Abstract
The genus Paeonia, also known as the “King of Flowers” in China, is an important source of traditional Chinese medicine (TCM). Plants of this genus have been used to treat a range of cardiovascular and gynecological diseases. However, the potential pharmacological activity of one particular species, Paeonia rockii, has not been fully investigated. In the first part of the present study, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) acid (ABTS), reducing power assays, and metal ion chelating assays were used to investigate the in vitro antioxidant activities of Paeonia rockii. In the second portion of the study, a mouse model of d-galactose-induced aging was used to validate the antioxidant effects of the flowers from Paeonia rockii in vivo. Lastly, potential antioxidant constituents were screened and identified by ultra-high pressure liquid chromatography and electrospray ionization coupled with high-resolution mass spectrometry (UHPLC-ESI-HRMSn) combined with the DPPH assay. Results indicated that the flowers and leaves exhibited stronger antioxidant activity than ascorbic acid in vitro. The therapeutic effect of Paeonia rockii was determined in relation to the levels of biochemical indicators, such as 8-iso-prostaglandin F2α (8-iso PGF2α) in the serum, superoxide dismutase (SOD), protein carbonyl, malondialdehyde (MDA), and glutathione (GSH) in the liver and brain, after daily intra-gastric administration of different concentrations of extracts (100, 200 and 400 mg/kg) for three weeks. The levels of 8-iso PGF2α (p < 0.01) and protein carbonyl groups (p < 0.01) were significantly reduced, whereas those of SOD (p < 0.05) had significantly increased, indicating that components of the flowers of Paeonia rockii had favorable antioxidant activities in vivo. Furthermore, UHPLC-ESI-HRMSn, combined with pre-column DPPH reaction, detected 25 potential antioxidant compounds. Of these, 18 compounds were tentatively identified, including 11 flavonoids, four phenolic acids, two tannins, and one monoterpene glycoside. This study concluded that the leaves and flowers from Paeonia rockii possess excellent antioxidant properties, highlighting their candidacy as “new” antioxidants, which can be utilized therapeutically to protect the body from diseases caused by oxidative stress.
1. Introduction
Oxidative stress is an excess of free radical production caused by a series of complex cellular processes, such as the accumulation of hydrogen peroxide, hydroxyl radicals, and superoxide anions during normal cellular metabolism. However, oxidative stress is thought to be involved in the development of a range of human diseases including aging, liver damage, cardiovascular disease, and cancer [1,2,3,4]. According to previous reports, antioxidants are able to prevent the production of free radicals [5,6] and the chain reaction which occurs before vital molecules are damaged, thus protecting the body from subsequent oxidative stress and further tissue injury [7,8]. There are two basic categories of antioxidants: synthetic and natural. In general, synthetic antioxidants are compounds with phenolic structures of varying degrees of alkyl substitution, while natural antioxidants may be flavonoids and phenolic compounds [9]. Due to the toxicity of synthetic antioxidants and their undesirable effects on human health [10], interest in natural antioxidants has increased considerably [11]. Natural antioxidants originating from Traditional Chinese medicinal herbs Bletilla striata, Lonicera japonica, and Paeonia have been reported to possess favorable antioxidant activity [12,13,14].
The genus Paeonia features 35 species [15], originates from Europe and Asia and is referred to as the “King of Flowers” in China. Paeonia is an ornamental shrub and one of the most important sources of traditional Chinese medicine (TCM) [14]. Paeonia predominantly contain monoterpenes, triterpenoids, flavonoids, phenols, and tannins [16,17] and exhibit antioxidative, anti-inflammatory, antimicrobial, analgesic, sedative, cardioprotective, and gynecological effects [18,19]. Earlier studies have isolated and identified five flavonoids from the flowers of Paeonia ostii, which are responsible for its strong antioxidant activities [20]. Seeds from the nine tree peony species have been shown to contain phenolic compounds which confer antioxidant activity, and have subsequently gained attention as a health food [21]. The chemical components and pharmacological activities of Paeonia roots have also been investigated recently, including its antioxidant activity [17,22].
Paeoni rockii (S.G. Haw & Lauener) T. Hong & J.J. Li ex D.Y. Hong (Paeoniaceae) is an important ancestral species of cultivated tree peony which naturally thrives in the provinces of Gansu, Henan, and Sichuan, Tibet, and several regions in Northern China. Paeonia rockii grows in areas at an altitude of 1100–2800 m [23]. Although local indigenous people use the flowers and leaves from Paeonia rockii as a decoction for the treatment of acne and gynecological diseases, its application in other medical scenarios has yet to be documented. Existing studies on the efficacy of Paeonia rockii suggest that its roots and seeds contain antioxidant compounds; oil extract from seeds, fruits, and root bark is already utilized as a component of moisturizing cosmetics and health-care capsules and as a new health food [14,24,25]. Given the rarity of Paeonia rockii, it is important to carry out specific studies of its flowers and leaves in order to make better use of this important resource.
However, information relating to the antioxidant activities of the flowers and leaves from Paeonia rockii is very scarce. Therefore, this study aims to investigate the potential antioxidant activities of leaves and flowers from Paeonia rockii, and to identify antioxidant compounds by UHPLC-ESI-HRMSn with a pre-column DPPH reaction.
2. Results
2.1. Total Phenolic and Flavonoid Content
Most antioxidant activities from plant sources correlate with phenolic and flavonoid contents [26,27,28]. In this study, we determined the TPC and TFC of MF and ML (Table 1). The TPC of ML at 693.93 ± 0.82 mg GAE/g extract was higher than that of MF at 540.38 ± 1.83 mg GAE/g, whereas the TFC of MF at 130.40 ± 0.41 mg RE/g extract was lower than that of ML at 180.8 ± 0.37 mg RE/g extract. TPC was also higher than TFC in both MF and ML extracts.

Table 1.
Total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activity of extracts prepared from Paeonia rockii.
