J = 7.1 Hz, 2H), 7.34 (d, J = 7.4 Hz, 1H), 7.24 (dd, J = 7.5, 4.7 Hz, 3H), 7.13 (t, J = 7.5 Hz, 1H), 6.89 (d, J = 7.8 Hz, 1H), 5.78 (d, J = 8.9 Hz, 1H), 4.02 (t, J = 8.4 Hz, 1H), 3.53 (d, J = 7.9 Hz, 1H), 3.24 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.6, 173.9, 173.2, 143.7, 140.6, 131.4, 130.3, 130.0, 129.9, 129.2, 128.6, 127.1, 126.4, 126.2, 125.0, 122.9, 108.4, 68.0, 60.1, 50.7, 49.4, 26.2; HRMS (ESI): m/z calcd for C26H20BrN3NaO3+ [M + Na]+ 524.0586, found 524.0570.
3′-(2-Bromophenyl)-1-methyl-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4k). White solid, 90.2 mg, 90% yield. 93:7 dr. 1H-NMR (400 MHz, CDCl3): δ 7.70–7.65 (m, 1H), 7.62 (d, J = 7.9 Hz, 1H), 7.41 (d, J = 7.7 Hz, 3H), 7.39–7.29 (m, 3H), 7.19 (d, J = 7.5 Hz, 3H), 7.13 (t, J = 7.5 Hz, 1H), 6.90 (d, J = 7.8 Hz, 1H), 6.07 (d, J = 8.3 Hz, 1H), 4.34 (t, J = 8.2 Hz, 1H), 3.60 (d, J = 8.0 Hz, 1H), 3.25 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.6, 173.8, 173.5, 143.8, 137.6, 132.7, 130.3, 129.4, 129.1, 128.5, 127.4, 127.2, 126.8, 126.1, 124.3, 122.9, 108.5, 67.7 , 59.9, 50.8, 46.9, 26.2; HRMS (ESI): m/z calcd for C26H20BrN3NaO3+ [M + Na]+ 524.0586, found 524.0561.
1-Methyl-3′-(4-nitrophenyl)-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4l). White solid, 79.6 mg, 85% yield. >95:5 dr. 1H-NMR (400 MHz, CDCl3): δ 8.24 (d, J = 8.6 Hz, 2H), 7.72 (d, J = 8.6 Hz, 2H), 7.47 (dd, J = 12.9, 5.2 Hz, 2H), 7.41 (dd, J = 7.6, 2.2 Hz, 2H), 7.32 (d, J = 7.3 Hz, 1H), 7.23 (d, J = 7.6 Hz, 2H), 7.16 (t, J = 7.5 Hz, 1H), 6.92 (d, J = 7.8 Hz, 1H), 5.94 (d, J = 8.5 Hz, 1H), 4.06 (t, J = 8.3 Hz, 1H), 3.62 (d, J = 8.0 Hz, 1H), 3.26 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.5, 173.8, 173.1, 147.7, 145.6, 143.7, 131.4, 130.5, 129.3, 128.9, 128.1, 126.8, 126.1, 125.0, 123.6, 123.5, 123.1, 108.7, 68.0, 60.0, 50.7, 49.5, 26.3; HRMS (ESI): m/z calcd for C26H20N4NaO5+ [M + Na]+ 491.1331, found 491.1331.
1-Methyl-3′-(3-nitrophenyl)-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4m). White solid; 77.7 mg, 83% yield; >95:5 dr; 1H-NMR (400 MHz, CDCl3): δ 8.43 (s, 1H), 8.17 (d, J = 7.9 Hz, 1H), 7.84 (d, J = 7.6 Hz, 1H), 7.55 (t, J = 7.9 Hz, 1H), 7.45 (d, J = 7.8 Hz, 2H), 7.40 (dd, J = 7.3, 3.6 Hz, 2H), 7.33 (d, J = 7.3 Hz, 1H), 7.23 (d, J = 7.7 Hz, 2H), 7.15 (t, J = 7.5 Hz, 1H), 6.90 (d, J = 7.7 Hz, 1H), 5.93 (d, J = 8.5 Hz, 1H), 4.04 (t, J = 8.2 Hz, 1H), 3.60 (d, J = 7.9 Hz, 1H), 3.25 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.6, 174.0, 173.2, 148.4 , 143.7, 140.4, 133.9, 130.5, 129.4, 129.3, 129.2, 128.9, 126.9, 126.4, 126.3, 123.3, 123.1, 122.0, 108.6, 67.9, 59.9, 50.7, 49.4, 26.2; HRMS (ESI): m/z calcd for C26H20N4NaO5+ [M + Na]+ 491.1331, found 491.1332.
