Physicochemical Properties and Oxidative Storage Stability of Milled Roselle (Hibiscus sabdariffa L.) Seeds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
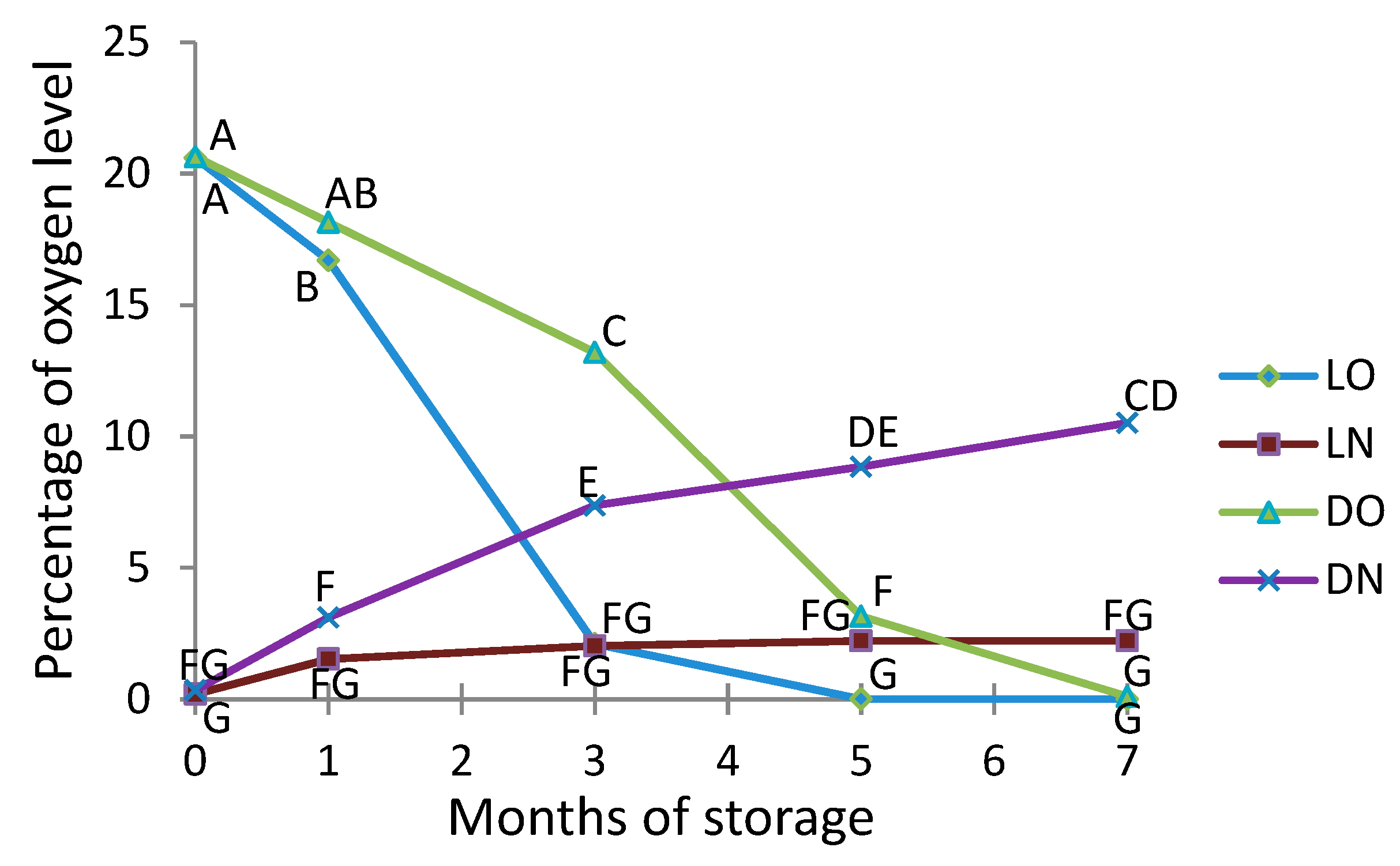
2.2. Monitoring of Oxygen and Nitrogen Levels in Packages
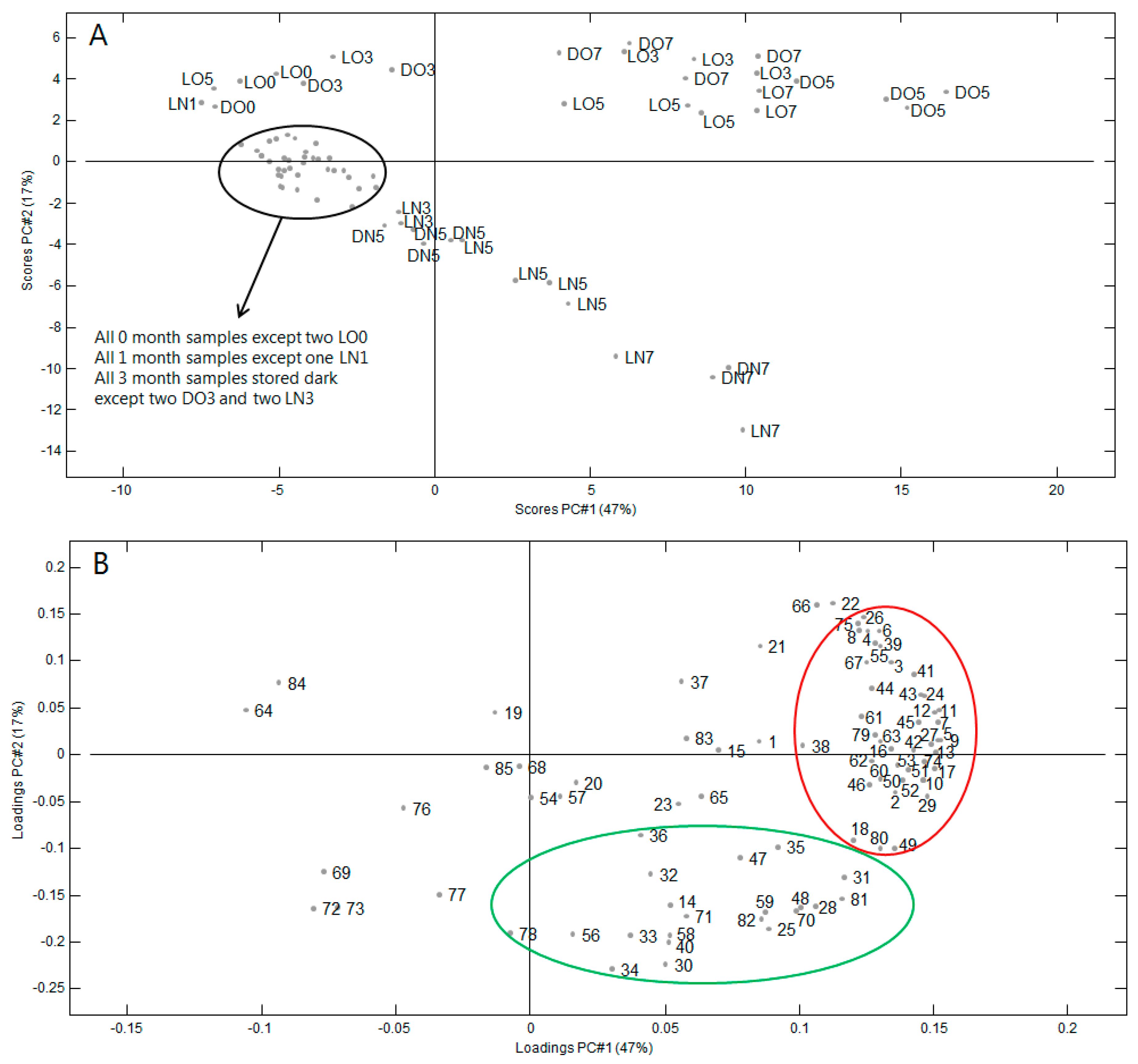
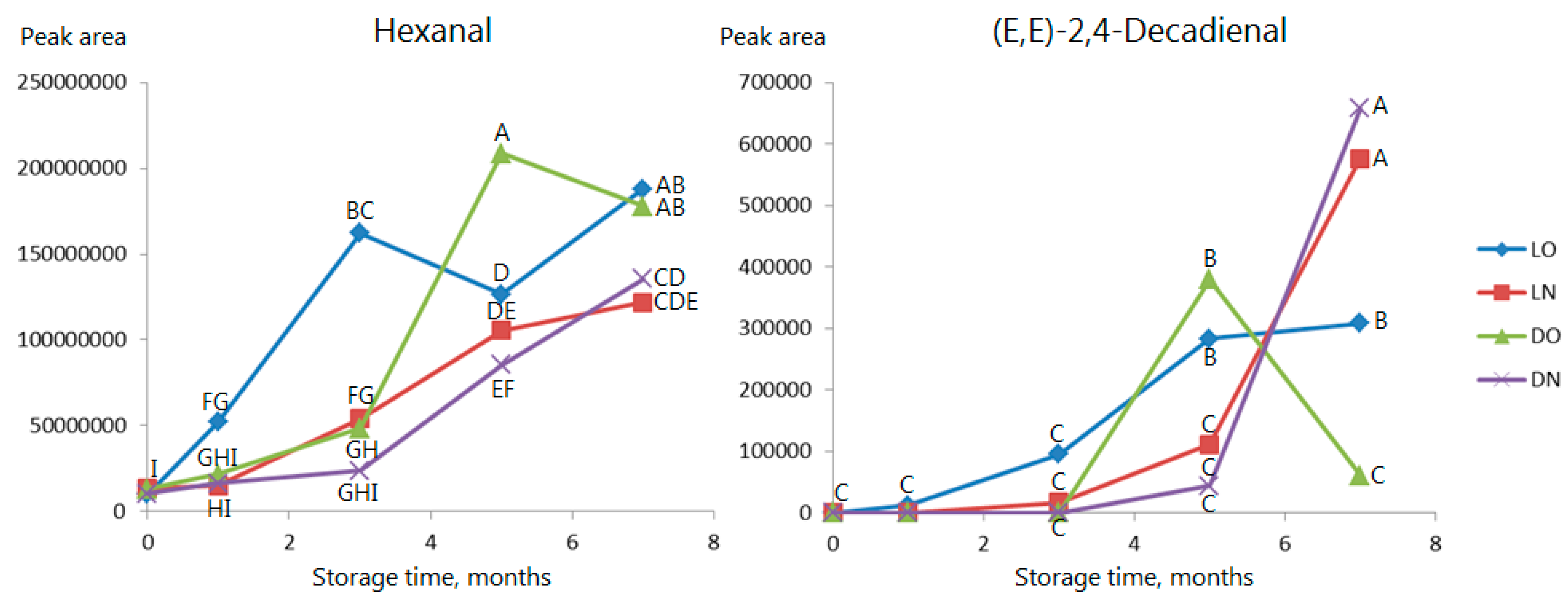
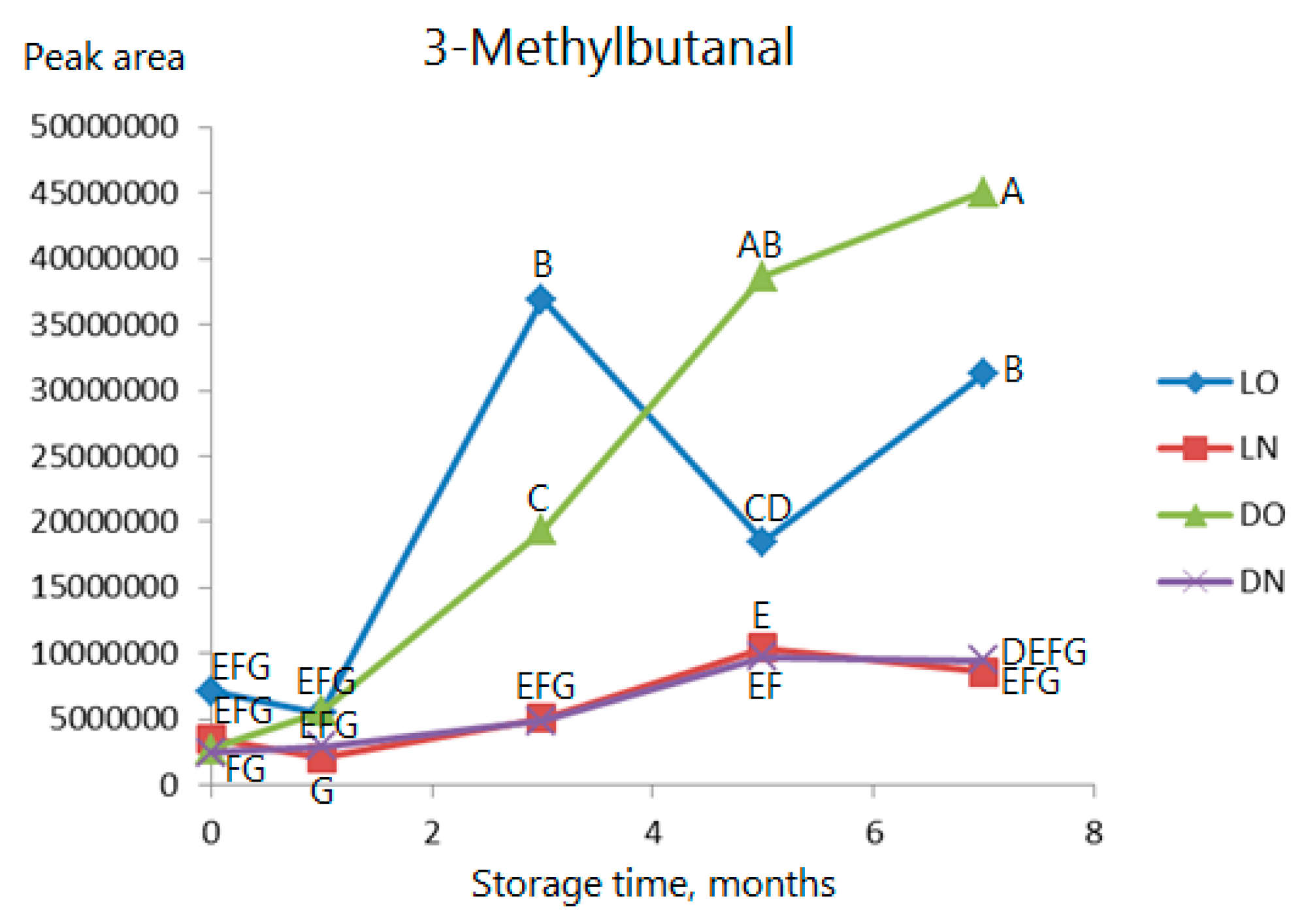
2.3. Volatile Compounds
3. Materials and Methods
3.1. Chemical Standards
3.2. Sample Materials
3.3. Chemical and Physical Analyses
3.3.1. Proximate Analysis
3.3.2. Water and Oil Absorption Capacities
3.4. Packaging and Storage Conditions
- LO (Light/Oxygen): samples stored in light in atmospheric air,
- LN (Light/Nitrogen): samples stored in light in nitrogen,
- DO (Darkness/Oxygen): samples stored in darkness in atmospheric air,
- DN (Darkness/Nitrogen): samples stored in darkness in nitrogen.
3.5. Gas Composition
3.6. Volatile Compound Analysis
3.7. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grubben, G.; Denton, O. Resources of Tripical Africa 2. Vegetables; PROTA Foundation: Wageningen, The Netherlands; Backhuys Publishers: Leiden/Wageningen, The Netherlends, 2004. [Google Scholar]
- Rao, P. Nutrient composition and biological evaluation of mesta (Hibiscus sabdariffa) seeds. Plant Foods Hum. Nutr. 1996, 49, 27–34. [Google Scholar] [PubMed]