2.2. In Vitro Assays
2.2.1. DPPH Radical Scavenging Activity
The DPPH radical is a stable free radical which is widely used in the evaluation of antioxidant capacity [29]. In the present study, the DPPH radical-scavenging activity of extracts was measured using spectrometric methods. Figure 1a shows dose-dependent activity, while Table 1 shows the IC50; a low IC50 value indicates high antioxidant activity. The DPPH radical-scavenging activity can be represented as follows: ML > MF > ascorbic acid.
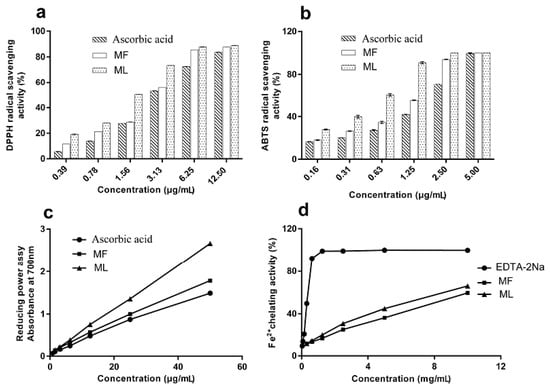
Figure 1.
Determination of the antioxidant activities of extracts from Paeonia rockii leaves and flowers using (a) DPPH radical scavenging assay; (b) ABTS radical scavenging assay; (c) reducing power assay; and (d) Fe2+ chelating assay.
2.2.2. ABTS Radical Scavenging Activity
The ABTS radical cation is generated when the ABTS radical cation reacts with oxidants and its absorbance is measure at 734 nm [5]. Antioxidants from Paeonia rockii extracts are known to reduce the ABTS radical cation. Figure 1b presents the ABTS radical-scavenging activity of Paeonia rockii extracts while Table 1 shows the relevant IC50 values. The ABTS radical-scavenging activity is as follows: ML > MF > ascorbic acid. Hence, Paeonia rockii extracts exhibit excellent ABTS radical cation scavenging ability.
2.2.3. Reducing Power
The reducing power assay determines antioxidant activity based on the reducing potential, or the ability of the extract to convert Fe3+ to Fe2+ [30]. The reducing potential was monitored by measuring the formation of a Prussian blue product at 700 nm. Figure 1c shows that the reducing power of MF and ML extracts increased with increasing concentrations. The ascorbic acid equivalents (AAE) given in μg AAE/100 μg of MF and ML were 119.85 ± 0.00 and 178.81 ± 0.00, respectively. The order of reducing power was ML > MF > ascorbic acid, indicating that ML had a higher antioxidant activity than MF and ascorbic acid.
2.2.4. Metal Ion Chelating Ability
Metal chelation is an important antioxidant property, as it reduces the concentration of the catalyzing transition metal in lipid peroxidation [31,32]. In this study, EDTA-Na2 was used as a positive control. ML showed a significantly stronger Fe2+ chelating activity than MF (p < 0.05). Figure 1d shows the Fe2+ chelating activity as EDTA-Na2 > ML > MF. The values demonstrate that EDTA has the strongest chelating capacity and the chelation rate than ML and MF. The results suggest that the ML and MF complexes with the ferrous ion in a dose-dependent manner [32].
2.3. In Vivo Assays
2.3.1. Establishment of an Animal Model
As shown in Table 2 and Table 3, the levels of 8-iso PGF2α in serum, and the levels of MDA and protein carbonyl in the liver and brain of the d-galactose-induced group of mice were significantly increased (p < 0.01), except for MDA in the liver (p < 0.05). MDA and protein carbonyl were reported as the major marker of endogenous lipid peroxidation, the increase of the levels of MDA and protein carbonyl showed d-galactose-induced benefit to the degree of oxidation [33,34]. Furthermore, SOD and GSH levels in the liver and brain of the d-galactose group were significantly reduced (p < 0.01), except for GSH in the brain (p < 0.05). This indicates that d-galactose can cause oxidative stress or damage.

Table 2.
Effects of Paeonia rockii flower extracts on 8-iso-PG from blood serum, brain and liver MDA, and protein carbonyl in d-galactose-induced mice.

Table 3.
Effects of Paeonia rockii flower extracts on brain and liver SOD and GSH in d-galactose-induced mice.
2.3.2. Biochemical Indices
The in vivo antioxidant effects of treatment with extracts prepared from the flowers of Paeonia rockii are shown in Table 2. Notably, treatment significantly reversed the antioxidant activity of 8-iso PGF2α, MDA and protein carbonyl in d-galactose-induced mice. Of these indices, 8-iso PGF2α in the serum, and protein carbonyl in the liver and brain were the biochemical markers indicative of the most extensive change. Moreover, treatment with flower extract markedly increased the levels of antioxidants, including SOD and GSH, compared with the d-galactose-induced model mice, especially in terms of SOD in the liver and brain (Table 3). The flower extract reduced oxidative damage by changing the levels of important biochemical indices, presumably due to its powerful antioxidant properties. The phytochemicals found to be present in the flower extracts, such as flavonoids and phenolic components, are likely to be responsible for the observed antioxidant properties, which have been reported previously.