1-Methyl-3′-(2-nitrophenyl)-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4n). White solid; 80.5 mg, 86% yield; 94:6 dr; 1H-NMR (400 MHz, CDCl3): δ 8.13 (d, J = 8.1 Hz, 1H), 8.06 (d, J = 7.8 Hz, 1H), 7.62 (t, J = 7.6 Hz, 1H), 7.44–7.38 (m, 3H), 7.38–7.32 (m, 3H), 7.17 (d, J = 7.6 Hz, 2H), 7.13 (t, J = 7.5 Hz, 1H), 6.89 (d, J = 7.8 Hz, 1H), 6.23 (dd, J = 8.2, 2.8 Hz, 1H), 4.51 (t, J = 8.2 Hz, 1H), 3.60 (d, J = 8.1 Hz, 1H), 3.22 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.5, 174.2, 173.4, 148.7, 143.8, 134.5, 133.4, 130.4, 129.2, 128.8, 128.7, 128.1, 126.7, 126.1, 125.3, 122.8, 108.6, 67.7, 56.9, 50.7, 48.5, 26.2; HRMS (ESI): m/z calcd for C26H20N4NaO5+ [M + Na]+ 491.1331, found 491.1350.
1-Methyl-5′-phenyl-3′-(p-tolyl)-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4o). White solid; 68.2 mg, 78% yield; 82:18 dr; 1H-NMR (400 MHz, CDCl3): δ 7.43 (dd, J = 14.0, 6.1 Hz, 3H), 7.38 (d, J = 3.3 Hz, 1H), 7.35 (d, J = 3.5 Hz, 4H), 7.23 (d, J = 7.7 Hz, 2H), 7.12 (dd, J = 8.7, 5.2 Hz, 2H), 6.88 (d, J = 8.0 Hz, 1H), 5.76 (d, J = 8.9 Hz, 1H), 4.00 (t, J = 8.4 Hz, 1H), 3.50 (d, J = 7.9 Hz, 1H), 3.23 (s, 3H), 2.36 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.8, 174.2, 173.5, 143.8, 138.0, 137.9, 130.2, 129.1, 128.5, 128.3, 128.0, 127.1, 126.2, 124.5, 122.8, 108.4, 68.1 , 60.7, 50.9, 49.5, 26.2, 21.6; HRMS (ESI): m/z calcd for C27H23N3NaO3+ [M + Na]+ 460.1637, found 460.1658.
1-Methyl-5′-phenyl-3′-(m-tolyl)-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4p). White solid; 62.1 mg, 71% yield; 75:25 dr; 1H-NMR (400 MHz, CDCl3): δ 7.46–7.40 (m, 4H), 7.37 (d, J = 7.4 Hz, 2H), 7.34 (d, J = 7.3 Hz, 1H), 7.24 (d, J = 7.5 Hz, 2H), 7.18 (d, J = 7.8 Hz, 2H), 7.12 (t, J = 7.5 Hz, 1H), 6.88 (d, J = 7.7 Hz, 1H), 5.78 (d, J = 8.7 Hz, 1H), 3.98 (t, J = 8.4 Hz, 1H), 3.52 (d, J = 7.9 Hz, 1H), 3.23 (s, 3H), 2.35 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.8, 174.2, 173.6, 143.7, 137.9, 134.9, 130.2, 129.2, 129.1, 128.5, 127.2, 127.0, 126.7, 126.2, 125.5, 122.8, 108.4, 67.9, 60.6, 51.0, 49.5, 26.2, 21.3; HRMS (ESI): m/z calcd for C27H24N3O3+ [M + H]+ 438.1818, found 438.1825.
