1. Introduction
The
Asteraceae family, which comprises more than 1600 genera, with over 23,000 species, widespread in different types of climates and regions all over the world, is the largest family of flowering plants [
1,
2]. The diversity and heterogeneity of this family justifies the great importance of its individual members, which are known and used from ancient times, not only as food sources or as spices, but also for medicinal purposes [
3,
4]. Several classes of compounds from
Asteraceae species were studied and tested for different bio-activities and were reported as having medicinal potential [
3,
4,
5]. Among these compounds, a special attention has been given to polyphenols, and especially to flavonoids, which provide for these species important uses in the pharmaceutical, cosmetics and food industry, that are due to their important medicinal properties as the antioxidant, anti-inflammatory, antifungal or antibacterial ones [
4]. In this context and taking into consideration the fact that in the last decades these compounds have shown a significant importance in the field of medicinal compounds, the
Asteraceae species should be reconsidered as possible sources of flavonoids and polyphenols, generally.
Antennaria dioica (L.) Gaertn. and
Helichrysum arenarium (L.) Moench. (
Figure 1) are two species belonging to the same tribe (
Gnaphalieae) [
6,
7] of the
Asteraceae family [
8,
9]. These species are widespread across the European continent, especially in Central and Eastern countries and are known in traditional medicine for their use in the treatment of different pathologies. The connection between these species relies on several common morphological characters and it is confirmed by phylogenetic studies [
10,
11], which certify their common taxonomic classification. The folk medicine of different countries cites common uses for herbal preparations obtained from the flowers of these species, exploited for their diuretic [
12,
13], choleretic and anti-inflammatory properties [
13,
14,
15]. Romanian sources mention both under the same phytonyme, describing their use for the treatment of jaundice [
16]. Few scientific evidence exists up to date in order to support these data and a more detailed study of the two species becomes therefore important.
A. dioica is a perennial herb, commonly found in dry grasslands and sandy or stony places from Eurasian areas, where it is traditionally used to treat biliary and respiratory ailments [
13,
15], and also for its astringent and hemostatic properties [
13,
17]. To date, few scientific data were reported regarding the chemical composition of this species. Meriҫli et al. [
15] studied the extracts obtained from the flowers of
A. dioica and isolated ursolic acid, chlorogenic acid, apigenin-7-
O-glucoside and luteolin-7-
O-glucoside. To the best of our knowledge, this is the only existing scientific study that describes the flavonoid composition of this species, and it does not establish a connection between the potential bioactivities of the species and the isolated compounds.
Helichrysum species were known from ancient times due to a large distribution and diversity and are cited as being a potent remedy for various pathologies [
12,
14,
18]. Among the species of this genus, a special importance was given to
H. arenarium, which is the most popular species, having monographs in different pharmacopoeias, such as the Russian Pharmacopoeia [
14]. Several studies aimed to investigate the phytochemical profile of
H. arenarium and to evaluate its potential bio-activities [
16,
17,
18,
19,
20]. Major constituents of the extracts obtained from the flowers of
H. arenarium are polyphenols, especially flavonoids [
19,
20,
21,
22,
23], usually found as glycosides. The connection between the phenolic content of this species and its antioxidant, anti-inflammatory and antimicrobial activities was studied by different authors [
19,
20,
21]. Other studies focused on the investigation of the volatile compounds. In this regard, Rančić et al. [
24] showed that the essential oil from
H. arenarium flowers has an important antimicrobial potential. All existing data remain nevertheless scarce and do not establish a clear chemical composition of the flowers and their connection with the cited biological activities.
Both species are used for different purposes in Romanian traditional medicine, but, despite this fact, few scientific data about the chemical composition and biological activities of
A. dioica and
H. arenarium collected from the Romanian spontaneous flora were reported so far. Grădinaru et al. investigated the phenolic content and antibacterial activity of a methanolic extract obtained from the inflorescences of
Helichrysum arenarium (L.) Moench subsp.
arenarium against lower respiratory tract pathogens, being the only existing scientific study about a Romanian
Helichrysum sample [
22]. Nonetheless, no scientific data with regard to Romanian
A. dioica exist to date.
Within this frame, the present study aimed to investigate the phenolic, flavonoidic and sterolic composition of A. dioica (L.) Gaertn. and H. arenarium (L.) Moench. collected from the Romanian spontaneous flora and to assess their antioxidant and antimicrobial properties. Thus, the present study represents a starting point for a most detailed study of the two species, demonstrating their important potential as sources of bioactive compounds.
