Cell Migration Related to MDR—Another Impediment to Effective Chemotherapy?
Abstract
:1. Introduction
2. ATP-Binding Cassette (ABC) Proteins—Structure and Function
3. ABC Transporters from Discovery to Clinical Testing
3.1. The Largest Multidrug Resistance Protein Family (MRP)
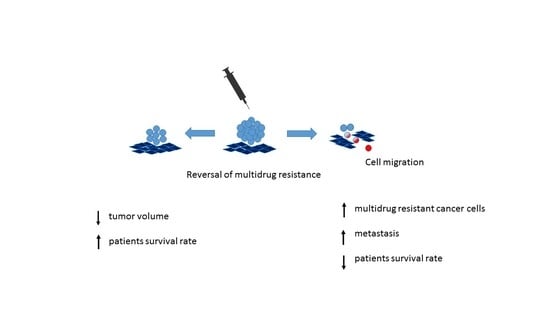
3.2. Reversal of Multidrug Resistance
3.3. Physiological Aspects of ABC Protein Modulation
4. Benefits and Downsides of ABC Modulation in Cancer
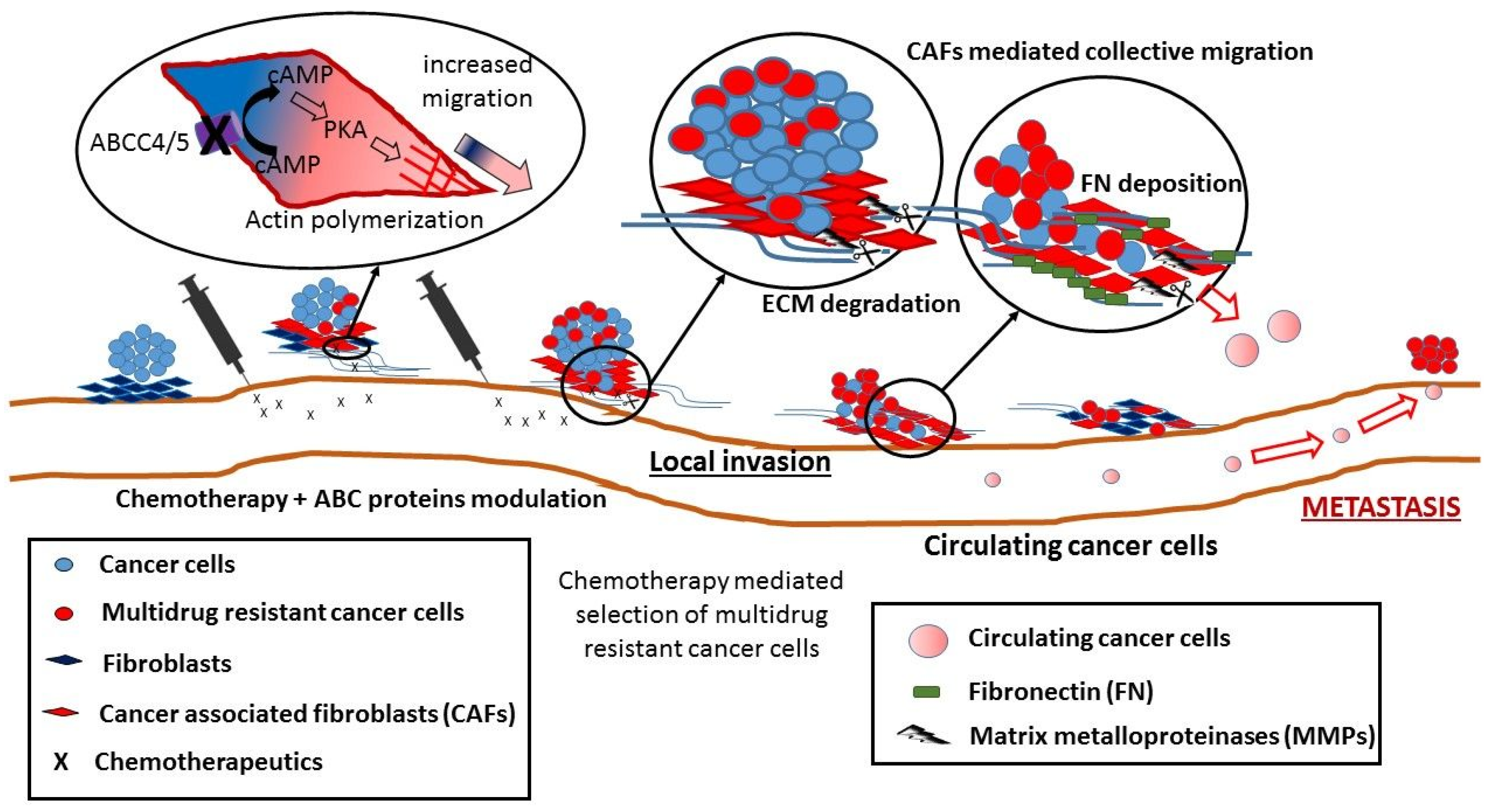
5. Non-Canonical Activity of ABC Transporters in Cell Migration
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rodeck, U. Growth factor independence and growth regulatory pathways in human melanoma development. Cancer Metastasis Rev. 1993, 12, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.; Cheepala, S.B.; Wang, Y.; Neale, G.; Adachi, M.; Nachagari, D.; Leggas, M.; Zhao, W.; Boyd, K.; Venkataramanan, R.; et al. Deregulated hepatic metabolism exacerbates impaired testosterone production in MRP4-deficient mice. J. Biol. Chem. 2012, 287, 14456–14466. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, G.S.; Pastoriza, J.M.; Wang, Y.; Harney, A.S.; Entenberg, D.; Pignatelli, J.; Sharma, V.P.; Xue, E.A.; Cheng, E.; D’Alfonso, T.M.; et al. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci. Transl. Med. 2017, 9, eaan0026. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Ren, J.; Zhang, D.; Li, Y.; Huang, X.; Ji, J.; Hu, Q.; Wang, H.; Ni, Y.; Hou, Y. The TLR3 agonist inhibit drug efflux and sequentially consolidates low-dose cisplatin-based chemoimmunotherapy while reducing side effects. Mol. Cancer Ther. 2017, 16, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Borst, P.; Evers, R.; Kool, M.; Wijnholds, J. The multidrug resistance protein family. Biochim. Biophys. Acta. 1999, 1461, 347–357. [Google Scholar] [CrossRef]
- Roy, U.; Barber, P.; Tse-Dinh, Y.-C.; Batrakova, E.V.; Mondal, D.; Nair, M. Role of MRP transporters in regulating antimicrobial drug inefficacy and oxidative stress-induced pathogenesis during HIV-1 and TB infections. Front. Microbiol. 2015, 6, 948. [Google Scholar] [CrossRef] [PubMed]
- Khamisipour, G.; Jadidi-Niaragh, F.; Jahromi, A.S.; Zandi, K.; Hojjat-Farsangi, M. Mechanisms of tumor cell resistance to the current targeted-therapy agents. Tumour Biol. 2016. [CrossRef] [PubMed]
- Glavinas, H.; Kis, E.; Pál, A.; Kovács, R.; Jani, M.; Vági, E.; Molnár, E.; Bánsághi, S.; Kele, Z.; Janáky, T.; et al. ABCG2 (breast cancer resistance protein/mitoxantrone resistance-associated protein) ATPase assay: A useful tool to detect drug-transporter interactions. Drug Metab. Dispos. 2007, 35, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Feng, J.; Yuan, D.; Zhou, J.; Miao, W. Tracing the structural evolution of eukaryotic ATP binding cassette transporter superfamily. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, S. Structure and mechanism of ABC transporters. F1000Prime Rep. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, S.V.; Kim, I.-W.; Xia, D.; Sauna, Z.E. The A-loop, a novel conserved aromatic acid subdomain upstream of the Walker A motif in ABC transporters, is critical for ATP binding. FEBS Lett. 2006, 580, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.F.; Linton, K.J. The ATP switch model for ABC transporters. Nat. Struct. Mol. Biol. 2004, 11, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Randak, C.O.; Welsh, M.J. Adenylate kinase activity in ABC transporters. J. Biol. Chem. 2005, 280, 34385–34388. [Google Scholar] [CrossRef] [PubMed]