1-Methyl-5′-phenyl-3′-(o-tolyl)-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4q). White solid; 69.9 mg, 80% yield; 77:23 dr; 1H-NMR (400 MHz, CDCl3): δ 7.75–7.68 (m, 1H), 7.40 (t, J = 7.7 Hz, 4H), 7.36–7.30 (m, 1H), 7.21 (d, J = 7.8 Hz, 3H), 7.13 (t, J = 8.4 Hz, 3H), 6.90 (d, J = 7.7 Hz, 1H), 5.94 (d, J = 9.0 Hz, 1H), 4.13 (t, J = 8.4 Hz, 1H), 3.53 (d, J = 7.9 Hz, 1H), 3.25 (s, 3H), 2.54 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.8, 173.9, 173.5, 143.8, 136.6, 130.3, 130.2, 129.1, 128.5, 127.8, 127.3, 126.2, 125.8, 125.1, 122.8, 108.4, 67.8, 57.1, 50.9, 47.2, 26.2, 19.5; HRMS (ESI): m/z calcd for C27H23N3NaO3+ [M + Na]+ 460.1637, found 460.1646.
3′-(4-Methoxyphenyl)-1-methyl-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4r). White solid; 76.1 mg, 84% yield; 83:17 dr; 1H-NMR (400 MHz, CDCl3): δ 7.46 (t, J = 7.7 Hz, 4H), 7.41–7.35 (m, 3H), 7.28–7.24 (m, 2H), 7.13 (t, J = 7.6 Hz, 1H), 6.94–6.88 (m, 3H), 5.78 (d, J = 8.8 Hz, 1H), 3.99 (t, J = 8.5 Hz, 1H), 3.82 (s, 3H), 3.53 (d, J = 7.9 Hz, 1H), 3.25 (s, 3H); 13C-NMR (100MHz, CDCl3): δ 178.8, 174.2, 173.5, 143.8, 138.0, 137.9, 129.1, 128.5, 128.3, 128.0, 127.1, 126.2, 124.5, 122.8, 108.4, 68.1, 60.7, 50.9, 49.5, 26.2, 21.6; HRMS (ESI): m/z calcd for C27H23N3NaO4+ [M + Na]+ 476.1586, found 476.1597.
3′-(3-Methoxyphenyl)-1-methyl-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4s). White solid; 68.9 mg, 76% yield; 80:20 dr; 1H-NMR (400 MHz, CDCl3): δ 7.48–7.42 (m, 2H), 7.41–7.30 (m, 4H), 7.27–7.22 (m, 2H), 7.14 (dd, J = 12.9, 5.2 Hz, 3H), 6.89 (d, J = 7.9 Hz, 1H), 6.86 (dd, J = 7.9, 2.2 Hz, 1H), 5.79 (d, J = 8.9 Hz, 1H), 4.02 (t, J = 8.4 Hz, 1H), 3.81 (s, 3H), 3.53 (d, J = 7.9 Hz, 1H), 3.25 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.7, 174.0, 173.5, 143.7, 139.8, 130.2, 129.4, 129.1, 128.5, 127.1, 126.7, 126.2, 125.3, 122.8, 119.8, 108.4, 68.0, 60.6, 55.2, 50.9, 49.4, 26.2; HRMS (ESI): m/z calcd for C27H23N3NaO4+ [M + Na]+ 476.1586, found 476.1605.
