Preparation of Novel Homodimers Derived from Cytotoxic Isoquinolinequinones. A Twin Drug Approach
Abstract
:1. Introduction
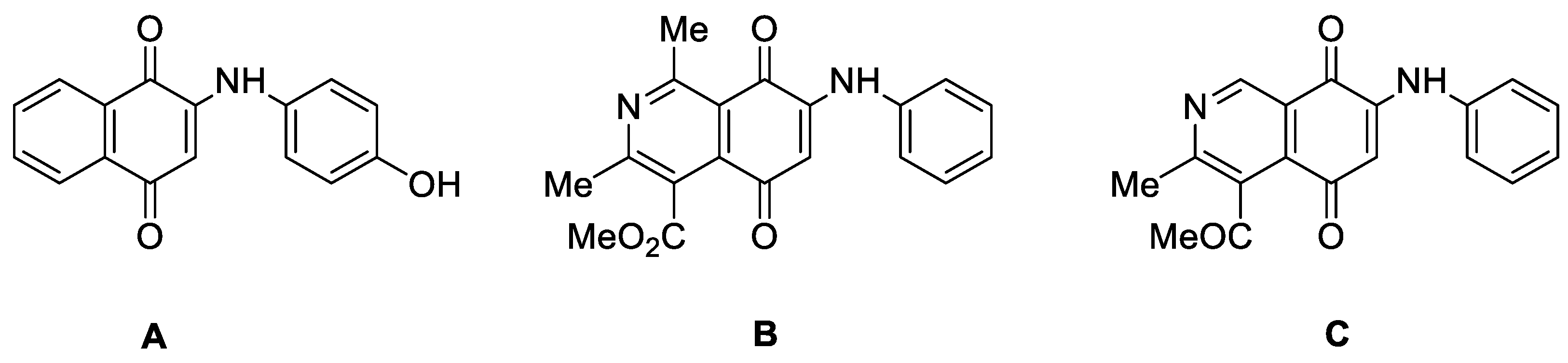
2. Results and Discussion
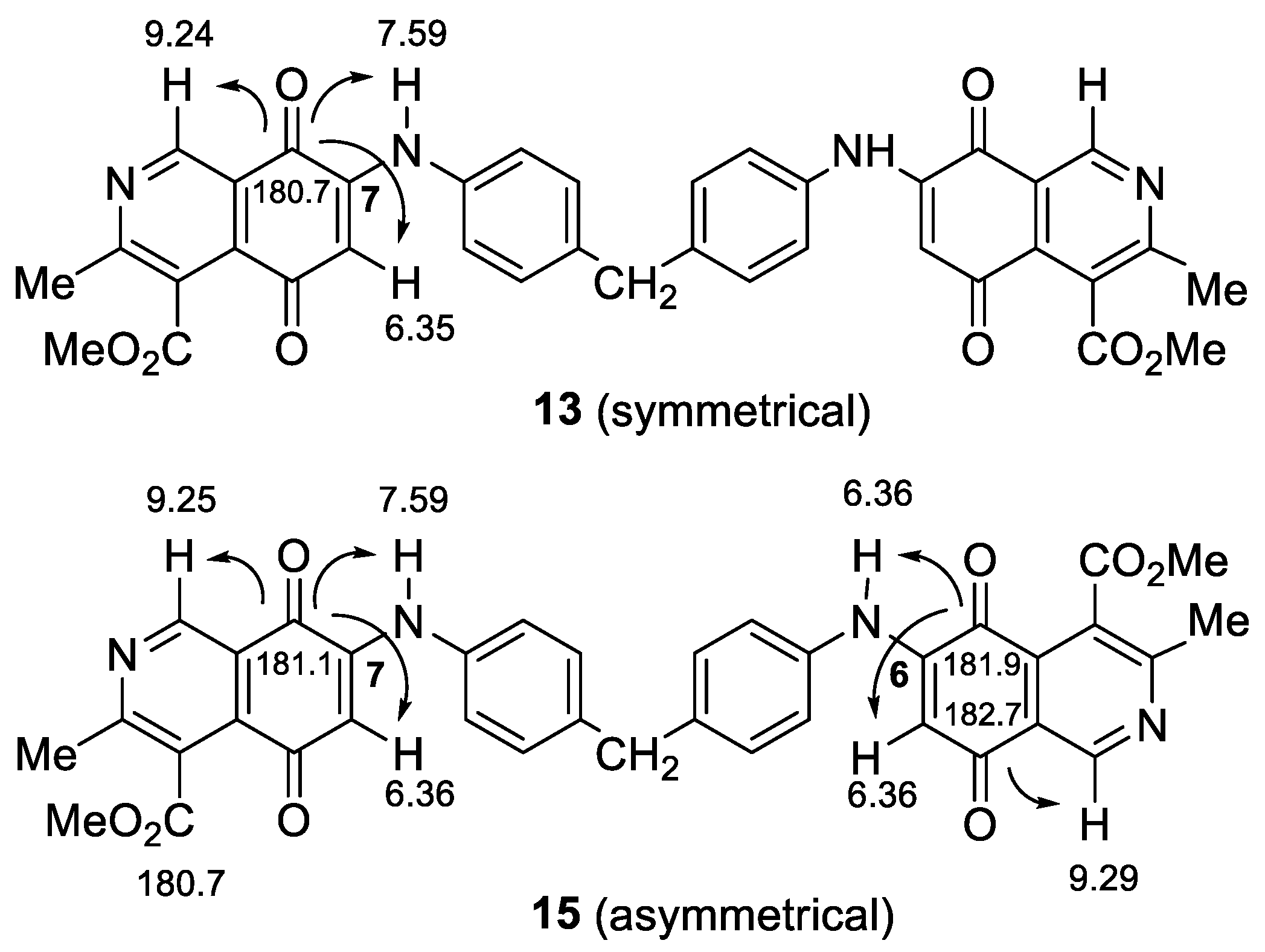
2.1. Chemistry
2.2. Biological Results
3. Materials and Methods
3.1. General
3.2. Chemistry
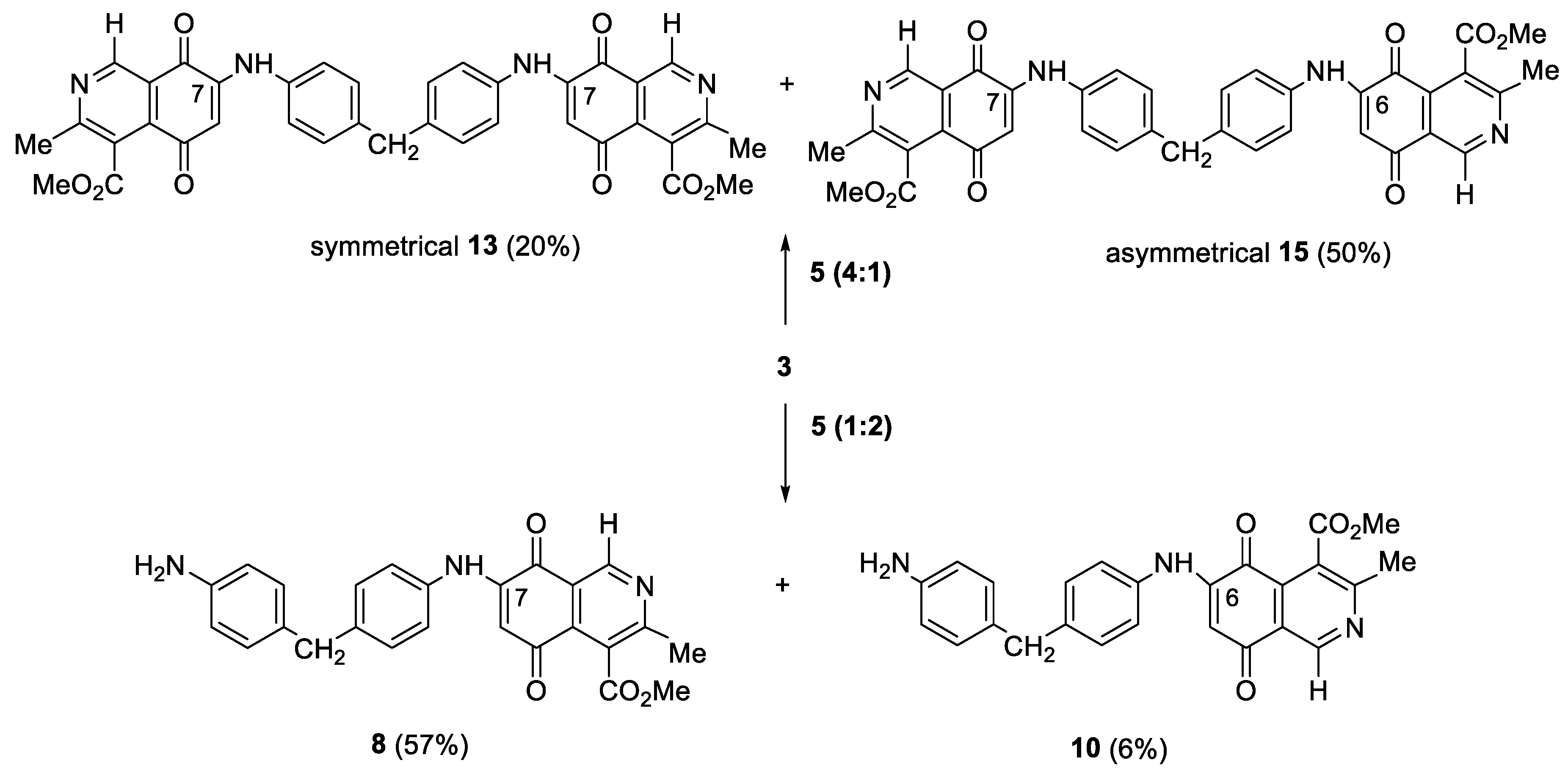
Preparation of Compounds 6–10 and Homodimers 11–15, General Procedure
3.3. Cell Growth Inhibition Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bolton, J.L.; Trush, M.A.; Penning, T.M.; Dryhurst, G.; Monks, T.J. Rol of Quinones in Toxicology. Chem. Res. Toxicol. 2000, 13, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Powis, G. Metabolism and reactions of quinoid anticancer agents. Pharmacol. Ther. 1987, 35, 57–62. [Google Scholar] [CrossRef]
- O’Brien, P.J. Molecular mechanisms of quinone cytotoxicity. Chem. Biol. Interact. 1991, 80, 1–41. [Google Scholar] [CrossRef]
- Paz, M.M.; Das, A.; Palom, Y.; He, Q.-Y.; Tomasz, M. Selective activation of mitomycin a by thiols to form DNA Cross-links and monoadducts: Biochemical basis for the modulation of mitomycin cytotoxicity by the quinone redox potential. J. Med. Chem. 2001, 44, 2834–2842. [Google Scholar] [CrossRef] [PubMed]