2.4. Screening Antioxidants by DPPH-UHPLC-ESI-HRMSn Analysis
DPPH-UHPLC-ESI-HRMSn can be used for the rapid screening of antioxidants from complex mixtures, based on the fact that the level of antioxidant compounds decreases or disappears completely following the pre-column reaction with DPPH [35,36]. In the current study, potential antioxidant compounds were identified by UHPLC-ESI-HRMSn in negative ion mode, and their retention time (tR), molecular formula, and MS fragmentation patterns were compared with published data, MS/MS data and identified compounds were presented in Table 4. Figure 2 shows chromatograms generated by extracts prepared from the flowers and leaves from Paeonia rockii before and after reaction with a DPPH radical. Twenty-five potential antioxidant compounds were screened based on a 46 compound database created in a preliminary study carried out by our group [37]; 18 of these compounds were identified in Paeonia rockii. Of these, 11 (quercetin-7-O-glucoside, quercetin-3-O-glucoside, kaempferol-7-O-glucoside, kaempferol galloylglucoside, astragalin, apigenin rhamnoglucoside, isorhamnetin-3-O-glucoside, apigenin galloylglucoside isomer, isorhamnetin, and apigenin), are flavonoids; four (gallic acid, hydroxybenzoic acid, methyl gallate, and methyl digallate) are phenolic acids; two (digalloyl glucose and tetragalloylglucose) are tannins; and one, benzoyloxypaeoniflorin, is the only monoterpene glycoside [17,37]. The results arising from the in vitro antioxidant activity tests, and the DPPH-UHPLC-ESI-HRMSn assay, show that leaves have stronger antioxidant activity than flowers—this may be owing to the structure and concentration of the individual constituents.

Table 4.
List of partially-identified antioxidant compounds from the flowers and leaves of Paeonia rockii by UHPLC-ESI-HRMSn analysis.

Figure 2.
Base peak chromatogram (BPC) of the extracts from Paeonia rockii flowers and leaves (negative mode). B-MF: Methanol extracts from flowers without reaction with DPPH; A-MF: Methanol extracts from flowers reacted with DPPH; B-ML: Methanol extracts from leaves without reaction with DPPH; A-ML: Methanol extracts of leaves after reaction with DPPH.
3. Discussion
Previous reports showed that extracts prepared from Paeonia seeds and roots exhibited antioxidant activity [21,22,38] and that seeds can be used as a source of functional food [25,39]. However, the biological activities of the Paeonia rockii plant have been scarcely investigated, and this study is the first to establish that Paeonia rockii leaf and flower extracts possess excellent DPPH radical scavenging activity, ABTS radical scavenging activity, and reducing power. In comparison with other members of the Paeonia genus, the antioxidant activity of flowers from Paeonia rockii was superior to that of Paeonia ostii and other Paeonia [20,40,41]. Earlier reports demonstrated the IC50 values of Paeonia ostii in DPPH and ABTS radical scavenging assays were in the range of 20 µg/mL and 40 µg/mL, respectively [21]. In this study, the IC50 values of Paeonia rockii in DPPH and ABTS radical scavenging assay were lower (3 µg/mL). Compared with Paeonia rockii roots, the extracts of flowers and leaves also demonstrated a stronger DPPH radical scavenging effect (13.3 µg/mL) [14]. Moreover, the antioxidant ability of extracts from flowers and leaves in vitro was stronger than that of ascorbic acid.
In the present study, the flowers and leaves from Paeonia rockii contain large amounts of total flavonoids and total phenolic acids. Flavonoids and phenolic acids are known to act as antioxidants, not only for their ability to donate hydrogen or electrons, but also for their role as stable radical intermediates [42]. Other previous results revealed that the radical scavenging activities of the flavonoids tested were correlated with the number and position of phenolic hydroxyl groups in the molecules [42]. Furthermore, the presence of at least one ortho-dihydroxy group in the benzene ring of the polyphenols was involved in the stability of electrons and free radicals [43]. DPPH-UHPLC-ESI-HRMSn assay confirmed that flavonoids and phenolic acids play a certain role in the extract’s antioxidant activity.
DPPH is one of the known stable radical species used to measure the radical-scavenging potential of various antioxidants, changing the molecular structure of the antioxidant in the process [44,45]. The DPPH-HPLC method can be used as a rapid screening tool for radical scavenging in complex mixtures, particularly plant extracts with minimal sample preparation. It was hypothesized that the antioxidant structure would change following a reaction with DPPH. Thus, the peak areas of compounds would be reduced or disappear completely [35,36,46]. As seen from the DPPH-UHPLC-ESI-HRMSn assay, our data highlights potential antioxidant compounds containing 11 flavonoids, four phenolic acids, two tannins, and one monoterpene glycoside. It is likely that flavonoids and phenolic acids represent the major compounds responsible for the antioxidant activity of Paeonia rockii flowers and leaf extracts [14,47].
Previous studies demonstrated that 10 of the 18 potential antioxidant compounds identified using the DPPH-UHPLC-ESI-HRMSn assay showed significant antioxidant activity by in vitro assessments: quercetin [21], gallic acid, methyl gallate [14], apigenin [36], methyl digallate [48], isorhamnetin [49], kaempferol-7-O-glucoside, isorhamnetin-3-O-glucoside [50], quercetin-7-O-glucoside, and quercetin-3-O-glucoside [51]. These results are consistent with previous reports. Notably, gallic acid, the ortho-hydroxyl group on the benzene ring, was shown to be the active group and the more hydroxyl groups, the stronger the oxidation resistance [43]. Furthermore, eight potential antioxidant components were identified in Paeonia rockii for the first time in this study: hydroxybenzoic acid, astragalin, benzoyloxypaeoniflorin, tetragalloylglucose, apigenin galloylglucoside isomer, apigenin rhamnoglucoside, kaempferol galloylglucoside, isorhamnetin-3-O-glucoside, and digalloyl glucose. UHPLC-ESI-HRMSn with a pre-column DPPH reaction as a method to identify antioxidant compounds from complex natural extraction is fast, low cost, high-throughput, and yields an index value [36]. In addition, qualitative and quantitative analyses of the antioxidant components, along with intensive studies of the mechanisms involved, are necessary.