3. Materials and Methods
3.1. Plant Material
Flowers of H. arenarium were collected from Botoșani county (North Eastern Romania), while flowers of A. dioica were collected from Suceava county, (North Eastern Romania). The species were identified by Dr. Ramona Păltinean, and voucher specimens were deposited in the Herbarium of the Department of Pharmaceutical Botany, Faculty of Pharmacy, “Iuliu Hațieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania (H. arenarium—Voucher No. 143.17.3.1 and A. dioica—Voucher No. 143.14.1.1).
3.2. Extraction Procedure
Dried flowers belonging to the two species were ground to a fine powder. This powder (1.00 g) was mixed with the appropriate solvent (20.00 mL) in a round-bottom flask and stirred for 20 min at 600 rpm on a mechanical stirrer. The solutions were then extracted by sonication for 30 min, at 70 °C, using as solvents methanol (99.98%), ethanol (96%) and 70% v/v ethanol in water. Subsequently, extracts were filtered and evaporated to dryness under reduced pressure, using a rotary evaporator. The crude extracts were weighed and stored in the refrigerator until they were analyzed. All analyses were performed in triplicate on the three types of extracts and the results were presented as mean ± SD.
In order to obtain more accurate data on flavonoid glycosides and aglycones concentration, each sample was analyzed before and after acid hydrolysis. Extractive solution (2.00 mL) was treated with 2 M hydrochloric acid (2.00 mL) and a 100 mg/mL ascorbic acid solution (0.20 mL). The mixtures were heated at 80 °C on a water bath for 30 min, ultrasonicated for 15 min, and re-heated for another 30 min at 80 °C. During the heating, 1.00 mL of each solvent was added to the extraction mixture every 10 min, in order to ensure a constant volume. The mixtures were centrifuged at 4000 rpm and the solutions were diluted with distilled water in a 10.00 mL volumetric flask and filtered through a 0.45 µm filter before injection [
31]. For the antimicrobial activity, the obtained extracts were evaporated to dryness under reduced pressure and re-suspended in bi-distilled water.
3.3. Chemicals
References used for the HPLC-MS analysis were purchased from Sigma Aldrich (St. Louis, MO, USA): Chlorogenic acid, p-coumaric acid, caffeic acid, rutin, apigenin, quercetin, isoquercitrin, quercitrin, hyperoside, kaempferol, myricetol, and fisetin, Roth (Karlsruhe, Germany): Ferulic acid, sinapic acid, gentisic acid, gallic acid, patuletin, luteolin or from Dalton (Toronto, ON, Canada): cichoric acid, caftaric acid. HPLC grade solvents, analytical grade acids used for mobile phases and Folin-Ciocâlteu reagent were purchased from Merck (Darmstadt, Germany), together with sodium carbonate, dipotassium hydrogen phosphate, potassium dihydrogen phosphate and aluminium chloride used for antioxidant assays. ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) ≥98% purity, potassium peroxodisulfate (≥99% purity), DPPH, and Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid; ≥97% purity) also used in antioxidant tests were purchased from Sigma Aldrich (Schnelldorf, Germany). Gallic acid monohydrate (99.5%) was purchased from Serva, (Heidelberg, Germany).
3.4. HPLC-MS Analysis
3.4.1. Apparatus and Chromatographic Conditions
Polyphenols, methoxylated flavones and phytosterols were analyzed and quantified using an HPLC-MS method. The apparatus consisted in an 1100 HPLC Series Agilent Technologies system (Agilent, Santa Clara, CA, USA), equipped with a G1322A degasser, G13311A binary gradient pump, column thermostat, G1313A autosampler and G1316A UV detector. The HPLC system was coupled with an Agilent 1100 mass spectrometer (LC/MSD Ion Trap VL). The chromatographic data were processed using ChemStation and DataAnalysis software from Agilent [
25,
32,
33,
34].
3.4.2. HPLC-MS Analysis of Phenolic Compounds
Analysis of phenols was carried out on by injecting 5 µL of the flowers extracts of
H. arenarium and
A. dioica on a Zorbax SB-C18 reverse-phase analytical column (100 × 3.0 mm i.d., 3.5 µm particles) and separation of compounds was performed using as a mobile phase a mixture of methanol and acetic acid 0.1%
v/
v. The flow rate was set at 1 mL/min and working temperature was 48 °C. The binary gradient that allowed the elution of compounds started with 5% methanol in a linear mode and ended with 42% methanol, for 35 min. The last 3 min of the gradient were set at 42% methanol. Detection of the compounds was performed on UV, at 330 nm until 17.5 min and at 370 nm until the end of the analysis time. The MS was also used for detection of compounds and was operated in a negative mode, using an electrospray ion source. Using these conditions, all polyphenols could be eluted in less than 40 min (
Table 9) [
25,
32,
33,
34].