- Jedlitschky, G.; Leier, I.; Buchholz, U.; Center, M.; Keppler, D. ATP-dependent transport of glutathione S-conjugates by the multidrug resistance-associated protein. Cancer Res. 1994, 54, 4833–4836. [Google Scholar] [PubMed]
- Leier, I.; Jedlitschky, G.; Buchholz, U.; Cole, S.P.; Deeley, R.G.; Keppler, D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J. Biol. Chem. 1994, 269, 27807–27810. [Google Scholar] [PubMed]
- Keppler, D.; Leier, I.; Jedlitschky, G.; Mayer, R.; Büchler, M. The function of the multidrug resistance proteins (MRP and cMRP) in drug conjugate transport and hepatobiliary excretion. Adv. Enzyme Regul. 1996, 36, 17–29. [Google Scholar] [CrossRef]
- Jedlitschky, G.; Hoffmann, U.; Kroemer, H.K. Structure and function of the MRP2 (ABCC2) protein and its role in drug disposition. Expert Opin. Drug Metab. Toxicol. 2006, 2, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Borst, P.; Zelcer, N.; van de Wetering, K.; Poolman, B. On the putative co-transport of drugs by multidrug resistance proteins. FEBS Lett. 2006, 580, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Ling, V.; Thompson, L.H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J. Cell. Physiol. 1974, 83, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Kartner, N.; Shales, M.; Riordan, J.R.; Ling, V. Daunorubicin-resistant Chinese hamster ovary cells expressing multidrug resistance and a cell-surface P-glycoprotein. Cancer Res. 1983, 43, 4413–4419. [Google Scholar] [PubMed]
- Cole, S.P.; Bhardwaj, G.; Gerlach, J.H.; Mackie, J.E.; Grant, C.E.; Almquist, K.C.; Stewart, A.J.; Kurz, E.U.; Duncan, A.M.; Deeley, R.G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 1992, 258, 1650–1654. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.P.; Sparks, K.E.; Fraser, K.; Loe, D.W.; Grant, C.E.; Wilson, G.M.; Deeley, R.G. Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res. 1994, 54, 5902–5910. [Google Scholar] [PubMed]
- Grant, C.E.; Valdimarsson, G.; Hipfner, D.R.; Almquist, K.C.; Cole, S.P.; Deeley, R.G. Overexpression of multidrug resistance-associated protein (MRP) increases resistance to natural product drugs. Cancer Res. 1994, 54, 357–361. [Google Scholar] [PubMed]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef] [PubMed]
- Miyake, K.; Mickley, L.; Litman, T.; Zhan, Z.; Robey, R.; Cristensen, B.; Brangi, M.; Greenberger, L.; Dean, M.; Fojo, T.; Bates, S.E.; et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: Demonstration of homology to ABC transport genes. Cancer Res. 1999, 59, 8–13. [Google Scholar] [PubMed]
- Allikmets, R.; Schriml, L.M.; Hutchinson, A.; Romano-Spica, V.; Dean, M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998, 58, 5337–5339. [Google Scholar] [PubMed]
- Janke, D.; Mehralivand, S.; Strand, D.; Gödtel-Armbrust, U.; Habermeier, A.; Gradhand, U.; Fischer, C.; Toliat, M.R.; Fritz, P.; Zanger, U.M.; et al. 6-mercaptopurine and 9-(2-phosphonyl-methoxyethyl) adenine (PMEA) transport altered by two missense mutations in the drug transporter gene ABCC4. Hum. Mutat. 2008, 29, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-K.; Wang, Y.-J.; Gupta, P.; Chen, Z.-S. Multidrug resistance proteins (MRPs) and cancer therapy. AAPS J. 2015, 17, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Meng, F.; Wang, L.; Mao, Y.; Zhou, H.; Hua, D.; Zhang, H.; Wang, W. A polymorphism in ABCC4 is related to efficacy of 5-FU/capecitabine-based chemotherapy in colorectal cancer patients. Sci. Rep. 2017, 7, 7059. [Google Scholar] [CrossRef] [PubMed]