In this study, in vivo antioxidant activity was validated using an established mouse model of d-galactose-induced aging to determine the levels of serum 8-iso PGF2α, SOD, protein carbonyl, MDA, and levels of GSH in the liver and brain as biochemical indicators. The d-galactose-induced aging mouse model has been utilized extensively for evaluating the antioxidant effects of drugs and plant extracts [33,34,52]. d-galactose treatment can increase the levels of 8-iso PGF2α, MDA, and protein carbonyl, and reduce the levels of SOD and GSH [34,53]. Antioxidants can prevent increases in the levels of biochemical indicators resulting from d-galactose treatment [33]. Extracts from Paeonia rockii flowers decreased levels of the biochemical indicators which had increased following d-galactose induction, particularly levels of 8-iso PGF2α, protein carbonyl, and SOD. In the present study, extracts of flowers from Paeonia rockii showed antioxidant activity in vivo. However, whether the 18 potential antioxidant compounds in vitro are effective antioxidants in vivo remains to be seen. Furthermore, studies are needed to isolate and identify the significant activities of compounds present in extracts of Paeonia rockii flowers and leaves and to elucidate their molecular mechanisms. Several previous in-depth investigations have indicated that oxidative stress was related to inflammation, cardiovascular disease, and Alzheimer’s disease [54,55,56]; moreover, biochemical indicators, such as increased GSH and SOD, and reduced levels of MDA, were identified as effective indices of inflammation and cardiovascular disease. Therefore, effective antioxidant activities in natural products and resources could play an important role in the prevention or treatment of diseases caused by oxidative stress.
4. Material and Methods
4.1. Chemicals and Reagents
The following chemicals were purchased from Chengdu Must Biotechnology Co., Ltd. (Chengdu, China): quercetin-3-O-glucoside, kaempferol, isorhamnetin, DPPH, ABTS, Folin-Ciocalteu reagent, and ascorbic acid. All other analytical reagents used for plant extraction and antioxidant analyses were obtained from Chengdu Kelong Chemical Co., Ltd. (Chengdu, China). UHPLC-ESI-HRMSn analysis was carried out with HPLC-grade acetonitrile (Sigma-Aldrich, St. Louis, MO, USA) and deionized water purified using a Milli-Q system (Millipore, Bedford, MA, USA).
4.2. Plant Material and Extraction
Samples of Paeonia rockii were collected from Songpan County (Northwestern Sichuan, China, and Tibetan areas) in May 2014, and the plants were identified by Professor Linfang Huang from the Institute of Medicinal Plant Development, Peking Union Medical College and Chinese Academy of Medical Sciences (Beijing 100193, China). The plant material consisted of individually-separated leaves and flowers and was preserved in the Herbarium of Southwest Minzu University.
The leaves and flowers were initially freeze-dried and then ground to a fine powder in a mechanical grinder with a 65-µm mesh size. To prepare extracts, 100 g of the plant powder was sonicated three times at room temperature for 1 h with 1000 mL of methanol. Filtered solutions were then concentrated using a rotary evaporator. The residue was lyophilized, and the resulting dry powder was stored at 4 °C. Methanol extracts of Paeonia rockii flowers (MF) and leaves (ML) yielded 20.53% and 19.62% dry matter, respectively, relative to the dry starting material. For UHPLC-ESI-HRMSn analysis, and in vitro studies, the dry extracts were re-dissolved in methanol (to a concentration of 5 mg/mL) and filtered through 0.22-μm nylon microporous membranes.
4.3. Determination of Total Phenolic Content (TPC)
The total phenolic content (TPC) of each extract was determined using Folin-Ciocalteu reagent according to a previously described method [5,57] with minor modifications. First, 0.2 mL of each sample (0.1 mg/mL) or standard (gallic acid; 3.125–100 μg/mL) was mixed with 2 mL of Folin-Ciocalteu reagent. After 5 min, 4 mL of 10% Na2CO3 was then added to the mixture, which was incubated at room temperature for 120 min before the absorbance was measured at 765 nm. TPC was expressed as mg gallic acid equivalents (GAE)/g dry weight of the extracts.
4.4. Determination of Total Flavonoid Content (TFC)
The total flavonoid content (TFC) of MF and ML was determined by a colorimetric assay using rutin as a standard [58]. Briefly, 5 mL of MF and ML (0.1 mg/mL) or rutin (8–48 μg/mL) was mixed with 1 mL of 5% NaNO2. After 6 min, 1 mL of 10% aluminum nitrate was added, and the mixture was allowed to stand for another 6 min. Afterwards, 10 mL of 10% NaOH was added to the mixture. The absorbance was measured at 510 nm after incubating the mixture at room temperature for 15 min. TFC was expressed as mg rutin equivalents (RE)/g dry weight of the extracts.
4.5. In Vitro Antioxidant Activity Testing
4.5.1. DPPH Radical Scavenging Assay
The DPPH radical-scavenging activity assay was performed as previously reported [29]. The extracts (2 mL) were mixed at different concentrations (0.39–12.50 μg/mL), with 2 mL of DPPH solution (0.1 mM, in methanol). The mixture was then shaken and incubated at room temperature for 30 min, and the absorbance was measured at 517 nm. Ascorbic acid was used as a reference [19,28]. Then, radical scavenging activity was calculated as follows:
where Ai is the absorbance in the presence of the extract. As is the absorbance in the presence of the sample background; and Ac is the absorbance of the negative control (without the extracts). IC50 was determined by probit regression using IBM’s Statistical Program for Social Sciences (SPSS, IBM, Armonk, NY, USA) analysis of variance (20.0).
DPPH radical scavenging activity (%) = [1 − (Ai − As)/Ac] × 100
4.5.2. ABTS Radical Scavenging Assay
The ability of the extracts to scavenge ABTS radical cations was evaluated using a previously described method [5] with some modifications. First, an ABTS radical cation solution was prepared by reacting 7 mM ABTS radical cation with 2.45 mM potassium persulphate at room temperature in the dark for 12–16 h. The solution was then diluted with distilled water to obtain an absorbance of 0.7 ± 0.02 at 734 nm. Aliquots (2 mL) of extracts at various concentrations (0.16–5 μg/mL) were then mixed with 2 mL of diluted ABTS radical cation solution. Absorbance was measured at 734 nm after incubating the samples at room temperature for 3 min. Ascorbic acid was used as a reference. ABTS radical cation scavenging activity was calculated using the same formula as that used to measure DPPH radical-scavenging activity. IC50 was determined by probit regression in SPSS (IBM, Armonk, NY, USA).