- Lim, T. Edible Medicinal and Non Medicinal Plants; Springer: Dordrecht, The Netherlends, 2012; Volume 3, ISBN 978-94-007-2533-1. [Google Scholar]
- Wilson, F.D.; Menzel, M.Y. Kenaf (Hibiscus cannabinus), roselle (Hibiscus sabdariffa). Econ. Bot. 1964, 18, 80–91. [Google Scholar] [CrossRef]
- Hainida, E.; Ismail, A.; Hashim, N.; Mohd-Esa, N.; Zakiah, A. Effects of defatted dried roselle (Hibiscus sabdariffa L.) seed powder on lipid profiles of hypercholesterolemia rats. J. Sci. Food Agric. 2008, 1050, 1043–1050. [Google Scholar] [CrossRef]
- El-Adawy, T.A.; Khalil, A.H. Characteristics of roselle seeds as a new source of protein and lipid. J. Agric. Food Chem. 1994, 42, 1896–1900. [Google Scholar] [CrossRef]
- Tounkara, F.; Amza, T.; Lagnika, C.; Le, G.W.; Shi, Y.H. Extraction, characterization, nutritional and functional properties of roselle (Hibiscus sabdariffa Linn) seed proteins. Songklanakarin J. Sci. Technol. 2013, 35, 159–166. [Google Scholar]
- Nyam, K.-L.; Leao, S.-Y.; Tan, C.-P.; Long, K. Functional properties of roselle (Hibiscus sabdariffa L.) seed and its application as bakery product. J. Food Sci. Technol. 2014, 15, 3830–3837. [Google Scholar] [CrossRef] [PubMed]
- Gaya, I.; Mohammad, O.; Suleiman, A.; Maje, M.; Adekunle, A. Toxicological and lactogenic studies on the seeds of Hibiscus sabdariffa Linn (Malvaceae) extract on serum prolactin levels of albino wistar rats. Internet J. Endocrinol. 2009, 5, 1–6. [Google Scholar]