- Hlavata, I.; Mohelnikova-Duchonova, B.; Vaclavikova, R.; Liska, V.; Pitule, P.; Novak, P.; Bruha, J.; Vycital, O.; Holubec, L.; Treska, V.; et al. The role of ABC transporters in progression and clinical outcome of colorectal cancer. Mutagenesis 2012, 27, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Wijnholds, J.; Evers, R.; van Leusden, M.R.; Mol, C.A.; Zaman, G.J.; Mayer, U.; Beijnen, J.H.; van der Valk, M.; Krimpenfort, P.; Borst, P. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat. Med. 1997, 3, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Lorico, A.; Rappa, G.; Finch, R.A.; Yang, D.; Flavell, R.A.; Sartorelli, A.C. Disruption of the murine MRP (multidrug resistance protein) gene leads to increased sensitivity to etoposide (VP-16) and increased levels of glutathione. Cancer Res. 1997, 57, 5238–5242. [Google Scholar] [PubMed]
- Zhang, W.; Deng, J.; Sunkara, M.; Morris, A.J.; Wang, C.; Clair, D.S.; Vore, M. Loss of multidrug resistance–associated protein 1 potentiates chronic doxorubicin-induced cardiac dysfunction in mice. J. Pharmacol. Exp. Ther. 2015, 355, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Leggas, M.; Adachi, M.; Scheffer, G.L.; Sun, D.; Wielinga, P.; Du, G.; Mercer, K.E.; Zhuang, Y.; Panetta, J.C.; Johnston, B.; et al. MRP4 confers resistance to topotecan and protects the brain from chemotherapy. Mol. Cell. Biol. 2004, 24, 7612–7621. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Morgan, J.A.; Scheffer, G.L.; Adachi, M.; Stewart, C.F.; Sun, D.; Leggas, M.; Ejendal, K.F.K.; Hrycyna, C.A.; Schuetz, J.D. Substrate overlap between MRP4 and ABCG2/BCRP affects purine analogue drug cytotoxicity and tissue distribution. Cancer Res. 2007, 67, 6965–6972. [Google Scholar] [CrossRef] [PubMed]
- Elsnerova, K.; Bartakova, A.; Tihlarik, J.; Bouda, J.; Rob, L.; Skapa, P.; Hruda, M.; Gut, I.; Mohelnikova-Duchonova, B.; Soucek, P.; et al. Gene expression profiling reveals novel candidate markers of ovarian carcinoma intraperitoneal metastasis. J. Cancer 2017, 8, 3598–3606. [Google Scholar] [CrossRef] [PubMed]
- Hedditch, E.L.; Gao, B.; Russell, A.J.; Lu, Y.; Emmanuel, C.; Beesley, J.; Johnatty, S.E.; Chen, X.; Harnett, P.; George, J.; et al. ABCA transporter gene expression and poor outcome in epithelial ovarian cancer. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Auner, V.; Sehouli, J.; Oskay-Oezcelik, G.; Horvat, R.; Speiser, P.; Zeillinger, R. ABC transporter gene expression in benign and malignant ovarian tissue. Gynecol. Oncol. 2010, 117, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.M.T.; Huynh, T.; Truong, A.M.; Haber, M.; Norris, M.D. ABC transporters and neuroblastoma. Adv. Cancer Res. 2015, 125, 139–170. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Shin, N.; Oh, J.-H.; Lee, Y.-J. High-dose metformin may increase the concentration of atorvastatin in the liver by inhibition of multidrug resistance-associated protein 2. J. Pharm. Sci. 2017, 106, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Westover, D.; Li, F. New trends for overcoming ABCG2/BCRP-mediated resistance to cancer therapies. J. Exp. Clin. Cancer Res. 2015, 34, 159. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-P.; Calcagno, A.M.; Ambudkar, S.V. Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: Evaluation of current strategies. Curr. Mol. Pharmacol. 2008, 1, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Ween, M.P.; Armstrong, M.A.; Oehler, M.K.; Ricciardelli, C. The role of ABC transporters in ovarian cancer progression and chemoresistance. Crit. Rev. Oncol. Hematol. 2015, 96, 220–256. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Tsuruo, T. Functional role for the 170- to 180-kDa glycoprotein specific to drug-resistant tumor cells as revealed by monoclonal antibodies. Proc. Natl. Acad. Sci. USA 1986, 83, 7785–7789. [Google Scholar] [CrossRef] [PubMed]