- Tudor, G.; Gutierrez, P.; Aguilera-Gutierrez, A.; Sausville, E.A. Cytotoxicity and apoptosis of benzoquinones: Redox cycling, cytochrome c release, and BAD protein expression. Biochem. Pharmacol. 2003, 65, 1061–1075. [Google Scholar] [CrossRef]
- Wellington, K.W. Understanding cancer and the anticancer activities of naphthoquinones—A review. RSC Adv. 2015, 5, 20309–20338. [Google Scholar] [CrossRef]
- Benites, J.; Valderrama, J.A.; Bettega, J.; Pedrosa, R.C.; Buc, P.; Verrax, J. Biological evaluation of donor-acceptor aminonaphtoquinones as antitumor agents. Eur. J. Med. Chem. 2010, 45, 6052–6057. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.I.; Casselato, A.; Neves, A.P.; Carneiro, J.W.; Vargas, M.D.; Visentin, L.; Magalhaes, A.; Camara, C.A.; Pessoa, C.; Costa-Lotufo, L.V.; et al. Novel 2-(R-phenyl)amino-3-(2-methylpropenyl)-[1,4]-naphtoquinones: Synthesis, characterization, electrochemical behavior and antitumor activity. J. Braz. Chem. Soc. 2010, 21, 169–178. [Google Scholar]