- Mohamed, B.B.; Sulaiman, A.A.; Dahab, A.A. Roselle (Hibiscus sabdariffa L.) in Sudan, cultivation and their uses. Bull. Environ. Pharmacol. Life Sci. 2012, 1, 48–54. [Google Scholar]
- Eltayeib, A.A.; Elaziz, A.A. Physicochemical properties of Roselle (Hibiscus sabdariffa L.) seeds oil ( Elrahad-1) in North Kordofan, Sudan. J. Sci. Innov. Res. 2014, 3, 578–582. [Google Scholar]
- Dhar, P.; Kar, C.; Ojha, D.; Pandey, S.; Mitra, J. Chemistry, phytotechnology, pharmacology and nutraceutical functions of kenaf (Hibiscus cannabinus L.) and roselle (Hibiscus sabdariffa L.) seed oil: An overview. Ind. Crops Prod. 2015, 77, 323–332. [Google Scholar] [CrossRef]
- Holse, M.; Skov, T.; Hansen, Å. Oxidative storage stability of roasted marama beans (Tylosema esculentum). Food Res. Int. 2012, 47, 385–391. [Google Scholar] [CrossRef]
- Ismail, A.; Ikram, E.H.K.; Nazri, H.S.M. Roselle (Hibiscus sabdariffa L.) Seeds-nutritional composition, protein quality and health benefits. Food 2008, 2, 1–16. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). Improving Nutrition through Home Gardening—A Training Package for Preparing Field Workers in Africa; Food and Nutrition Division in Collaboration with Plant Production and Protection Division, FAO: Rome, Italy, 2001; Available online: http://www.fao.org/es/ESN/Educate.htm (accessed on 25 October 2017).