4.5.3. Reducing Power Assay
The reducing power of the extracts was determined according to a previously described procedure [57] with some modifications. Extracts (1 mL) were mixed at various concentrations (0.78–50 μg/mL) with 2 mL of phosphate buffer (0.2 M, pH 6.6) and 1 mL of 1% potassium ferric cyanide [K3Fe(CN)6]. After incubation at 50 °C for 30 min, 2 mL of 10% trichloroacetic acid (TCA) was added. A portion of the solution (2 mL) was mixed with 2 mL of distilled water and 1 mL of 0.1% ferric chloride (FeCl3). The absorbance was then measured at 700 nm. Ascorbic acid was used as a reference. Ascorbic acid equivalent values were calculated from linear equations for the samples and reducing power was expressed as ascorbic acid equivalents (AAE) given in μg AAE/100 μg of the extracts.
4.5.4. Assay for Metal Ion Chelating Activity
Fe2+-chelating activity was measured according to a previously described method [31] with some modifications. Extracts (1 mL) at various concentrations (0.31–10 mg/mL) were mixed with 0.2 mL of FeSO4 (1 mM) and 2.3 mL of distilled water. The mixture was shaken vigorously and left at room temperature for 5 min. Afterwards, 1 mL of ferrozine (1 mM in methanol) was added to the mixture, which was mixed and left for another 5 min to react with the residual Fe2+. The absorbance of the Fe2+ ferrozine complex was measured at 562 nm against a blank; EDTA-Na2 was used as a reference. Fe2+ chelating activity was calculated using the same formula as that used for the DPPH assay. IC50 was determined by probit regression in SPSS (IBM, Armonk, NY, USA).
4.6. In Vivo Antioxidant Activity Testing
4.6.1. Animals
Specific-pathogen-free (SPF) male mice (20 ± 2 g) were purchased from the Experimental Animal Company of Chendu Dashuo (Chendu, China). Mice were allowed to acclimate to room conditions for three days prior to experimentation and were kept in a controlled environment at 25 ± 2 °C under a 12 h light/12 h dark cycle and in 60 ± 2% relative humidity. Mice were fed a standard rodent pellet diet and had access to fresh water at all times.
4.6.2. Establishment of the Mouse Model of d-Galactose-Induced Aging
Mice were randomly divided into six groups, each containing ten mice. Group I was a blank control group that was subcutaneously injected with normal saline (100 mg/kg) once a day for six weeks. Groups II–VI were intraperitoneally injected with 100 mg/kg doses of d-galactose (0.1 mL/10 g) once a day for 6 weeks [33]. Group II was intragastrically administered with normal saline (100 mg/kg), and Group III was given ascorbic acid (100 mg/kg in saline water). Groups IV–VI were intragastrically administered with high (400 mg/kg), moderate (200 mg/kg), and low (100 mg/kg) doses of MF. Each group received intragastric administration for three weeks. Two days after the end of the experiment, the animals were anaesthetized through inhalation of ethyl ether. Eyeball blood was collected and allowed to clot, and the serum was separated for the assessment of enzyme activity. The mice were then sacrificed by cervical dislocation. Liver and brain samples were dissected, cleaned of blood with ice-cold saline, and immediately stored in a refrigerator for the determination of biochemical indices.
4.6.3. Determination of Biochemical Indices
The collected eyeball blood was allowed to naturally sediment for 2 h. The resulting suspension was centrifuged at 3000 rpm for 10 min at 4 °C, and the supernatant was collected for 8-iso PGF2α analysis. Liver and brain homogenates (10% w/v) were prepared in cold saline. The resulting suspension was centrifuged at 3000 rpm for 10 min at 4 °C and frozen at −80 °C until the assay was conducted as described below. The activities of 8-iso PGF2α from blood serum, along with SOD, protein carbonyl, MDA, and GSH from liver and brain, were assayed using commercial reagent kits obtained from the Institute of Biological Engineering of Chengdu Kelong (Chengdu, China) according to the manufacturer’s instructions and using standard assay procedures [34,52].
4.7. DPPH-UHPLC-ESI-HRMSn Analysis
MF and ML extracts (5.0 mg/mL in methanol) were reacted with DPPH (0.2 mM in methanol) at 37 °C for 30 min; the volume ratio of the extracts and DPPH solutions was 1:1. The resulting mixtures were filtered through a 0.22 μm filter prior to UHPLC-ESI-HRMSn analysis. A sample (2.5 mg/mL) without DPPH was used as a blank control. Peaks representing antioxidant components reduced or disappeared after reacting with DPPH. UHPLC analyses were performed using an Ultimate 3000 system (Dionex, Sunnyvale, CA, USA) equipped with an online vacuum degasser, a quaternary pump, an autosampler, and a temperature-controlled column compartment. Chromatographic separation was performed on a SunFireTM C18 (100 mm × 2.1 mm, 1.7 μm, Waters, MA, USA) column. The eluent consisted of solvents A (0.1% aqueous formic acid in water) and B (acetonitrile); flow rate was 0.3 mL/min. Gradient elution was programmed as follows: 0–2 min, 5% B; 2–10 min, 5%–15% B; 10–30 min, 15%–40% B; and 30–40 min, 40%–80% B. The injection volume was 2 μL, and the injection temperature was 15 °C.
Tandem mass spectrometry was performed in a Q-Exactive Orbitrap mass spectrometer (MS) (Thermo Fisher, Waltham, MA, USA) using a heated electrospray ionization source for the ionization of target compounds. MS data were acquired across a range of 80–1200 m/z in both positive and negative ion modes. The operating conditions were as follows: capillary voltage, 2.00 kV; pressure of nebulizer, 30 psi; auxiliary gas pressure, 10 L/min; capillary temp, 320 °C; auxiliary gas heater temp, 350 °C. MS/MS spectra were acquired with collision energy at 35 eV to achieve the maximum number of characteristic fragments for structural elucidation.