- Valderrama, J.A.; Ibacache, J.A.; Arancibia, V.; Rodriguez, J.; Theoduloz, T. Studies on quinones. Part 45: Novel 7-aminoisoquinoline-5,8 -quinone derivatives with antitumor properties on cancer cell lines. Bioorg. Med. Chem. 2009, 17, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Ibacache, J.A.; Delgado, V.; Benites, J.; Theoduloz, C.; Arancibia, V.; Muccioli, G.; Valderrama, J.A. Synthesis, half wave potentials and antiproliferative activity of 1-aryl-substituted Aminoisoquinolinequinones. Molecules 2014, 19, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; Ibacache, J.A.; Arancibia, V.; Theoduloz, C.; Valderrama, J.A. Synthesis and in vitro antiproliferative activity of new phenylaminoisoquinolinequinones against cancer cell lines. Molecules 2013, 18, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Ibacache, J.A.; Valderrama, J.A.; Montoya, M.; Segura, R.; Faundes, J.; Mejias, S. Synthesis and antiproliferative evaluation of new aminoisoquinolinequinones derived from p-phenylenediamine, benzidine and dapsone. J. Chem. Pharm. Res. 2017, 9, 127–134. [Google Scholar]
- Contreras, J.M.; Sippl, W. Homo and heterodimer ligands: The twin drug approach. In The Practice of Medicinal Chemistry, 3nd ed.; Wermuth, C.G., Ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Tosco, P.; Ahring, P.K.; Dyhring, T.; Peters, D.; Harpsoe, K.; Liljefors, T.; Balle, T. Complementary three-dimensional quantitative structure-activity relationship modeling of binding affinity and functional potency: a study on alpha4beta2 nicotinic ligands. J. Med. Chem. 2009, 52, 2311. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, W.; Rodriguez, D.; de Oliveira, R.; Mendes, R.; Olegário, T.; Rocha, J.; Keesen, T.; Lima-Junior, C.; Vasconcellos, M. Synthesis and activity of novel homodimers of Morita–Baylis–Hillman adducts against Leishmania donovani: A twin drug approach. Bioorg. Med. Chem. Lett. 2016, 26, 4523–4526. [Google Scholar] [CrossRef] [PubMed]
- Fujizaki, F.; Kurutachi, M.; Fujiwara, R.; Okabe, M.; Aki, H.; Kashige, N.; Miake, F.; Sumoto, K. Preparation and antibacterial evaluation of some symmetrical twin-drug type bivalent molecules. Heterocycles 2015, 91, 1668–1677. [Google Scholar]