- Bhat, R.; Yahya, N.B. Evaluating belinjau (Gnetum gnemon L.) seed flour quality as a base for development of novel food products and food formulations. Food Chem. 2014, 156, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Samy, M. Chemical and nutritional studies on roselle seeds (Hibiscus sabdariffa L.). Zeitschris fiir Erniihrungswissenschas 1980, 19, 47–49. [Google Scholar] [CrossRef]
- Emmy Hainida, K.; Amin, I.; Normah, H.; Mohd-Esa, N. Nutritional and amino acid contents of differently treated Roselle (Hibiscus sabdariffa L.) seeds. Food Chem. 2008, 111, 906–911. [Google Scholar] [CrossRef]
- Juhari, N.H.; Petersen, M.A. Aroma profile and proximate composition of Roselle seeds: Effects of different origins and different sample preparation methods. In Proceedings of the 15th Weurman Flavour Research Symposium, Graz, Austria, 18–22 September 2017. [Google Scholar]
- Jensen, P.N.; Sørensen, G.; Engelsen, S.B.; Bertelsen, G. Evaluation of quality changes in walnut kernels (Juglans regia L.) by Vis/NIR spectroscopy. J. Agric. Food Chem. 2001, 49, 5790–5796. [Google Scholar] [CrossRef] [PubMed]
- Mexis, S.F.; Kontominas, M.G. Effect of oxygen absorber, nitrogen flushing, packaging material oxygen transmission rate and storage conditions on quality retention of raw whole unpeeled almond kernels (Prunus dulcis). LWT Food Sci. Technol. 2010, 43, 1–11. [Google Scholar] [CrossRef]
- Wietstock, P.C.; Kunz, T.; Methner, F.J. Relevance of oxygen for the formation of Strecker aldehydes during beer production and storage. J. Agric. Food Chem. 2016, 64, 8035–8044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, H.; Sun, B.; Mao, X.; Zhang, Y.; Zhou, Y. Comparative analysis of volatile composition in Chinese truffles via GCxGC/HR-TOF/MS and electronic nose. Int. J. Mol. Sci. 2016, 17, 412. [Google Scholar] [CrossRef] [PubMed]
- AOAC. AOAC Official Methods of Analysis, 20th ed.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Megazyme, I.I.L. Total Dietary Fibre Assay Procedure; Megazyme International Ireland Limited: Wicklow, Ireland, 2005; pp. 1–19. [Google Scholar]
- Johnsen, L.G.; Skou, P.B.; Khakimov, B.; Bro, R. Gas chromatography–mass spectrometry data processing made easy. J. Chromatogr. A 2017, 1503, 57–64. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples are not available from authors. |
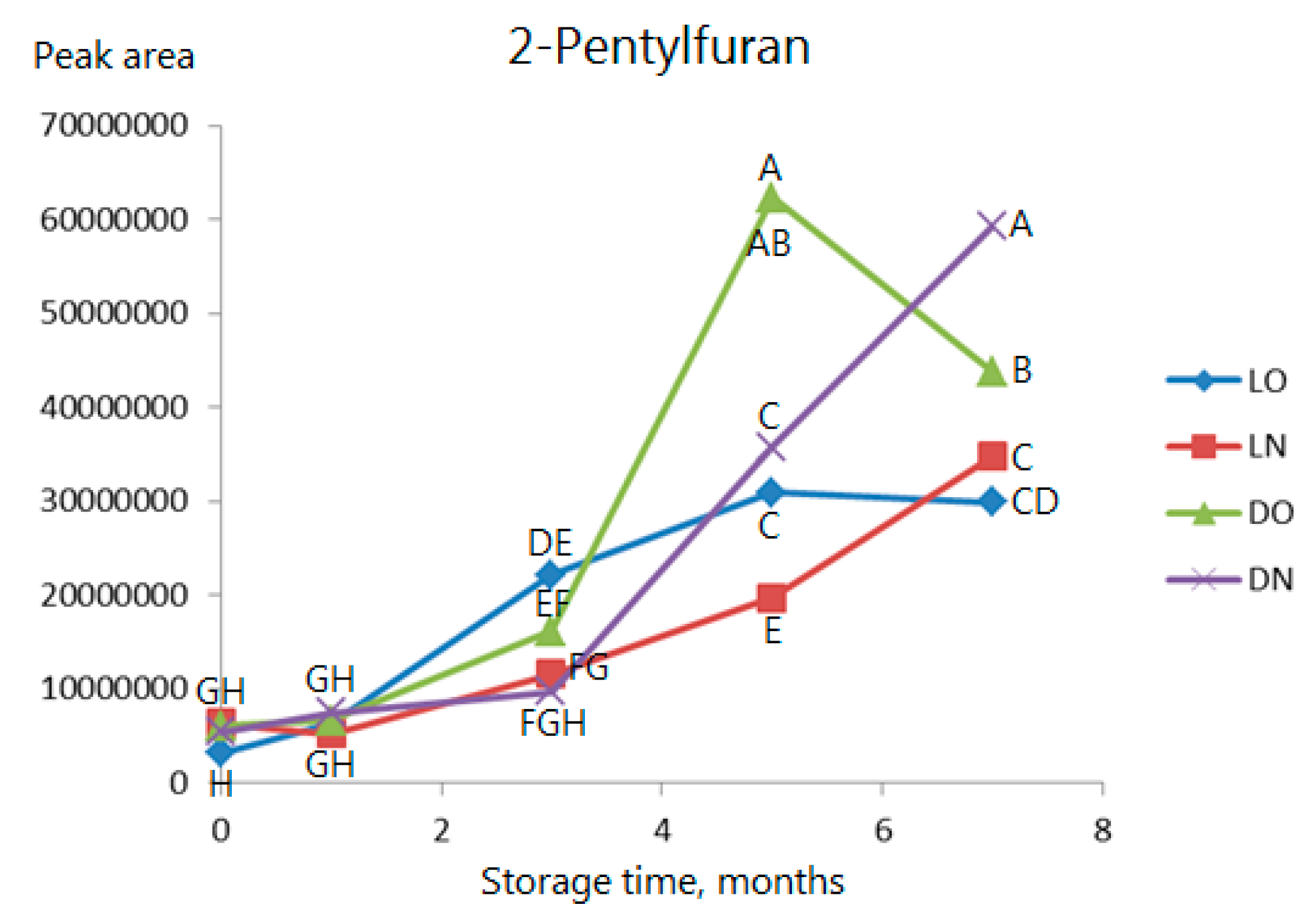
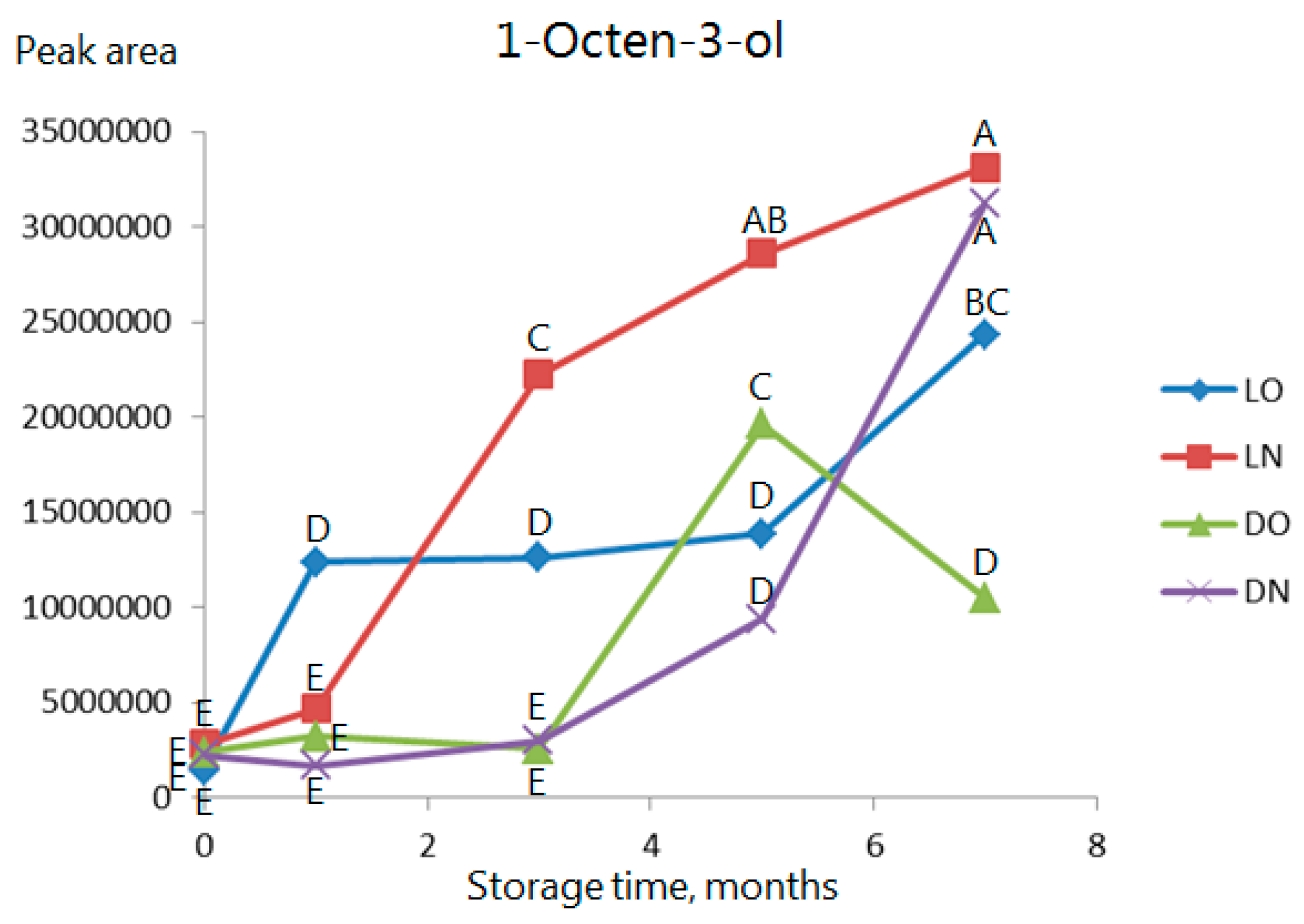
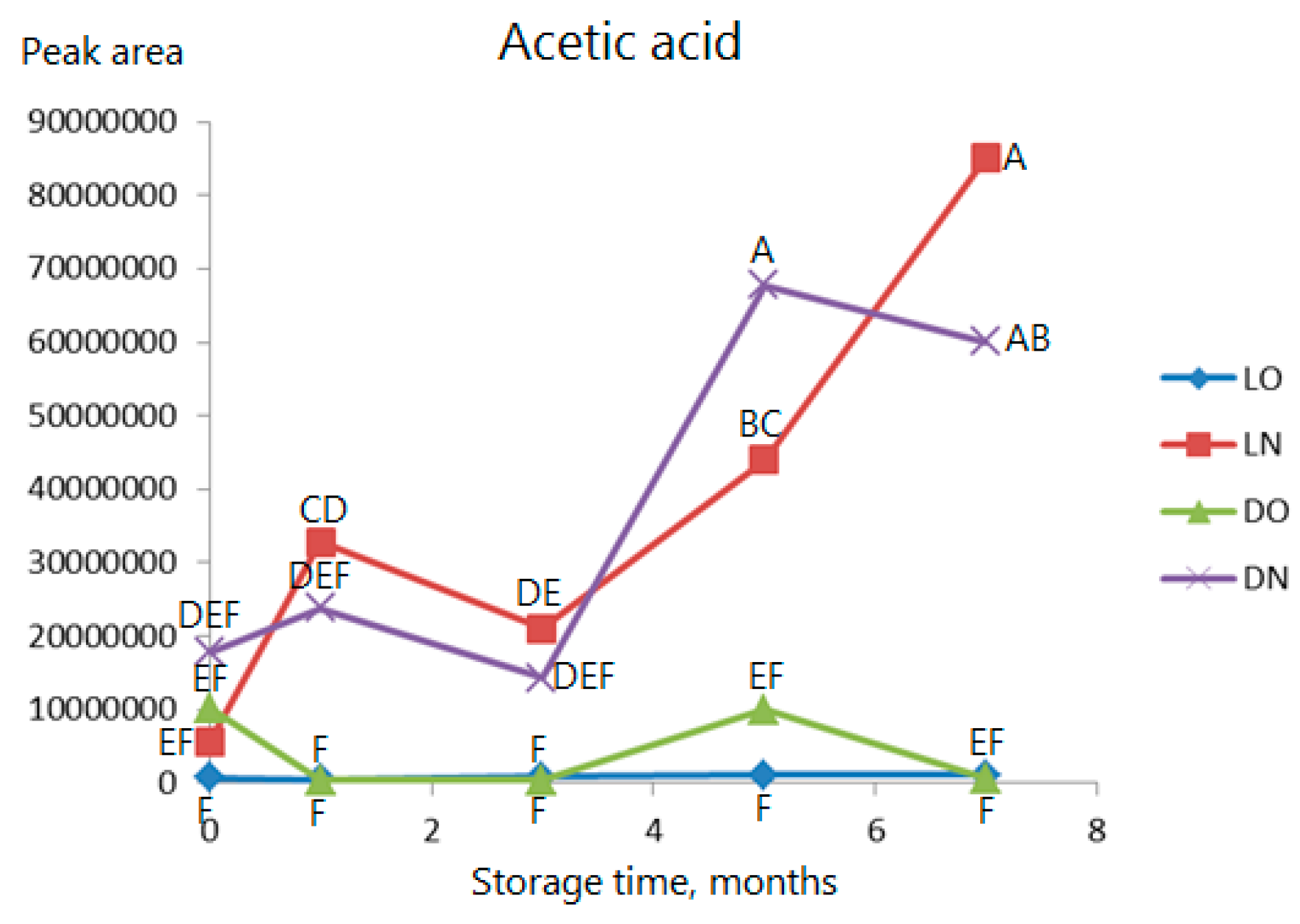