The specific mass spectra of each polyphenol were recorded in a library and the MS traces/spectra of the compounds that were found in samples were compared with spectra existing in the library. Qualitative analysis of polyphenols was performed based on the MS signal, used for identification of compounds; only compounds identified on basis of their MS spectra were further quantified using the UV signal. Quantitative analyses were performed using the external standard method, with calibration curves of 5 points, ranging between 0.5–50 µg/mL, having a good linearity (
R2 > 0.999). Limit of quantification in this method was 0.5 µg/mL, and the limit of detection was 0.1 µg/mL [
25,
32,
33,
34].
3.4.3. HPLC-MS Analysis of Methoxylated Flavones
Analysis of methoxylated flavones was carried out on the same HPLC instrument, using the same analytical column. Mobile phase also consisted in the mixture of 0.1% (
v/
v) acetic acid and methanol, but ratios of solvents were changed in order to better perform the separation of compounds. Total duration of the method was less than 10 min and gradient began with 45% methanol and ended at 50% methanol, with a flow rate of 0.9 mL/min. Injection volume was 5 µL and temperature was set at 48 °C. Detection was performed on the same MS/MS system, using an electrospray ionization (ESI) source, in negative mode. Conditions used for detection were set as following: nebulizer pressure at 60 psi, gas (nitrogen) temperature at 325 °C with a flow rate of 12 L/min, and capillary voltage +2500 V. Specific fragments were monitored. Identification was achieved by comparison of retention times and mass spectra for the existing references (
Table 10) and compounds identified in samples. Parent ions were detected as forms of the molecules, which have lost a proton. The multiple reaction monitoring mode was used in order to avoid background interferences [
33,
34].
3.4.4. HPLC-MS Analysis of Phytosterols
Conditions for the analysis of phytosterols were also slightly different from the ones described in the analysis of polyphenols and methoxylated flavones. Same apparatus was used for the separation of compounds. Chromatographic analytical column was the same, but elution of compounds was performed in an isocratic mode, in a mixture of 10:90 (
v/