1-Methyl-3′-(naphthalen-1-yl)-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4t). White solid; 88.0 mg, 93% yield; 94:6 dr; 1H-NMR (400 MHz, CDCl3): δ 8.26 (d, J = 8.4 Hz, 1H), 7.90 (d, J = 8.4 Hz, 2H), 7.83 (d, J = 8.1 Hz, 1H), 7.64 (t, J = 7.5 Hz, 1H), 7.54 (t, J = 7.5 Hz, 1H), 7.48 (d, J = 7.8 Hz, 1H), 7.41 (dd, J = 14.6, 7.3 Hz, 5H), 7.18–7.12 (m, 3H), 6.93 (d, J = 7.9 Hz, 1H), 6.57 (d, J = 8.5 Hz, 1H), 4.32 (t, J = 8.2 Hz, 1H), 3.64 (d, J = 7.8 Hz, 1H), 3.28 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.7, 173.6, 173.5, 143.8,130.3, 129.1, 129.0, 128.6, 128.5, 127.1, 126.5, 126.2, 125.8, 125.2, 122.9, 108.5, 67.7, 56.5, 51.1, 48.4, 26.2; HRMS (ESI): m/z calcd for C30H23N3NaO3+ [M + Na]+ 496.1637, found 496.1651.
3′-(Furan-2-yl)-1-methyl-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4u). White solid; 57.8 mg, 70% yield; 94:6 dr; 1H-NMR (400 MHz, CDCl3): δ 7.48 (d, J = 8.0 Hz, 3H), 7.39 (dd, J = 16.4, 7.7 Hz, 3H), 7.32 (d, J = 7.5 Hz, 2H), 7.10 (t, J = 7.6 Hz, 1H), 6.88 (d, J = 7.8 Hz, 1H), 6.43 (d, J = 3.1 Hz, 1H), 6.40–6.36 (m, 1H), 5.78 (d, J = 8.8 Hz, 1H), 4.02 (t, J = 8.4 Hz, 1H), 3.51 (d, J = 8.0 Hz, 1H), 3.21 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.3, 174.4, 173.5, 151.2, 143.8, 142.7, 131.9, 130.3, 129.2, 128.7, 126.9, 126.3, 124.8, 122.8, 110.4, 108.4, 108.1, 67.9, 55.8, 50.7, 48.6, 26.2; HRMS (ESI): m/z calcd for C24H19N3NaO4+ [M + Na]+ 436.1273, found 436.1290.
1-Methyl-5′-phenyl-3′-(thiophen-2-yl)-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4v). White solid; 67.8 mg, 79% yield; 93:7 dr; 1H-NMR (400 MHz, CDCl3): δ 7.47 (t, J = 7.6 Hz, 2H), 7.39 (dd, J = 7.5, 5.4 Hz, 2H), 7.34 (d, J = 7.6 Hz, 1H), 7.29 (s, 1H), 7.28 (d, J = 2.1 Hz, 2H), 7.22 (d, J = 3.2 Hz, 1H), 7.13 (t, J = 7.5 Hz, 1H), 7.08-7.04 (m, 1H), 6.89 (d, J = 7.7 Hz, 1H), 6.11 (d, J = 9.1 Hz, 1H), 4.02 (t, J = 8.5 Hz, 1H), 3.49 (d, J = 1.7 Hz, 1H), 3.24 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.5, 173.8, 173.4, 143.7, 142.7, 131.8, 130.3, 129.2, 128.6, 127.3, 127.2, 126.7, 126.4, 125.5, 125.0, 124.8, 122.8, 108.4, 67.8, 57.1, 50.5, 49.3, 26.2; HRMS (ESI): m/z calcd for C24H19N3NaO3S+ [M + Na]+ 452.1045, found 452.1066.
3′-Benzyl-1-methyl-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4w). White solid; 66.5 mg, 76% yield; 77:23 dr; 1H-NMR (400 MHz, CDCl3): δ 7.56 (t, J = 7.6 Hz, 2H), 7.46 (dd, J = 12.7, 7.4 Hz, 4H), 7.31 (t, J = 7.2 Hz, 5H), 7.23 (dd, J = 13.3, 6.6 Hz, 2H), 6.82 (d, J = 7.7 Hz, 1H), 4.87–4.78 (m, 1H), 3.77 (t, J = 7.8 Hz, 1H), 3.57 (d, J = 8.0 Hz, 1H), 3.29–3.23 (m, 1H), 3.15 (s, 3H), 2.80–2.71 (m, 1H); 13C-NMR (100 MHz, CDCl3): δ 178.5, 175.4, 173.9, 143.6, 139.4, 131.9, 130.1, 129.4, 129.1, 128.8, 128.7, 128.4, 126.5, 126.3, 122.8, 108.4, 67.7, 58.8, 51.7, 47.6, 38.2, 26.1; HRMS (ESI): m/z calcd for C27H23N3NaO3+ [M + Na]+ 460.1637, found 460.1647.