| Type of Analysis | Current Study | Previous Studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Malaysian | Egyptian 1 | Egyptian 2 | Indian 3 | Malaysian 4 | ||||||
| Sun Dried | Light Red | Early Dark Red | Late Dark Red | Amw-2 | Bhimili-1 | Raw Freeze Dried | Sun Dried | Boiled Sun Dried | ||
| Moisture content (%) | 8.4 ± 0.1 | 7.6 | 9.3 | 11.7 | 11.5 | 8.6 | 6.7 | 6.8 | 9.9 | 9.8 |
| Ash (%) | 6.5 ± 0.0 | 7.0 | 6.9 | 5.8 | 6.5 | 5.4 | 5.4 | 7.4 | 7.5 | 6.6 |
| Lipid (%) | 16.2 ± 0.5 | 22.3 | 21.6 | 22.5 | 23.3 | 19.1 | 22.8 | 27.22 | 22.1 | 29.6 |
| Protein (%) | 21.3 ± 0.9 | 15.4 | 31.0 | 30.1 | 30.9 | 18.8 | 22.3 | 35.4 | 33.5 | 30.6 |
| Total dietary fiber (%) | 47.3 ± 1.4 | ND | ND | ND | ND | 42.6 | 39.5 | 25.5 | 18.3 | 19.2 |
| Crude fiber (%) | ND | 15.3 | 4.1 | 3.4 | 1.2 | ND | ND | ND | ND | ND |
| Carbohydrate (%) | 0.3 ± 0.1 | ND | 36.4 | 38.1 | 38.1 | ND | ND | 2.3 | 13.0 | 4.0 |
| Water absorption capacity (%) | 174.5 ± 2.8 | ND | 254 | 254 | 220 | ND | ND | ND | ND | ND |
| Oil absorption capacity (%) | 144.0 ± 4.2 | ND | 159 | 158 | 125 | ND | ND | ND | ND | ND |
| Compounds | Retention Index | Significance h | Odor Description (from Literature) | ||||
|---|---|---|---|---|---|---|---|
| Exp | Auth/Litt a | Storage | Oxygen | Light | |||
| Aldehydes | |||||||
| 1 | 2-Methylpropanal | 811 | 770–834 | *** | * | ns | Pungent, burnt, malty, grain e |
| 2 | Butanal | 873 | 830–911 | *** | ns | *** | Green, plastic-like c, floral d |
| 3 | 2-Methylbutanal | 911 | Auth | *** | *** | ns | Cocoa, almond, malty b, fermented e |
| 4 | 3-Methylbutanal | 915 | Auth | *** | *** | ns | Fruity, toasted, malty, green c |
| 5 | Pentanal | 979 | Auth | *** | *** | * | Almond, malty, pungent c, oily, green e |
| 6 | 2-Methylpentanal | 989 | *** | *** | ns | Unpleasant, rotten apples c | |
| 7 | Hexanal | 1093 | Auth | *** | *** | ns | Grassy, tallow, fatty b |
| 8 | 2-Methyl-(E)-2-butenal | 1092 | *** | *** | ns | Green, pungent, nutty, ethereal d | |
| 9 | Octanal | 1303 | Auth | *** | *** | * | Citrus, waxy d |
| 10 | (E)-2-Heptenal | 1335 | 1273–1366 | *** | ** | *** | Green, vegetable, fatty d |
| 11 | 2-Ethyl-2-hexenal | 1345 | 1322 | *** | *** | ns | Fatty, citrus, green b, grassy, soapy e |
| 12 | Nonanal | 1399 | Auth | *** | *** | ns | Fatty e |
| 13 | (E)-2-Octenal | 1437 | 1393–1467 | *** | ** | ns | Green, nutty, fatty b, waxy, mushroom c |
| 14 | Benzaldehyde | 1532 | Auth | *** | ns | * | Almond, burnt, sugar b |