- Lhommé, C.; Joly, F.; Walker, J.L.; Lissoni, A.A.; Nicoletto, M.O.; Manikhas, G.M.; Baekelandt, M.M.O.; Gordon, A.N.; Fracasso, P.M.; Mietlowski, W.L.; et al. Phase III study of valspodar (PSC 833) combined with paclitaxel and carboplatin compared with paclitaxel and carboplatin alone in patients with stage IV or suboptimally debulked stage III epithelial ovarian cancer or primary peritoneal cancer. J. Clin. Oncol. 2008, 26, 2674–2682. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, M.; Mross, K.; Schueller, J.; Thuerlimann, B.; Kroeger, N.; Kupper, H. Phase II trial of dexverapamil and epirubicin in patients with non-responsive metastatic breast cancer. Br. J. Cancer 1998, 77, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Seiden, M.V.; Swenerton, K.D.; Matulonis, U.; Campos, S.; Rose, P.; Batist, G.; Ette, E.; Garg, V.; Fuller, A.; Harding, M.W.; et al. A phase II study of the MDR inhibitor biricodar (INCEL, VX-710) and paclitaxel in women with advanced ovarian cancer refractory to paclitaxel therapy. Gynecol. Oncol. 2002, 86, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Harding, M.W.; Neubauer, M.; Langer, C.J.; Moore, M.; Ross, H.J.; Johnson, B.E.; Lynch, T.J. A phase II study of the safety and efficacy of the multidrug resistance inhibitor VX-710 combined with doxorubicin and vincristine in patients with recurrent small cell lung cancer. Cancer 2007, 109, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.J.; Rodarte, J.C.; Benbatoul, K.D.; Romano, S.J.; Zhang, C.; Krane, S.; Moran, E.J.; Uyeda, R.T.; Dixon, R.; Guns, E.S.; et al. Discovery and characterization of OC144-093, a novel inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Res. 2000, 60, 2964–2972. [Google Scholar] [PubMed]
- Shepard, R.L.; Cao, J.; Starling, J.J.; Dantzig, A.H. Modulation of P-glycoprotein but not MRP1- or BCRP-mediated drug resistance by LY335979. Int. J. Cancer 2003, 103, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Evers, R.; Kool, M.; Smith, A.J.; van Deemter, L.; de Haas, M.; Borst, P. Inhibitory effect of the reversal agents V-104, GF120918 and Pluronic L61 on MDR1 Pgp-, MRP1- and MRP2-mediated transport. Br. J. Cancer 2000, 83, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Telu, S.; Shukla, S.; Ambudkar, S.V.; Pike, V.W.; Halldin, C.; Gottesman, M.M.; Innis, R.B.; Hall, M.D. The “specific” P-glycoprotein inhibitor Tariquidar is also a substrate and an inhibitor for breast cancer resistance protein (BCRP/ABCG2). ACS Chem. Neurosci. 2011, 2, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Vezmar, M.; Georges, E. Reversal of MRP-mediated doxorubicin resistance with quinoline-based drugs. Biochem. Pharmacol. 2000, 59, 1245–1252. [Google Scholar] [CrossRef]