4.8. Statistical Analysis
All antioxidant experiments were performed in triplicate. SPSS (IBM, New York, NY, USA).and Graph Pad Prism were used to analyze the data statistically. Data were expressed as mean ± standard deviation (SD). The statistical evaluations used one-way analysis of variance (ANOVA) with multiple comparisons, followed by Dunnett’s t-tests, and the means compared using Duncan’s multiple range test. Statistical significance was determined at p < 0.05.
5. Conclusions
This study first evaluated the antioxidant activities of leaves and flowers from Paeonia rockii and revealed the presence of antioxidant compounds. The flowers and leaves from Paeonia rockii showed stronger antioxidant activity than ascorbic acid in vitro in reducing power, DPPH, and ABTS radical scavenging assay. The antioxidant ability of Paeonia rockii flowers was evidenced by the reduction of 8-iso PGF2α and protein carbonyl and a concurrent increase in SOD levels in vivo. In addition, 25 potential antioxidant compounds in the leaves and flowers were screened, and 18 compounds were identified by DPPH-UHPLC-ESI-HRMSn methodology in vitro. The result demonstrated that the flavonoids and phenolic acids might be the major compounds responsible for the strong antioxidant activity of the flowers and leaves from Paeonia rockii. This study calls attention to the important antioxidant properties of Paeonia rockii and highlights the therapeutic potential of this traditional Chinese herb in preventing and treating diseases caused by oxidative stress.
Acknowledgments
This research was supported by the National Key Research and Development Program of China(2017YFC1700705), the Program of Study Abroad for Young Scholar Sponsored by China Scholarship Council (CSC201500850007), Sichuan Province Department of Basic Research Project (2015jy0009), Key Technologies R and D Program of Sichuan (2014SZ0131), the key projects of central universities(2018NZD18) and Innovative Scientific Research Project for graduate students of Southwest University for Nationalities (CX2017SZ097).
Author Contributions
Rui Zeng and Yan Qu designed the research; Rui Zeng, Zhanguo Wang and Xiaodong Ren revised the manuscript; Jinhua Li, Yanfang Li and Ruiping Li performed in vitro antioxidant experiment and checked manuscript; Katherine G. Maffucci helped in language revise and study design; Yating Bao performed in vitro and in vivo antioxidant experiment, analyzed the mass spectra data and wrote the paper; and all authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| TCM | traditional Chinese medicine |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) acid |
| UHPLC-ESI-HRMSn | ultra high pressure liquid chromatography and electrospray ionization coupled with high resolution mass spectrometry |
| 8-iso-PGF2α | 8-iso-prostaglandin F2α |
| SOD | superoxide dismutase |
| MDA | malondialdehyde |
| GSH | glutathione |
| ML | methanol extracts of Paeonia rockii leaves |
| MF | methanol extracts of Paeonia rockii flowers |
| TPC | total phenolic content |
| TFC | total flavonoid content |
| GAE | gallic acid equivalents |
| RE | rutin equivalents |
| TCA | trichloroacetic acid |
| AAE | ascorbic acid equivalents |
| EDTA-Na2 | ethylenediaminetetraacetic acid disodium salt |
| tR | retention time |
| B-MF | Methanol extracts from flowers without reaction with DPPH |
| A-MF | Methanol extracts from flowers reacted with DPPH |
| B-ML | Methanol extracts from leaves without reaction with DPPH |
| A-ML | Methanol extracts of leaves after reaction with DPPH |
| SD | standard deviation |
| SPSS | Statistical Program for Social Sciences |
References
- Venkataraman, K.; Khurana, S.; Tai, T.C. Oxidative stress in aging—Matters of the heart and mind. Int. J. Mol. Sci. 2013, 14, 17897–17925. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.H.; Drummond, G.R.; Sobey, C.G.; De Silva, T.M.; Kemp-Harper, B.K. The opposing roles of NO and oxidative stress in cardiovascular disease. Pharmacol. Res. 2016, 116, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Oxidative stress as an iceberg in carcinogenesis and cancer biology. Arch. Biochem. Biophys. 2016, 595, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Zhang, Y.T.; Guo, Y.R.; Huang, Q.L.; Peng, T.; Xu, Y.; Tang, L.; Chen, F. Antioxidant and anti-inflammatory activities of the phenolic extracts of Sapium sebiferum (L.) Roxb. leaves. J. Ethnopharmacol. 2013, 147, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Nayki, C.; Nayki, U.; Gunay, M.; Kulhan, M.; Cankaya, M.; Humeyra Taskin Kafa, A.; Balci, G. Oxidative and antioxidative status in the endometrium of patients with benign gynecological disorders. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Chen, J.; Cai, Y.; Lei, Y.; Chen, L.; Pei, L.; Zhou, D.; Liang, X.; Ruan, J. Antioxidant, free radical scavenging, anti-inflammatory and hepatoprotective potential of the extract from Parathelypteris nipponica (Franch. et Sav.) Ching. J. Ethnopharmacol. 2010, 130, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Alarcon, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Sharma, A.; Kang, S.C.; Baek, K.-H. Antioxidant, lipid peroxidation inhibition and free radical scavenging efficacy of a diterpenoid compound sugiol isolated from Metasequoia glyptostroboides. Asian Pac. J. Trop. Med. 2014, 7, 9–15. [Google Scholar] [CrossRef]