3′-(tert-Butyl)-1-methyl-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (4x). White solid; 58.1 mg; 72% yield; 72:28 dr; 1H-NMR (400 MHz, CDCl3): δ 7.50 (d, J = 7.7 Hz, 3H), 7.37–7.30 (m, 4H), 6.87 (d, J = 7.9 Hz, 2H), 4.38 (d, J = 7.9 Hz, 1H), 3.75 (t, J = 8.0 Hz, 1H), 3.58 (d, J = 8.1 Hz, 1H), 3.22 (s, 3H), 1.19 (s, 9H); 13C-NMR (100 MHz, CDCl3): δ 178.8, 176.3, 173.5, 143.6, 130.0, 129.3, 128.8, 126.5, 126.2, 122.8, 108.4, 68.4, 67.3, 52.2, 47.0, 33.2, 29.7, 26.2; HRMS (ESI): m/z calcd for C24H25N3NaO3+ [M + Na]+ 426.1794, found 426.1808.
5-Methoxy-1-methyl-3′,5′-diphenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (5a). White solid; 74.3 mg; 82% yield; 83:17 dr; 1H-NMR (400 MHz, CDCl3): δ 7.54 (d, J = 7.5 Hz, 2H), 7.42 (dd, J = 14.5, 6.9 Hz, 3H), 7.39–7.32 (m, 3H), 7.23 (d, J = 7.8 Hz, 2H), 6.94 (s, 1H), 6.89 (d, J = 8.5 Hz, 1H), 6.78 (d, J = 8.4 Hz, 1H), 5.80 (d, J = 8.6 Hz, 1H), 3.99 (t, J = 8.3 Hz, 1H), 3.76 (s, 3H), 3.55 (d, J = 7.9 Hz, 1H), 3.21 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.6, 174.1, 173.4, 155.9, 138.0, 137.1, 129.1, 128.5, 128.3, 128.2, 127.3, 127.0, 126.2, 114.5, 114.3, 108.7, 68.2, 60.9, 55.8, 51.1, 49.6, 26.2; HRMS (ESI): m/z calcd for C27H23N3NaO4+ [M + Na]+ 476.1586, found 476.1605.
5-Methoxy-1-methyl-3′-(4-nitrophenyl)-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (5b). White solid; 79.7 mg; 80% yield; 82:18 dr; 1H-NMR (400 MHz, CDCl3): δ 8.26 (dd, J = 8.4, 4.9 Hz, 3H), 7.88 (d, J = 8.6 Hz, 2H), 7.48 (d, J = 7.7 Hz, 2H), 7.42 (d, J = 7.2 Hz, 1H), 6.91 (d, J = 8.4 Hz, 3H), 6.79 (d, J = 8.5 Hz, 1H), 5.63 (d, J = 6.8 Hz, 1H), 3.95 (t, J = 10.8 Hz, 1H), 3.86 (s, 3H), 3.57 (dd, J = 10.0, 7.0 Hz, 1H), 3.17 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 177.29 (s), 175.51 (s), 173.89 (s), 156.90 (s), 148.76 (s), 137.30 (s), 131.74 (s), 130.47 (s), 129.04 (d, J = 31.4 Hz), 128.76 (s), 127.66 (s), 126.87 (s), 126.41 (s), 123.94 (s), 114.48 (s), 111.45 (s), 109.36 (s), 69.20 (s), 61.17 (s), 55.97 (s), 53.48 (s), 52.95 (s), 26.26 (s). HRMS (ESI): m/z calcd for C27H22N4NaO6+ [M + Na]+ 521.1437, found 521.1437.