- Abe, T.; Koike, K.; Ohga, T.; Kubo, T.; Wada, M.; Kohno, K.; Mori, T.; Hidaka, K.; Kuwano, M. Chemosensitisation of spontaneous multidrug resistance by a 1,4-dihydropyridine analogue and verapamil in human glioma cell lines overexpressing MRP or MDR1. Br. J. Cancer 1995, 72, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Gekeler, V.; Ise, W.; Sanders, K.H.; Ulrich, W.R.; Beck, J. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem. Biophys. Res. Commun. 1995, 208, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Evers, R.; Zaman, G.J.; van Deemter, L.; Jansen, H.; Calafat, J.; Oomen, L.C.; Oude Elferink, R.P.; Borst, P.; Schinkel, A.H. Basolateral localization and export activity of the human multidrug resistance-associated protein in polarized pig kidney cells. J. Clin. Invest. 1996, 97, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Gollapudi, S.; Kim, C.H.; Tran, B.N.; Sangha, S.; Gupta, S. Probenecid reverses multidrug resistance in multidrug resistance-associated protein-overexpressing HL60/AR and H69/AR cells but not in P-glycoprotein-overexpressing HL60/Tax and P388/ADR cells. Cancer Chemother. Pharmacol. 1997, 40, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Franz, C.; Xiao, Z.; Mohr, E.; Serba, S.; Büchler, M.W.; Schemmer, P. Sorafenib modulates the gene expression of multi-drug resistance mediating ATP-binding cassette proteins in experimental hepatocellular carcinoma. Anticancer Res. 2010, 30, 4503–4508. [Google Scholar] [PubMed]
- Lin, S.; Hoffmann, K.; Xiao, Z.; Jin, N.; Galli, U.; Mohr, E.; Büchler, M.W.; Schemmer, P. MEK inhibition induced downregulation of MRP1 and MRP3 expression in experimental hepatocellular carcinoma. Cancer Cell Int. 2013, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Lage, H. Gene therapeutic approaches to overcome ABCB1-mediated drug resistance. Recent Results Cancer Res. 2016, 209, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.; Surowiak, P.; Lage, H. Reversal of different drug-resistant phenotypes by an autocatalytic multitarget multiribozyme directed against the transcripts of the ABC transporters MDR1/P-gp, MRP2, and BCRP. Mol. Ther. 2005, 11, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Materna, V.; Stege, A.; Surowiak, P.; Priebsch, A.; Lage, H. RNA interference-triggered reversal of ABCC2-dependent cisplatin resistance in human cancer cells. Biochem. Biophys. Res. Commun. 2006, 348, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Priebsch, A.; Rompe, F.; Tönnies, H.; Kowalski, P.; Surowiak, P.; Stege, A.; Materna, V.; Lage, H. Complete reversal of ABCG2-depending atypical multidrug resistance by RNA interference in human carcinoma cells. Oligonucleotides 2006, 16, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Huang, Q.; Wang, Z.; Song, Y.; Wang, L. Reversal of multi-drug resistance by vector-based-shRNA-mdr1 in vitro and in vivo. J. Huazhong Univ. Sci. Technol. Med. Sci. 2009, 29, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Germann, U.A.; Ford, P.J.; Shlyakhter, D.; Mason, V.S.; Harding, M.W. Chemosensitization and drug accumulation effects of VX-710, verapamil, cyclosporin A, MS-209 and GF120918 in multidrug resistant HL60/ADR cells expressing the multidrug resistance-associated protein MRP. Anticancer. Drugs 1997, 8, 141–155. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, A.A.K.; van den Heuvel, J.J.M.W.; Koenderink, J.B.; Russel, F.G.M. Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J. Pharmacol. Exp. Ther. 2007, 320, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Chen, Q.; Wang, Y.; Peng, W.; Cai, H. Effects of curcumin on ion channels and transporters. Front. Physiol. 2014, 5, 94. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; O’Loughlin, K.L.; Fricke, S.M.; Williamson, N.A.; Greco, W.R.; Minderman, H.; Baer, M.R. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin. Cancer Res. 2005, 11, 2320–2326. [Google Scholar] [CrossRef] [PubMed]