- Kumari, S.; Deori, M.; Elancheran, R.; Kotoky, J.; Devi, R. In vitro and In vivo Antioxidant, Anti-hyperlipidemic Properties and Chemical Characterization of Centella asiatica (L.) Extract. Front. Pharmacol. 2016, 7, 400. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Li, Y.; Bai, M.; Deng, Y.; Liang, G.; Wu, H. A comparative study of the dynamic accumulation of polyphenol components and the changes in their antioxidant activities in diploid and tetraploid Lonicera japonica. Plant Physiol. Biochem. 2017, 112, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Li, C.; Zhang, C.; Zeng, R.; Fu, C. Optimization of infrared-assisted extraction of Bletilla striata polysaccharides based on response surface methodology and their antioxidant activities. Carbohydr. Polym. 2016, 148, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Picerno, P.; Mencherini, T.; Sansone, F.; Del Gaudio, P.; Granata, I.; Porta, A.; Aquino, R.P. Screening of a polar extract of Paeonia rockii: Composition and antioxidant and antifungal activities. J. Ethnopharmacol. 2011, 138, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Duan, W.; Liu, P.; Deng, R.; Ren, Y.; Zhao, S. GC-MS analysis of essential oil of Paeonia suffruticosa Andrews from ZhaoFen and RouFurong flowers in China. Natl. Sci. 2012, 4, 552–554. [Google Scholar]
- Parker, S.; May, B.; Zhang, C.; Zhang, A.L.; Lu, C.; Xue, C.C. A Pharmacological Review of Bioactive Constituents of Paeonia lactiflora Pallas and Paeonia veitchii Lynch. Phytother. Res. 2016, 30, 1445–1473. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.D.; Jiang, L.L.; Li, H.Y.; Yan, P.F.; Zhang, Y.L. Chemical Components and Pharmacological Activities of Terpene Natural Products from the Genus Paeonia. Molecules 2016, 21, 1362. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Wu, D.G.; Chen, Y.W. Chemical Constituents and Bioactivities of Plants from the Genus Paeonia. Chem. Biodivers. 2010, 41, 90–104. [Google Scholar] [CrossRef] [PubMed]
- He, C.N.; Peng, Y.; Zhang, Y.C.; Xu, L.J.; Gu, J.; Xiao, P.G. Phytochemical and biological studies of Paeoniaceae. Chem. Biodivers. 2010, 41, 805–838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.; Wu, K.; Wang, M.; Liu, P.; Wang, X.; Deng, R. Antioxidant Activities and Chemical Constituents of Flavonoids from the Flower of Paeonia ostii. Molecules 2016, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Shi, Q.Q.; Ji, D.; Niu, L.X.; Zhang, Y.L. Determination of the phenolic content, profile, and antioxidant activity of seeds from nine tree peony (Paeonia section Moutan DC.) species native to China. Food Res. Int. 2017, 97, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.Y.; Chou, T.H.; Lin, R.J.; Chan, L.P.; Wang, G.H.; Liang, C.H. Antioxidant and antimelanogenic behaviors of Paeonia suffruticosa. Plant Foods Hum. Nutr. 2011, 66, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey C, C.S.; Yong, L. Flora of China. Flora Reipublicae Popul. Sin. 1979, 27, 45. [Google Scholar]
- Mencherini, T.; Picerno, P.; Festa, M.; Russo, P.; Capasso, A.; Aquino, R. Triterpenoid constituents from the roots of Paeonia rockii ssp. rockii. J. Natl. Prod. 2011, 74, 2116–2121. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Gao, J.; Lu, Z.; Yin, W.; Deng, R. Resveratrol trimers from seed cake of Paeonia rockii. Molecules 2014, 19, 19549–19556. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, B.; He, J.; Han, L.; Zhan, Y.; Wang, Y. In vitro and in vivo antioxidant effects of the ethanolic extract of Swertia chirayita. J. Ethnopharmacol. 2011, 136, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Ban, X.; He, J.; Tong, J.; Tian, J.; Wang, Y. Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves. Food Chem. 2010, 120, 873–878. [Google Scholar] [CrossRef]
- Motamed, S.M.; Naghibi, F. Antioxidant activity of some edible plants of the Turkmen Sahra region in northern Iran. Food Chem. 2010, 119, 1637–1642. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, M.; Wang, J.S.; Cui, C.; Yang, B.; Jiang, Y.; Zhao, Q. Antioxidant, immunomodulatory and anti-breast cancer activities of phenolic extract from pine (Pinus massoniana Lamb.) bark. Innov. Food Sci. Emerg. Technol. 2008, 9, 122–128. [Google Scholar] [CrossRef]
- Choudhury, B.; Kandimalla, R.; Bharali, R.; Monisha, J.; Kunnumakara, A.B.; Kalita, K.; Kotoky, J. Anticancer Activity of Garcinia morella on T-Cell Murine Lymphoma Via Apoptotic Induction. Front. Pharmacol. 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, X.D.; Zhou, G.C.; Cai, L.; Yao, W.B. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem. 2008, 106, 888–895. [Google Scholar] [CrossRef]
- He, J.; Huang, B.; Ban, X.; Tian, J.; Zhu, L.; Wang, Y. In vitro and in vivo antioxidant activity of the ethanolic extract from Meconopsis quintuplinervia. J. Ethnopharmacol. 2012, 141, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yu, Y.H.; Wang, S.T.; Ren, J.; Camer, D.; Hua, Y.Z.; Zhang, Q.; Huang, J.; Xue, D.L.; Zhang, X.F.; et al. Chlorogenic acid protects d-galactose-induced liver and kidney injury via antioxidation and anti-inflammation effects in mice. Pharm. Biol. 2016, 54, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.L.; Yin, Y.G. In vivo antioxidant activity of total flavonoids from indocalamus leaves in aging mice caused by d-galactose. Food Chem. Toxicol. 2012, 50, 3814–3818. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hu, X.; Chen, X.; Shi, S.; Jiang, X.; Liang, X.; Chen, W.; Zhang, S. Analysis and improved characterization of minor antioxidants from leaves of Malus doumeri using a combination of major constituents’ knockout with high-performance liquid chromatography-diode array detector-quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. A 2015, 1398, 57–65. [Google Scholar] [PubMed]