- Aguilar-Martínez, M.; Cuevas, G.; Jímenez-Estrada, M.; Gonzalez, I.; Lotina-Hennse, B.; Macías-Ruvalcaba, N. An experimental and theorical study of the substituent effects on the redox properties of 2-[(R-phenyl) amine]-1,4-naphtalenediones in acetonitrile. J. Org. Chem. 1999, 64, 3684–3694. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; Ibacache, V.; Theoduloz, C.; Valderrama, J. Synthesis and in vitro cytotoxic evaluation of aminoquinones structurally related to marine isoquinolinequinones. Molecules 2012, 17, 7042–7056. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.J.; Gray, M.; Yue, S.T.; Haugland, R.P.; Singer, V.L. Sensitive determination of cell number using the CyQUANT® cell proliferation assay. J. Immunol. Methods 2001, 254, 85–98. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 6, 8, 9, 11 and 12 are available from the authors. |
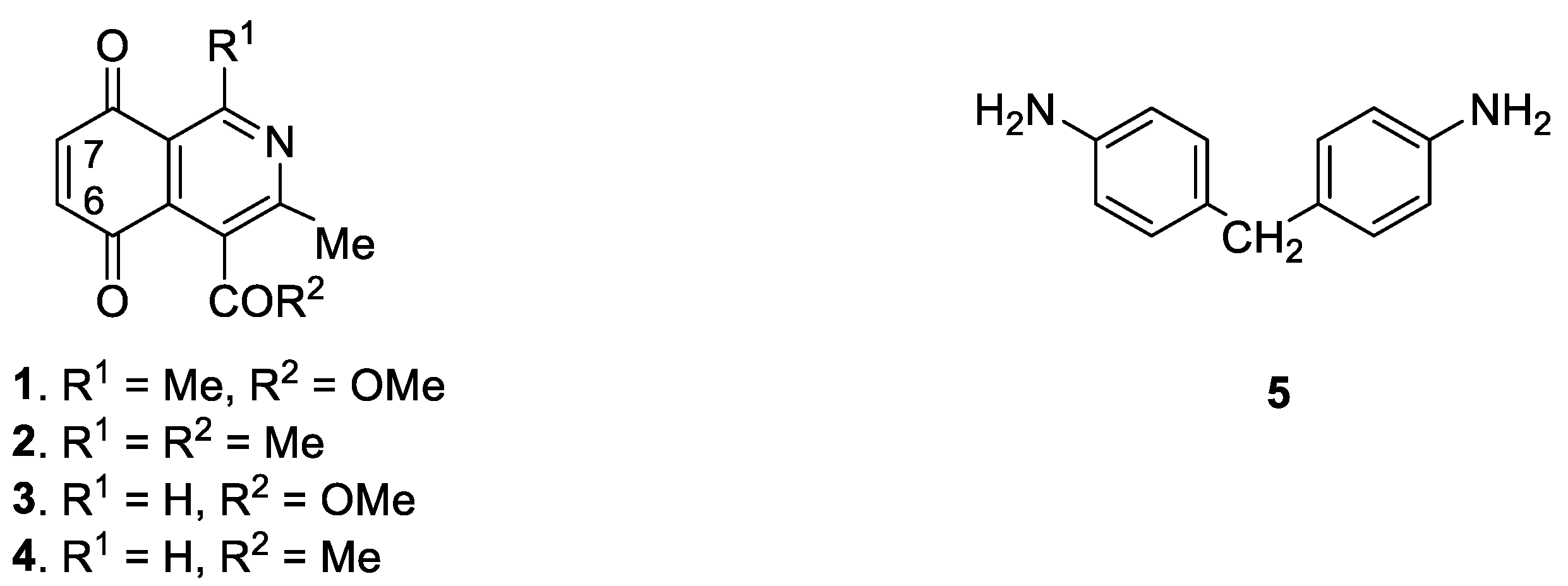
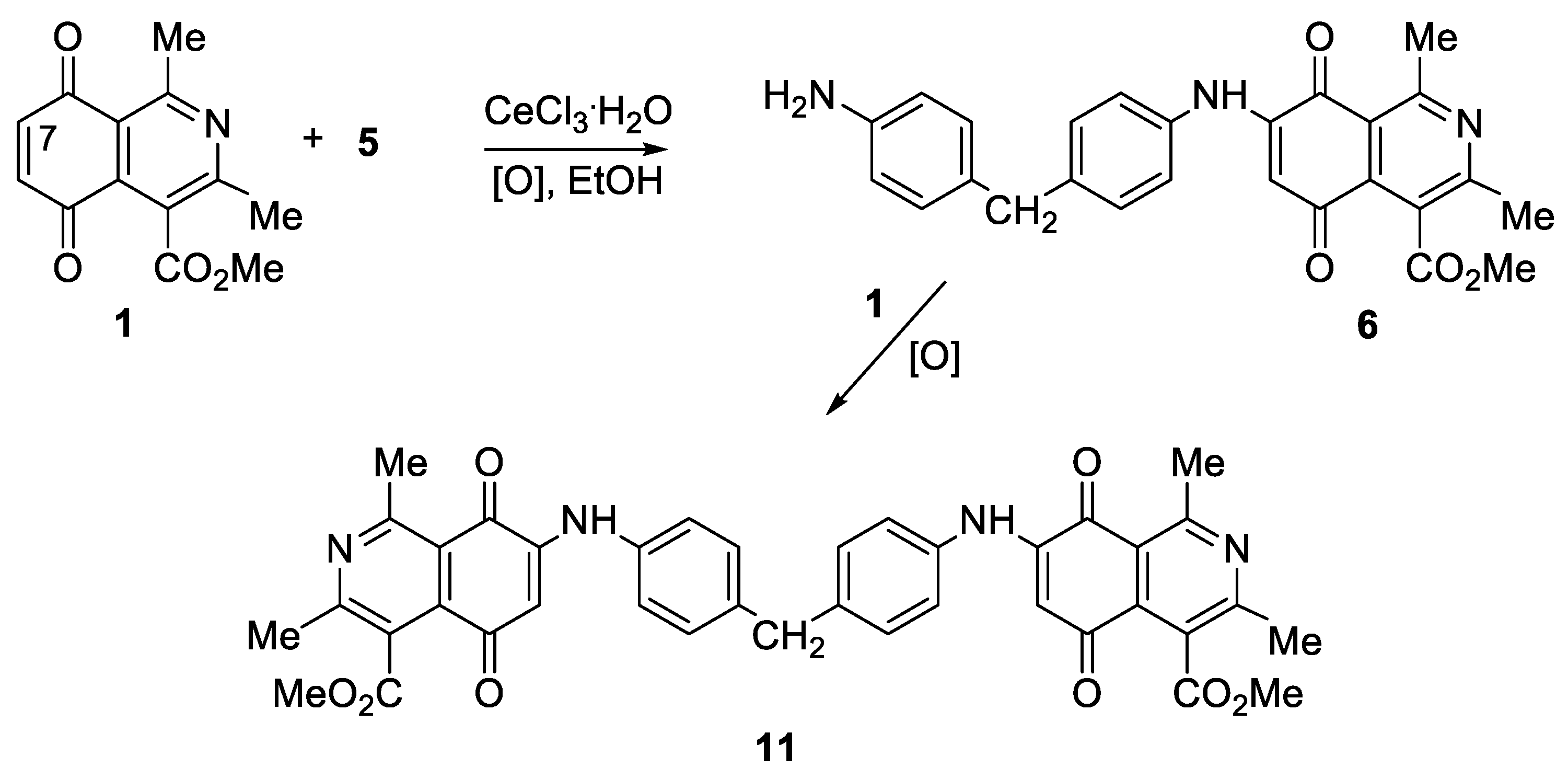
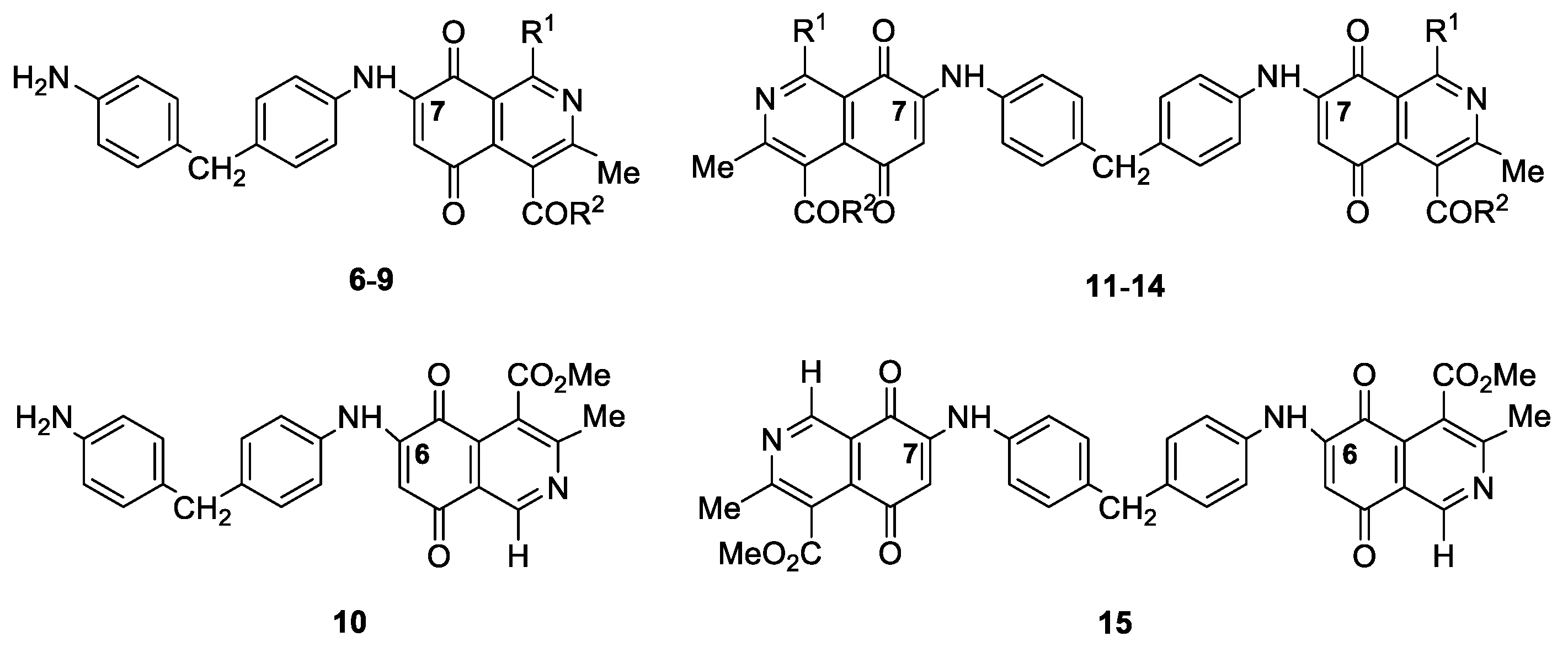
| Compound N° | R1 | R2 | Yield(%) * | N° | R1 | R2 | Yield(%) * |
|---|---|---|---|---|---|---|---|