- Feller, N.; Broxterman, H.J.; Währer, D.C.; Pinedo, H.M. ATP-dependent efflux of calcein by the multidrug resistance protein (MRP): No inhibition by intracellular glutathione depletion. FEBS Lett. 1995, 368, 385–388. [Google Scholar] [CrossRef]
- Reid, G.; Wielinga, P.; Zelcer, N.; de Haas, M.; van Deemter, L.; Wijnholds, J.; Balzarini, J.; Borst, P. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol. Pharmacol. 2003, 63, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Katayama, R.; Koike, S.; Sato, S.; Sugimoto, Y.; Tsuruo, T.; Fujita, N. Dofequidar fumarate sensitizes cancer stem-like side population cells to chemotherapeutic drugs by inhibiting ABCG2/BCRP-mediated drug export. Cancer Sci. 2009, 100, 2060–2068. [Google Scholar] [CrossRef] [PubMed]
- Touhey, S.; O’Connor, R.; Plunkett, S.; Maguire, A.; Clynes, M. Structure-activity relationship of indomethacin analogues for MRP-1, COX-1 and COX-2 inhibition. identification of novel chemotherapeutic drug resistance modulators. Eur. J. Cancer. 2002, 38, 1661–1670. [Google Scholar] [CrossRef]
- Sato, M.; Iwanaga, T.; Mamada, H.; Ogihara, T.; Yabuuchi, H.; Maeda, T.; Tamai, I. Involvement of uric acid transporters in alteration of serum uric acid level by angiotensin II receptor blockers. Pharm. Res. 2008, 25, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Soldner, A.; Benet, L.Z.; Mutschler, E.; Christians, U. Active transport of the angiotensin-II antagonist losartan and its main metabolite EXP 3174 across MDCK-MDR1 and caco-2 cell monolayers. Br. J. Pharmacol. 2000, 129, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.S.; Kawabe, T.; Ono, M.; Aoki, S.; Sumizawa, T.; Furukawa, T.; Uchiumi, T.; Wada, M.; Kuwano, M.; Akiyama, S.I. Effect of multidrug resistance-reversing agents on transporting activity of human canalicular multispecific organic anion transporter. Mol. Pharmacol. 1999, 56, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Tsuruo, T.; Rose, D.R. Mode of binding of anti-P-glycoprotein antibody MRK-16 to its antigen. A crystallographic and molecular modeling study. J. Biol. Chem. 1998, 273, 25413–25419. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lei, Y.; Jia, Y.; Li, N.; Wink, M.; Ma, Y. Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells. Phytomedicine 2011, 19, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Copsel, S.; Bruzzone, A.; May, M.; Beyrath, J.; Wargon, V.; Cany, J.; Russel, F.G.M.; Shayo, C.; Davio, C. Multidrug resistance protein 4/ATP binding cassette transporter 4: a new potential therapeutic target for acute myeloid leukemia. Oncotarget 2014, 5, 9308–9321. [Google Scholar] [CrossRef] [PubMed]
- Yarla, N.S.; Ganapaty, S. Bioactive flavonoids as ABC transporters inhibitors for reversion of multidrug resistance in cancer. J. Mar. Sci. Res. Dev. 2013, 4, 1–2. [Google Scholar] [CrossRef]