- Zhang, Y.; Shi, S.; Wang, Y.; Huang, K. Target-guided isolation and purification of antioxidants from Selaginella sinensis by offline coupling of DPPH-HPLC and HSCCC experiments. J. Chromatogr. B 2011, 879, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuang, G.; Chen, X.; Zeng, R. Identification of Chemical Composition of Leaves and Flowers from Paeonia rockii by UHPLC-Q-Exactive Orbitrap HRMS. Molecules 2016, 21, 947. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.J.; Zhang, J.G.; Sun, Y.H.; Qu, J.; Li, L.; Prasad, C.; Wei, Z.J. Physicochemical properties and antioxidant activities of polysaccharides sequentially extracted from peony seed dreg. Int. J. Biol. Macromol. 2016, 91, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Du, S.; Yuan, J.; Hu, Y. Fatty acid profile in the seeds and seed tissues of Paeonia L. species as new oil plant resources. Sci. Rep. 2016, 6, 26944. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Yang, J.; Jiang, Y.; Lu, B.; Hu, Y.; Zhou, F.; Mao, S.; Shen, C. Phenolic compounds and antioxidant capacities of 10 common edible flowers from China. J. Food Sci. 2014, 79, C517–C525. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Du, H.; Wang, L.; Shu, Q.; Zheng, Y.; Xu, Y.; Zhang, J.; Zhang, J.; Yang, R.; Ge, Y. Flavonoid composition and antioxidant activity of tree peony (Paeonia section moutan) yellow flowers. J. Agric. Food Chem. 2009, 57, 8496–8503. [Google Scholar] [CrossRef] [PubMed]
- Ammar, R.B.; Bhouri, W.; Sghaier, M.B.; Boubaker, J.; Skandrani, I.; Neffati, A.; Bouhlel, I.; Kilani, S.; Mariotte, A.-M.; Chekir-Ghedira, L.; et al. Antioxidant and free radical-scavenging properties of three flavonoids isolated from the leaves of Rhamnus alaternus L. (Rhamnaceae): A structure-activity relationship study. Food Chem. 2009, 116, 258–264. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Inhibitory activities of soluble and bound millet seed phenolics on free radicals and reactive oxygen species. J. Agric. Food Chem. 2011, 59, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Li, H.J.; Chen, J.; Guo, C.W.; Li, P. Rapid and simple method for screening of natural antioxidants from Chinese herb Flos Lonicerae japonicae by DPPH-HPLC-DAD-TOF/MS. J. Sep. Sci. 2008, 31, 3519–3526. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, L.; Shi, S.; Cai, P.; Liang, X.; Zhang, S. Antioxidant capacity and phenolic compounds of Lonicerae macranthoides by HPLC-DAD-QTOF-MS/MS. J. Pharm. Biomed. Anal. 2016, 124, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Ben Ahmed, Z.; Yousfi, M.; Viaene, J.; Dejaegher, B.; Demeyer, K.; Mangelings, D.; Vander Heyden, Y. Seasonal, gender and regional variations in total phenolic, flavonoid, and condensed tannins contents and in antioxidant properties from Pistacia atlantica ssp. leaves. Pharm. Biol. 2017, 55, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Asnaashari, M.; Farhoosh, R.; Sharif, A. Antioxidant activity of gallic acid and methyl gallate in triacylglycerols of Kilka fish oil and its oil-in-water emulsion. Food Chem. 2014, 159, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Zuo, A.; Yanying, Y.; Li, J.; Binbin, X.; Xiongying, Y.; Yan, Q.; Shuwen, C. Study on the relation of structure and antioxidant activity of isorhamnetin, quercetin, phloretin, silybin and phloretin isonicotinyl hydrazone. Free Radic. Antioxid. 2011, 1, 39–47. [Google Scholar] [CrossRef]
- Ruberto, G.; Renda, A.; Daquino, C.; Amico, V.; Spatafora, C.; Tringali, C.; Tommasi, N.D. Polyphenol constituents and antioxidant activity of grape pomace extracts from five Sicilian red grape cultivars. Food Chem. 2007, 100, 203–210. [Google Scholar] [CrossRef]
- Llorach, R.; Martinez-Sanchez, A.; Tomas-Barberan, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Delwing-de Lima, D.; Hennrich, S.B.; Delwing-Dal Magro, D.; Aurelio, J.G.; Serpa, A.P.; Augusto, T.W.; Pereira, N.R. The effect of d-galactose induced oxidative stress on in vitro redox homeostasis in rat plasma and erythrocytes. Biomed. Pharmacother. 2017, 86, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Fan, S.H.; Zheng, Y.L.; Lu, J.; Wu, D.M.; Shan, Q.; Hu, B. Purple sweet potato color attenuates oxidative stress and inflammatory response induced by d-galactose in mouse liver. Food Chem. Toxicol. 2009, 47, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Kelly, F.J.; Fussell, J.C. Role of oxidative stress in cardiovascular disease outcomes following exposure to ambient air pollution. Free Radic. Biol. Med. 2017, 110, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, F.; Tramutola, A.; Butterfield, D.A. Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic. Biol. Med. 2017, 111, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Pinter, O.; Hardi, P.; Nagy, T.; Gasz, B.; Kovacs, V.; Arato, E.; Sinay, L.; Lenard, L.; Jancso, G. The role of GST polymorphism in reperfusion induced oxidative stress, inflammatory responses and clinical complications after surgical and percutaneous coronary intervention. Clin. Hemorheol. Microcirc. 2017, 66, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Bursal, E.; Gülçin, İ. Polyphenol contents and in vitro antioxidant activities of lyophilised aqueous extract of kiwifruit (Actinidia deliciosa). Food Res. Int. 2011, 44, 1482–1489. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, J.; Lu, X.; Zhang, L.; Zhang, Y. Evaluation to the antioxidant activity of total flavonoids extract from persimmon (Diospyros kaki L.) leaves. Food Chem. Toxicol. 2011, 49, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).