5-Chloro-1-methyl-3′,5′-diphenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (5c). White solid; 77.7 mg; 85% yield; 75:25 dr; 1H-NMR (400 MHz, CDCl3): δ 7.53 (d, J = 7.2 Hz, 3H), 7.39-7.31 (m, 7H), 7.21 (d, J = 7.3 Hz, 3H), 5.76 (d, J = 8.8 Hz, 1H), 4.02 (t, J = 8.3 Hz, 1H), 3.53 (d, J = 7.9 Hz, 1H), 3.22 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.4, 174.0, 173.3, 142.3, 137.7, 130.1, 129.2, 128.8, 128.7, 128.6, 128.4, 128.3, 127.4, 127.3, 126.1, 109.4, 67.9, 60.9, 51.0, 49.4, 26.3; HRMS (ESI): m/z calcd for C26H20ClN3NaO3+ [M + Na]+ 480.1091, found 480.1103.
5-Chloro-1-methyl-3′-(4-nitrophenyl)-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (5d). White solid; 82.3 mg; 82% yield; 78:22 dr; 1H-NMR (400 MHz, CDCl3): δ 8.22 (d, J = 7.9 Hz, 2H), 7.84 (d, J = 7.7 Hz, 2H), 7.52–7.43 (m, 4H), 7.41 (d, J = 6.3 Hz, 2H), 6.78 (t, J = 8.4 Hz, 2H), 5.56 (d, J = 6.1 Hz, 1H), 3.85 (d, J = 9.9 Hz, 1H), 3.54 (t, J = 9.8 Hz, 1H), 3.14 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 177.2, 175.5, 173.9, 148.5, 147.6, 142.5, 130.3, 130.2, 129.3, 129.0, 127.7, 127.2, 126.8, 126.7, 124.6, 124.0, 110.0, 68.8, 61.2, 53.4, 52.8, 26.3; HRMS (ESI): m/z calcd for C26H19ClN4NaO5+ [M + Na]+ 525.0942, found 525.0962.
3′,5′-Diphenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (5e). White solid; 55.6 mg; 68% yield; 69:31 dr; 1H-NMR (400 MHz, DMSO-d6): δ 10.46 (s, 1H), 7.44 (d, J = 26.8 Hz, 6H), 7.24 (d, J = 36.5 Hz, 8H), 5.53 (s, 1H), 4.27 (s, 1H), 3.88 (s, 1H); 13C-NMR (100 MHz, DMSO-d6): δ 181.3, 174.9, 174.3, 142.8, 140.0, 129.6, 129.4, 128.2, 128.0, 127.7, 127.4,127.3, 121.5, 109.8, 68.1, 60.6, 51.8, 50.4; HRMS (ESI): m/z calcd for C25H19N3NaO3+ [M + Na]+ 432.1324, found 432.1341.
1-Benzyl-3′,5′-diphenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (5f). White solid; 79.9 mg; 80% yield; 83:17 dr; 1H-NMR (400 MHz, DMSO-d6): δ 7.50 (s, 5H), 7.42–7.18 (m, 12H), 7.00 (s, 1H), 6.85 (s, 1H), 5.60 (s, 1H), 4.90 (s, 2H), 3.94 (s, 1H), 3.57 (s, 1H); 13C-NMR (100 MHz, DMSO-d6): δ 179.4, 174.8, 174.1, 143.2, 139.9, 136.7, 133.3, 129.7, 129.4, 129.1, 129.0, 128.8, 128.2, 128.0, 127.7, 127.4, 127.2, 122.3, 109.4, 68.0, 60.8, 52.1, 50.4, 42.9; HRMS (ESI): m/z calcd for C32H25N3NaO3+ [M + Na]+ 522.1794, found 522.1799.