v) of methanol and acetonitrile. Flow rate was set at 1 mL/min, chromatographic system was operated at 40 °C and the injection volume was 5 µL. For detection of compounds, the same mass spectrometer was used, with an atmospheric pressure chemical ionization (APCI) interface, in a positive mode. Conditions were set as following: gas temperature (nitrogen) 325 °C at a flow rate of 7 L/min, nebulizer pressure 60 psi and capillary voltage −4000 V. Identification of compounds was performed on the same basis as the one performed for methoxylated flavones, using the multiple reactions monitoring analysis model (
Table 11) [
32].
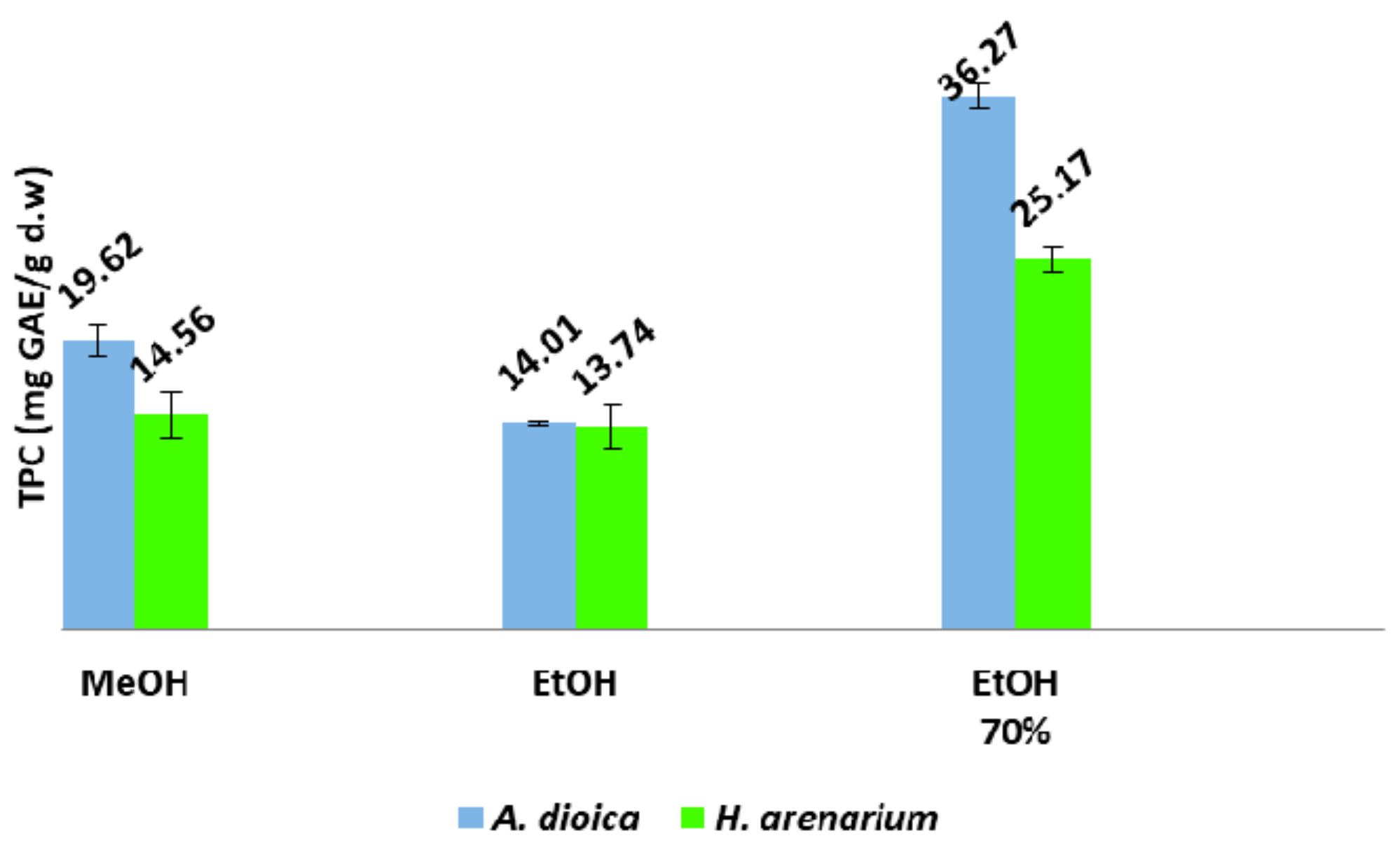
3.5. Determination of Total Phenolic Content
The TPC was determined using the Folin–Ciocâlteu method. For a high throughput of samples, a SPECTROstar Nano Multi—Detection Microplate Reader with 96-well plates (BMG Labtech, Ortenberg, Germany) was used. Briefly, a mixture solution consisting of 20 µL of extract, 100 µL of Folin-Ciocâlteu reagent and 80 µL of sodium carbonate (Na
2CO
3, 7.5%
w/
v) was homogenized and incubated at room temperature in the dark for 30 min. Afterwards, the absorbance of the samples was measured at 760 nm. Gallic acid was used as a reference standard, and the TPC was expressed as gallic acid equivalents (GAE) in mg/g dry weight (d.w.) of plant material [
35,
36].
3.6. Determination of Total Flavonoid Content
The total flavonoid content (TFC) was calculated and expressed as quercetin equivalents using a method previously described by Mocan et al. [
37,
38]. Briefly, a 100 µL aliquot of 2% AlCl
3 aqueous solution was mixed with 100 µL of sample. After an incubation time of 15 min, the absorbance of the sample was measured at 420 nm. Quercetin was used as a reference standard, and the TFC was expressed as quercetin equivalents (QE) in mg/g dry weight (d.w.) of plant material.
3.7. Antioxidant Activity Assays
3.7.1. TEAC Assay
The TEAC of the different
Helichrysum and
Antennaria extracts against the stable synthetic ABTS radical cation was tested using the method described by Martinez et al. [
39] and Savran et al. [
40]. A Trolox calibration curve was plotted as a function of the percentage of ABTS radical cation scavenging activity, and the results were expressed as milligrams of trolox equivalents (TE) per milliliter of herbal extract (mg TE/mL).