| 15 | Benzeneacetaldehyde | 1653 | 1592–1684 | ** | *** | ns | Green, hyacinth d |
| 16 | 2-Butyl-2-octenal | 1679 | 1640–1688 | *** | * | ns | Green, citrus, grassy, fruity g,* |
| 17 | 2,4-Nonadienal | 1714 | 1660–1740 | *** | ** | ns | Watermelon, fatty, waxy, green b |
| 18 | 2,4-Decadienal | 1826 | 1763–1858 | *** | ns | ns | Fatty, fried d |
| Alcohols | |||||||
| 19 | 2-Propanol | 935 | 884–975 | ns | ns | ns | Alcoholic, musty, woody d |
| 20 | 2-Butanol | 1036 | 989–1057 | ns | ns | *** | Fruity, sweet, apricot d |
| 21 | Propanol | 1049 | Auth | ** | *** | ns | Alcoholic, fermented d |
| 22 | 2-Methyl-1-propanol | 1105 | Auth | *** | *** | ns | Ethereal, winey, whiskey d |
| 23 | 1-Methoxy-2-propanol | 1138 | 1108–1160 | * | ns | ns | Mild, ethereal, weak pleasant f |
| 24 | Butanol | 1162 | Auth | *** | *** | ns | Fusel, oily d |
| 25 | 1-Penten-3-ol | 1175 | 1112–1207 | *** | *** | ns | Pungent, buttery, milky c |
| 26 | 3-Methyl-1-butanol | 1222 | Auth | *** | *** | ns | Fermented, fusel, alcoholic, whiskey d |
| 27 | Pentanol | 1271 | Auth | *** | ** | ns | Alcohol, pungent, fruity, balsamic b |
| 28 | 2-Heptanol | 1339 | 1280–1344 | *** | ** | * | Citrus, herbal, floral, green d |
| 29 | Hexanol | 1369 | Auth | *** | ns | * | Resin, flowery, green b |
| 30 | 2-Octanol | 1431 | 1380–1430 | *** | *** | * | Spicy, fresh, green, woody, earthy d |
| 31 | 1-Octen-3-ol | 1460 | Auth | *** | * | *** | Mushroom, earthy c |
| 32 | Octanol | 1570 | Auth | ns | ns | ns | Mushroom, moss, nutty, burnt b |
| 33 | 2,3-Butanediol | 1592 | 1492–1620 | *** | *** | ns | Fruity, creamy, buttery d |
| 34 | 2-Decanol | 1628 | 1585–1622 | ** | ** | ns | Fatty, waxy, floral, orange, sweet d |
| 35 | Benzyl alcohol | 1892 | Auth | *** | ns | * | Sweet, flowery b |
| 36 | Phenethyl alcohol | 1929 | Auth | ns | ns | ns | Honey, spice, rose, lilac b |
| Ketones | |||||||
| 37 | Acetone | 814 | 775–847 | *** | * | ns | Solvent, ethereal d |
| 38 | 2-Butanone | 903 | Auth | *** | * | *** | Chemical-like, ether-like, cheesy c |
| 39 | 3-Methyl-2-butanone | 925 | 918–989 | *** | *** | ns | Acetone-like f |
| 40 | 1-Penten-3-one | 1018 | 973–1056 | *** | *** | ns | Pungent, rotten, fruity, plastic-like c |
| 41 | 2-Octanone | 1298 | Auth | *** | *** | ns | Soap, gasoline-like b |
| 42 | 6-Methyl-5-hepten-2-one | 1349 | 1296–1368 | *** | *** | ns | Mushroom, woody, rubbery f, fruity e |