- Burkhart, C.A.; Watt, F.; Murray, J.; Pajic, M.; Prokvolit, A.; Xue, C.; Flemming, C.; Smith, J.; Purmal, A.; Isachenko, N.; et al. Small molecule MRP1 inhibitor Reversan increases the therapeutic index of chemotherapy in mouse model of neuroblastoma. Cancer Res. 2009, 69, 6573–6580. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Tiwari, A.K.; Shukla, S.; Robey, R.W.; Singh, S.; Kim, I.-W.; Bates, S.E.; Peng, X.; Abraham, I.; Ambudkar, S.V.; et al. Sildenafil reverses ABCB1- and ABCG2-mediated chemotherapeutic drug resistance. Cancer Res. 2011, 71, 3029–3041. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Berridge, G.; Mistry, P.; Higgins, C.; Charlton, P.; Callaghan, R. The molecular interaction of the high affinity reversal agent XR9576 with P-glycoprotein. Br. J. Pharmacol. 1999, 128, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Boesch, D.; Gavériaux, C.; Jachez, B.; Pourtier-Manzanedo, A.; Bollinger, P.; Loor, F. In vivo circumvention of P-glycoprotein-mediated multidrug resistance of tumor cells with SDZ PSC 833. Cancer Res. 1991, 51, 4226–4233. [Google Scholar] [PubMed]
- Keppler, D. Multidrug Resistance Proteins (MRPs, ABCCs): Importance for Pathophysiology and Drug Therapy. In Drug Transporters; MRP4 k of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 299–323. ISBN 978-3-642-14540-7. [Google Scholar]
- Van de Ven, R.; Scheffer, G.L.; Reurs, A.W.; Lindenberg, J.J.; Oerlemans, R.; Jansen, G.; Gillet, J.-P.; Glasgow, J.N.; Pereboev, A.; Curiel, D.T.; et al. A role for multidrug resistance protein 4 (MRP4; ABCC4) in human dendritic cell migration. Blood 2008, 112, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Wielinga, P.; Zelcer, N.; van der Heijden, I.; Kuil, A.; de Haas, M.; Wijnholds, J.; Borst, P. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc. Natl. Acad. Sci. USA 2003, 100, 9244–9249. [Google Scholar] [CrossRef] [PubMed]
- Kruh, G.D.; Guo, Y.; Hopper-Borge, E.; Belinsky, M.G.; Chen, Z.-S. ABCC10, ABCC11, and ABCC12. Pflugers Arch. 2007, 453, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.-K.; Zhou, Z.-W.; Wei, M.Q.; Liu, J.-P.; Zhou, S.-F. Modulators of multidrug resistance associated proteins in the management of anticancer and antimicrobial drug resistance and the treatment of inflammatory diseases. Curr. Top. Med. Chem. 2010, 10, 1732–1756. [Google Scholar] [CrossRef] [PubMed]
- Le Vee, M.; Gripon, P.; Stieger, B.; Fardel, O. Down-regulation of organic anion transporter expression in human hepatocytes exposed to the proinflammatory cytokine interleukin 1beta. Drug Metab. Dispos. 2008, 36, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.J.; Hossain, M.M.; Richardson, J.R.; Aleksunes, L.M. Inflammatory regulation of ATP binding cassette efflux transporter expression and function in microglia. J. Pharmacol. Exp. Ther. 2012, 343, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Leite, D.F.P.; Echevarria-Lima, J.; Ferreira, S.C.; Calixto, J.B.; Rumjanek, V.M. ABCC transporter inhibition reduces zymosan-induced peritonitis. J. Leukoc. Biol. 2007, 82, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Cash, J.L.; White, G.E.; Greaves, D.R. Chapter 17 Zymosan-Induced Peritonitis as a Simple Experimental System for the Study of Inflammation. In Methods in Enzymology; Chemokines, Part B; Academic Press: Cambridge, MA, USA, 2009; Volume 461, pp. 379–396. [Google Scholar]
- Van de Ven, R.; de Groot, J.; Reurs, A.W.; Wijnands, P.G.J.T.B.; van de Wetering, K.; Schuetz, J.D.; de Gruijl, T.D.; Scheper, R.J.; Scheffer, G.L. Unimpaired immune functions in the absence of MRP4 (ABCC4). Immunol. Lett. 2009, 124, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Kryczka, J.; Boncela, J. Leukocytes: The double-edged sword in fibrosis. Mediators Inflamm. 2015, 2015, 652035. [Google Scholar] [CrossRef] [PubMed]