3.7.2. DPPH Assay
The antioxidant capacity of the investigated samples against the DPPH radical was tested using the method previously described by Martins et al. and Mocan et al. [
41,
42]. A Trolox calibration curve was plotted as a function of DPPH consumption, and the results were expressed as milligrams of trolox equivalents (TE) per milliliter of herbal extract (mg TE/mL).
3.8. Assay of Antimicrobial Activity
3.8.1. Bacteria and Culture Conditions
For this bioassay, five aerobic bacterial strains were used, Staphylococcus aureus (ATCC 49444), Bacillus cereus (ATCC 11778), Listeria monocytogenes (ATCC 19114), Salmonella typhimurium (ATCC 14028), and Escherichia coli (ATCC 25922). All of the tested microorganisms were obtained from the Laboratory of Food Biotechnology, Life Sciences Institute, University of Agricultural Sciences and Veterinary Medicine, Cluj Napoca, Romania. The bacteria were cultured on Muller-Hinton Agar and stored at 4 °C and subcultured once a month.
The modified microdilution technique was used to evaluate antimicrobial activity as previously reported by Mocan et al. [
29,
38]. Bacterial species were cultured overnight at 37 °C in Tryptic Soy Broth (TSB) medium. The bacterial cell suspensions were adjusted with sterile saline to a concentration of approximately 3 × 10
5 CFU/mL in a final volume of 100 µL per well. The inoculum was stored at +4 °C for further use. Dilutions of the inoculum were cultured on solid Muller–Hinton (MH) for bacteria to verify the absence of contamination and to check the validity of the inoculum. Determinations of minimum inhibitory concentrations (MICs) were performed by a serial dilution technique using 96-well microtitre plates. The concentration range of extracts was 1000–0.03 mg/mL. Different solvent dilutions of ethanol: methanol extracts were carried out over the wells containing 100 µL of Tryptic Soy Broth (TSB) and afterwards, 10 µL of inoculum was added to all the wells. The microplates were incubated for 24–48 h at 37 °C. The MIC of the samples was detected following the addition of 20 µL (0.2 mg/mL) of resazurin solution to each well, and the plates were incubated 2 h at 37 °C. A change from blue to pink indicates reduction of resazurin and, therefore, bacterial growth. The MIC was defined as the lowest drug concentration that prevented this color change. The minimum bactericidal concentrations (MBCs) were determined by serial subcultivation of a 2 µL into microtitre plates containing 100 µL of broth per well and further incubation for 48 h at 37 °C. The lowest concentration with no visible growth was defined as MBC, indicating 99.5% killing of the original inoculum. Streptomycin (Sigma P 7794) (0.05–3 mg/mL) was used as positive control. Water was used as negative control.
3.8.2. Antifungal Activity
To investigate the antifungal activities, the following fungi were used:
Aspergillus flavus (ATCC 9643),
Aspergillus niger (ATCC 6275),
Candida albicans (ATCC 10231),
Candida parapsilosis (ATCC 22019) and
Penicillium funiculosum (ATCC 56755). These fungi were obtained from the Laboratory of Food Biotechnology, Life Sciences Institute, University of Agricultural Sciences and Veterinary Medicine, Cluj-Napoca, Romania. Cultures were maintained on malt agar at 4 °C and subcultured every month. Spore suspension (1.0 × 10
5) was obtained by washing agar plates with sterile solution containing (0.85% saline, 0.1% Tween 80 (
v/
v), then added to each well to a final volume of 100 µL. Inocula were screened for contamination by culturing on a solid medium. The minimum inhibitory (MIC) and minimum fungicidal (MFC) concentrations assays were performed using the microdilution method by preparing a serial of dilutions in 96-well microtiter plates. The extracts were diluted in 0.85% saline (10 mg/mL), then added to microplates containing Broth Malt medium with inoculum and incubated for 72 h at 28 °C on a rotary shaker. The lowest concentrations without visible growth (at the binocular microscope) were defined as minimal inhibitory concentrations (MICs). The fungicidal concentrations (MFCs) were determined by serial sub-cultivation of 2 µL of tested extracts dissolved in medium and inoculated for 72 h, into microtiter plates containing 100 µL of broth per well and further incubation 72 h at 28 °C. The lowest concentration with no visible growth was defined as MFC indicating 99.5% killing of the original inoculum. The fungicide fluconazole was used as positive control (1–3500 µg/mL). All experiments were performed in duplicate and repeated thrice [
29].
3.9. Statistical Analysis
The samples have been analyzed in triplicate; the average and the relative SD have been calculated using the Excel software package (Microsoft, Redmond, WA, USA).