| 43 | 2-Nonanone | 1396 | Auth | *** | *** | ns | Hot milk, soap, green, blue cheese b |
| 44 | 3-Octen-2-one | 1415 | 1392–1411 | *** | *** | ns | Nutty, blueberry, oily, fruity, green e |
| 45 | 2-Decanone | 1502 | 1463–1519 | *** | *** | ns | Floral, orange, fatty, peach d |
| 46 | 3-Nonen-2-one | 1522 | 1523 | *** | * | ** | Fruity, berry, brandy, mushroom d |
| 47 | 3,5-Octadien-2-one | 1581 | 1521–1610 | * | ns | ns | Fruity, green, grassy d |
| Furans | |||||||
| 48 | 2-Ethylfuran | 948 | 923–975 | *** | ** | ns | Rubbery, pungent, acid, sweet c |
| 49 | 2-Propylfuran | 1030 | 1011–1043 | *** | ns | ns | Heated peanut, apricot, plum f |
| 50 | 2-Butylfuran | 1143 | 1088–1140 | *** | ns | ns | Mild, fruity, wine, sweet, spice c |
| 51 | 2-Pentylfuran | 1242 | 1193–1258 | *** | ns | ** | Green bean, buttery b, green, pungent e |
| 52 | 2-Hexylfuran | 1343 | 1312–1345 | *** | ns | ns | - |
| 53 | 2-Heptylfuran | 1440 | 1416–1454 | *** | ns | ns | Green, fatty, oily, roasted nutty d |
| 54 | Furfural | 1472 | Auth | * | ns | ns | Bread, almond, sweet b, fruity, wood c |
| 55 | Dihydro-4,5-dimethyl-2(3H)-furanone | 1619 | 1590–1624 | *** | *** | ns | Caramel, sweet, candy d |
| Acids | |||||||
| 56 | Acetic acid | 1446 | 1401–1485 | *** | *** | ns | Vinegar b |
| 57 | Propanoic acid | 1545 | 1487–1570 | ns | ns | ns | Pungent, rancid, soy b |
| 58 | 2-Methylpropanoic acid | 1574 | 1520–1608 | *** | *** | ns | Acidic, sour, cheesy, dairy, rancid d |
| 59 | Butanoic acid | 1634 | 1556–1674 | *** | *** | ns | Cheesy, sharp, acetic, buttery, fruity d |
| 60 | 2-Methylbutanoic acid | 1678 | 1638–1706 | *** | ns | ns | Acidic, pungent, cheesy d |
| 61 | 3-Methylbutanoic acid | 1676 | 1631–1707 | *** | * | ns | Cheesy, sour, sweaty, tropical d |
| 62 | Pentanoic acid | 1745 | 1686–1766 | *** | ns | ns | Cheesy, acidic, sweaty, rancid d |
| 63 | Hexanoic acid | 1854 | 1797–1880 | *** | * | ns | Sweaty, cheesy, rancid, goat-like c |
| 64 | Heptanoic acid | 1961 | 1913–2000 | *** | ns | ns | Cheesy, rancid, sour, sweaty d |
| 65 | Octanoic acid | 2071 | 2011–2100 | * | ns | ns | Fatty, rancid, vegetable, cheesy d |
| Esters | |||||||
| 66 | Ethyl acetate | 891 | 850–914 | *** | *** | ns | Pineapple b, fruity, orange c |
| 67 | Ethyl pentanoate | 1147 | Auth | *** | *** | ns | Fruity, apple, pineapple, green d |
| 68 | Ethyl lactate | 1357 | 1342–1356 | ns | ns | ns | Fruity, creamy d |
| 69 | Methyl acetate | 826 | 782–877 | *** | *** | *** | Fruity, solvent-like, blackcurrant c |