1-Benzyl-3′-(4-nitrophenyl)-5′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (5g). White solid; 88.2 mg; 81% yield; 82:18 dr; 1H-NMR (400 MHz, DMSO-d6): δ 8.22 (d, J = 7.9 Hz, 2H), 7.85 (d, J = 7.9 Hz, 2H), 7.48 (d, J = 6.8 Hz, 2H), 7.41 (s, 3H), 7.37–7.32 (m, 2H), 7.30–7.21 (m, 5H), 7.04 (t, J = 6.8 Hz, 1H), 6.88 (d, J = 7.2 Hz, 1H), 5.05–4.85 (m, 2H), 4.70 (s, 1H), 4.13 (t, J = 7.7 Hz, 1H), 3.66 (d, J = 7.4 Hz, 1H); 13C-NMR (100 MHz, DMSO-d6): δ 179.3, 174.9, 174.0, 148.3, 147.2, 143.2, 136.6, 129.8, 129.5, 129.2, 129.1, 128.9, 127.9, 127.8, 127.4, 127.1, 127.0, 123.4, 122.5, 109.6, 68.0, 60.1, 52.2, 50.5, 43.0; HRMS (ESI): m/z calcd for C32H24N4NaO5+ [M + Na]+ 567.1644, found 567.1646.
5′-(4-Chlorophenyl)-1-methyl-3′-phenyl-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (5h). White solid; 60.3 mg; 66% yield; 67:33 dr; 1H-NMR (400 MHz, DMSO-d6): δ 7.55 (d, J = 7.9 Hz, 3H), 7.47 (d, J = 7.1 Hz, 3H), 7.28 (d, J = 7.1 Hz, 2H), 7.23 (d, J = 7.9 Hz, 3H), 7.01 (d, J = 8.2 Hz, 2H), 5.54 (d, J = 6.1 Hz, 1H), 3.90 (t, J = 8.1 Hz, 1H), 3.50 (d, J = 7.6 Hz, 1H), 3.14 (s, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 179.1, 174.7, 174.0, 144.4, 139.9, 133.2, 131.6, 129.7, 129.5, 129.1, 128.2, 127.9, 127.7, 127.1, 127.0, 122.2, 108.7, 67.9, 60.7, 51.8, 50.5, 26.3; HRMS (ESI): m/z calcd for C26H20ClN3NaO3+ [M + Na]+ 480.1091, found 480.1110.
5′-(4-Chlorophenyl)-1-methyl-3′-(4-nitrophenyl)-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (5i). White solid; 81.3 mg; 81% yield; 82:18 dr; 1H-NMR (400 MHz, CDCl3): δ 8.22 (d, J = 8.6 Hz, 2H), 7.68 (d, J = 8.6 Hz, 2H), 7.42 (t, J = 3.3 Hz, 2H), 7.28 (dd, J = 14.0, 7.8 Hz, 2H), 7.15 (dd, J = 16.8, 8.2 Hz, 3H), 6.91 (d, J = 7.8 Hz, 1H), 5.91 (d, J = 8.5 Hz, 1H), 4.03 (t, J = 8.2 Hz, 1H), 3.59 (d, J = 8.0 Hz, 1H), 3.24 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.5, 173.6, 172.9, 147.7, 145.5, 143.7, 134.5, 130.6, 129.9, 129.5, 129.4, 128.1, 128.0, 127.2, 126.6, 125.0, 123.6, 123.0, 108.7, 68.0, 60.0, 50.8, 49.5, 26.3; HRMS (ESI): m/z calcd for C26H19ClN4NaO5+ [M + Na]+ 525.0942, found 525.0964.
5′-(3-Chlorophenyl)-1-methyl-3′-(4-nitrophenyl)-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (5j). White solid; 80.3 mg; 80% yield; 81:19 dr; 1H-NMR (400 MHz, CDCl3): δ 8.24 (d, J = 8.7 Hz, 2H), 7.71 (d, J = 8.7 Hz, 2H), 7.46–7.35 (m, 3H), 7.28 (dd, J = 12.9, 4.8 Hz, 2H), 7.21–7.11 (m, 2H), 6.93 (d, J = 7.8 Hz, 1H), 5.94 (d, J = 8.5 Hz, 1H), 4.06 (t, J = 8.2 Hz, 1H), 3.61 (d, J = 8.0 Hz, 1H), 3.26 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.4, 173.5, 172.7, 147.7, 145.4, 143.7, 134.8, 130.6, 130.3, 129.0, 128.0, 126.7, 126.3, 124.9, 124.2, 123.7, 123.1, 108.7, 68.0, 60.0, 50.7, 49.5, 26.3; HRMS (ESI): m/z calcd for C26H19ClN4NaO5+ [M + Na]+ 525.0942, found 525.0965.