| 6 | Me | OMe | 74 | 11 | Me | OMe | 98 |
| 7 | Me | Me | 55 | 12 | Me | Me | 95 |
| 8 | H | OMe | 57 | 13 | H | OMe | 20 |
| 9 | H | Me | 32 | 14 | H | Me | 58 |
| 10 | - | - | 6 | 15 | - | - | 50 |
| IC50 ± SEM (µM) a | |||||
|---|---|---|---|---|---|
| Compound N | MEF b | MDA-MB 231 c | B16-F10 d | Mean IC50 | SI e |
| Homodimer | |||||
| 11 | 105.20 ± 13.86 | 66.90 ± 6.13 | 640 ± 12.66 | 353.45 | 0.30 |
| 12 | 71.56 ± 6.39 | 55.65 ± 5.95 | 48.61 ± 4.42 | 52.13 | 1.37 |
| 13 | 79.76 ± 9.27 | 24.81 ± 4.78 | 66.28 ± 5.61 | 45.55 | 1.75 |
| 14 | 32.75 ± 3.38 | 7.98 ± 1.43 | 5.83 ± 0.73 | 6.91 | 4.74 |
| 15 | 2.58 ± 0.33 | 0.29 ± 0.05 | 0.45 ± 0.08 | 0.37 | 6.97 |
| Monoamination product | |||||
| 6 | 8.87 ± 0.88 | 2.46 ± 0.39 | 6.16 ± 0.88 | 4.31 | 2.06 |
| 7 | 15.36 ± 1.97 | 19.54 ± 1.46 | 7.14 ± 0.95 | 13.34 | 1.15 |
| 8 | 5.54 ± 0.82 | 1.45 ± 0.31 | 3.31 ± 0.47 | 2.38 | 2.33 |
| 9 | 2.79 ± 0.44 | 1.59 ± 0.37 | 0.76 ± 0.21 | 1.18 | 2.36 |
| 10 | 5.14 ± 0.69 | 2.75 ± 0.42 | 2.17 ± 0.37 | 2.46 | 2.09 |
| Etoposide | 1.18 ± 0.40 | 5.34 ± 0.12 | 2.00 ± 0.44 | 3.67 | 0.32 |