| 70 | Methyl octanoate | 1397 | Auth | *** | * | ** | Orange b |
| 71 | Methyl nonanoate | 1500 | 1476–1536 | *** | ** | *** | Coconut b |
| Terpenes | |||||||
| 72 | α-Pinene | 1014 | Auth | *** | *** | * | Herbal, pine, woody d |
| 73 | Eucalyptol | 1207 | 1173–1246 | *** | ** | * | Herbal, eucalyptus, minty |
| 74 | α-Terpineol | 1711 | Auth | *** | *** | ns | Pine, lilac, citrus, woody, floral d |
| Pyrazines | |||||||
| 75 | 2,6-Dimethylpyrazine | 1342 | 1300–1355 | *** | *** | ns | Cocoa, roasted, nutty d |
| 76 | Tetramethyl pyrazine | 1490 | 1438–1486 | *** | ns | * | Nutty, musty, chocolate, coffee e |
| Sulfur-containing compounds | |||||||
| 77 | Dimethyl sulfide | 743 | 715–804 | ns | *** | ** | Sulfurous, onion, sweet corn d |
| 78 | Dimethyl disulfide | 1068 | 1036–1127 | * | *** | * | Sulfurous, cabbage, onion d |
| Lactones | |||||||
| 79 | gamma-Butyrolactone | 1642 | 1593–1673 | *** | ns | * | Creamy, oily, fatty d |
| 80 | gamma-Hexalactone | 1718 | 1655–1745 | *** | ns | ns | Creamy, vanilla d |
| 81 | gamma-Octalactone | 1938 | 1867–1945 | *** | * | ns | Creamy, coconut d |
| 82 | gamma-Nonalactone | 2052 | 1981–2062 | *** | ** | ns | Floral, waxy, metallic, plum d |
| Miscellaneous | |||||||
| 83 | Phenol | 2015 | 1949–2037 | ns | ns | ns | Phenolic, medicinal c |
| 84 | Ethyl ether | 618 | 570–619 | *** | ns | ns | Ethereal d |
| 85 | Theaspirane | 1555 | 1472–1553 | ns | ns | ns | Herbal, tea, green, woody, spicy d |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhari, N.H.; Petersen, M.A. Physicochemical Properties and Oxidative Storage Stability of Milled Roselle (Hibiscus sabdariffa L.) Seeds. Molecules 2018, 23, 385. https://doi.org/10.3390/molecules23020385
Juhari NH, Petersen MA. Physicochemical Properties and Oxidative Storage Stability of Milled Roselle (Hibiscus sabdariffa L.) Seeds. Molecules. 2018; 23(2):385. https://doi.org/10.3390/molecules23020385
Chicago/Turabian StyleJuhari, Nurul Hanisah, and Mikael Agerlin Petersen. 2018. "Physicochemical Properties and Oxidative Storage Stability of Milled Roselle (Hibiscus sabdariffa L.) Seeds" Molecules 23, no. 2: 385. https://doi.org/10.3390/molecules23020385
APA StyleJuhari, N. H., & Petersen, M. A. (2018). Physicochemical Properties and Oxidative Storage Stability of Milled Roselle (Hibiscus sabdariffa L.) Seeds. Molecules, 23(2), 385. https://doi.org/10.3390/molecules23020385