5′-(4-Methoxyphenyl)-1-methyl-3′-(4-nitrophenyl)-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (5k). White solid; 79.7 mg; 80% yield; 78:22 dr; 1H-NMR (400 MHz, DMSO-d6): δ 8.29 (s, 2H), 7.87 (s, 2H), 7.10 (d, J = 34.9 Hz, 8H), 5.26 (s, 1H), 4.48 (s, 1H), 3.99 (s, 1H), 3.78 (s, 3H), 3.09 (s, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 177.9, 177.1, 175.3, 159.5, 150.8, 147.3, 144.4, 130.1, 129.8, 128.7, 128.6, 128.3, 125.2, 124.6, 124.3,124.1, 123.4, 114.7, 109.3, 100.0, 69.0, 60.9, 55.9, 53.6, 26.3; HRMS (ESI): m/z calcd for C27H22N4NaO6+ [M + Na]+ 521.1437, found 521.1450.
5′-(3-Methoxyphenyl)-1-methyl-3′-(4-nitrophenyl)-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (5l). White solid; 84.7 mg; 85% yield; 84:16 dr; 1H-NMR (400 MHz, CDCl3): δ 8.21 (d, J = 8.6 Hz, 2H), 7.70 (d, J = 8.6 Hz, 2H), 7.36 (ddd, J = 20.8, 14.1, 7.5 Hz, 4H), 7.14 (t, J = 7.5 Hz, 1H), 6.91 (dd, J = 11.2, 4.7 Hz, 2H), 6.79 (d, J = 7.9 Hz, 1H), 5.90 (d, J = 8.5 Hz, 1H), 4.03 (t, J = 8.2 Hz, 1H), 3.77 (s, 3H), 3.58 (d, J = 7.9 Hz, 1H), 3.23 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.6, 173.8, 173.1, 147.7, 145.7, 143.7, 132.5, 130.5, 130.0, 129.9, 128.1, 126.8, 125.1, 123.6, 123.0, 118.3, 114.4, 112.2, 108.6, 68.0, 60.0, 55.4, 50.8, 49.5, 26.2; HRMS (ESI): m/z calcd for C27H22N4NaO6+ [M + Na]+ 521.1437, found 521.1461.
1-Methyl-3′-(4-nitrophenyl)-5′-(p-tolyl)-2′,3′,3a′,6a′-tetrahydro-4′H-spiro[indoline-3,1′-pyrrolo[3,4-c]pyrrole]-2,4′,6′(5′H)-trione (5m). White solid; 78.1 mg; 81% yield; 82:18 dr; 1H-NMR (400 MHz, CDCl3): δ 8.21 (d, J = 8.6 Hz, 2H), 7.69(d, J = 8.6 Hz, 2H), 7.40(t, J = 7.5 Hz, 1H), 7.30 (d, J = 7.3 Hz, 1H), 7.28–7.21 (m, 2H), 7.13 (t, J = 7.5 Hz, 1H), 7.08 (d, J = 8.2 Hz, 2H), 6.89 (d, J = 7.8 Hz, 1H), 5.90 (d, J = 8.5 Hz, 1H), 4.02 (t, J = 8.2 Hz, 1H), 3.57 (d, J = 7.9 Hz, 1H), 3.23 (s, 3H), 2.36 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 178.6, 173.9, 173.2, 147.7, 145.7, 143.7, 138.9, 130.4, 129.9, 128.9, 128.1, 126.8, 125.9, 125.1, 123.6, 123.0, 108.6, 68.0, 60.0, 50.7, 49.5, 26.2, 21.2; HRMS (ESI): m/z calcd for C27H22N4NaO5+ [M + Na]+ 505.1488, found 505.1510.