| Taxol | 0.32 ± 0.05 | 0.32 ± 0.07 | 0.38 ± 0.06 | 0.35 | 0.91 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibacache, J.A.; Faundes, J.; Montoya, M.; Mejías, S.; Valderrama, J.A. Preparation of Novel Homodimers Derived from Cytotoxic Isoquinolinequinones. A Twin Drug Approach. Molecules 2018, 23, 439. https://doi.org/10.3390/molecules23020439
Ibacache JA, Faundes J, Montoya M, Mejías S, Valderrama JA. Preparation of Novel Homodimers Derived from Cytotoxic Isoquinolinequinones. A Twin Drug Approach. Molecules. 2018; 23(2):439. https://doi.org/10.3390/molecules23020439
Chicago/Turabian StyleIbacache, Juana Andrea, Judith Faundes, Margarita Montoya, Sophia Mejías, and Jaime A. Valderrama. 2018. "Preparation of Novel Homodimers Derived from Cytotoxic Isoquinolinequinones. A Twin Drug Approach" Molecules 23, no. 2: 439. https://doi.org/10.3390/molecules23020439
APA StyleIbacache, J. A., Faundes, J., Montoya, M., Mejías, S., & Valderrama, J. A. (2018). Preparation of Novel Homodimers Derived from Cytotoxic Isoquinolinequinones. A Twin Drug Approach. Molecules, 23(2), 439. https://doi.org/10.3390/molecules23020439