- Donner, M.G.; Keppler, D. Up-regulation of basolateral multidrug resistance protein 3 (MRP3) in cholestatic rat liver. Hepatology 2001, 34, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Stöckel, B.; König, J.; Nies, A.T.; Cui, Y.; Brom, M.; Keppler, D. Characterization of the 5’-flanking region of the human multidrug resistance protein 2 (MRP2) gene and its regulation in comparison with the multidrug resistance protein 3 (MRP3) gene. Eur. J. Biochem. 2000, 267, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Rost, D.; Cui, Y.; Keppler, D. Characterization of the human multidrug resistance protein isoform MRP3 localized to the basolateral hepatocyte membrane. Hepatology 1999, 29, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Nies, A.T.; Keppler, D. The apical conjugate efflux pump ABCC2 (MRP2). Pflugers Arch. 2007, 453, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Köck, K.; Ferslew, B.C.; Netterberg, I.; Yang, K.; Urban, T.J.; Swaan, P.W.; Stewart, P.W.; Brouwer, K.L.R. Risk factors for development of cholestatic drug-induced liver injury: Inhibition of hepatic basolateral bile acid transporters multidrug resistance-associated proteins 3 and 4. Drug Metab. Dispos. 2014, 42, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.; Lynch, J.; Panetta, J.C.; Wang, Y.; Frase, S.; Bao, J.; Zheng, J.; Opferman, J.T.; Janke, L.; Green, D.M.; et al. Apoptosome activation, an important molecular instigator in 6-mercaptopurine induced Leydig cell death. Sci. Rep. 2015, 5, 16488. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nakadate, H.; Kondoh, K.; Nakamura, K.; Koh, K.; Manabe, A. Interaction between NUDT15 and ABCC4 variants enhances intolerability of 6-mercaptopurine in Japanese patients with childhood acute lymphoblastic leukemia. Pharmacogenomics J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Klement, J.F.; Matsuzaki, Y.; Jiang, Q.-J.; Terlizzi, J.; Choi, H.Y.; Fujimoto, N.; Li, K.; Pulkkinen, L.; Birk, D.E.; Sundberg, J.P.; et al. Targeted ablation of the abcc6 gene results in ectopic mineralization of connective tissues. Mol. Cell. Biol. 2005, 25, 8299–8310. [Google Scholar] [CrossRef] [PubMed]
- Favre, G.; Laurain, A.; Aranyi, T.; Szeri, F.; Fulop, K.; Le Saux, O.; Duranton, C.; Kauffenstein, G.; Martin, L.; Lefthériotis, G. The ABCC6 transporter: A new player in biomineralization. Int. J. Mol. Sci. 2017, 18, e1941. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.S.; Duijst, S.; Mahakena, S.; Sommer, D.; Szeri, F.; Váradi, A.; Plomp, A.; Bergen, A.A.; Oude Elferink, R.P.J.; Borst, P.; et al. ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1985–1989. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.E.; Rodriguez-Cruz, V.; Felmlee, M.A. SLC and ABC Transporters: Expression, localization, and species differences at the blood-brain and the blood-cerebrospinal fluid barriers. AAPS J. 2017, 19, 1317–1331. [Google Scholar] [CrossRef] [PubMed]
- Lingineni, K.; Belekar, V.; Tangadpalliwar, S.R.; Garg, P. The role of multidrug resistance protein (MRP-1) as an active efflux transporter on blood–brain barrier (BBB) permeability. Mol. Divers. 2017, 21, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Saidijam, M.; Karimi Dermani, F.; Sohrabi, S.; Patching, S.G. Efflux proteins at the blood-brain barrier: Review and bioinformatics analysis. Xenobiotica 2017, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Kanamitsu, K.; Kusuhara, H.; Schuetz, J.D.; Takeuchi, K.; Sugiyama, Y. Investigation of the importance of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the active efflux of anionic drugs across the blood–brain barrier. J. Pharm. Sci. 2017, 106, 2566–2575. [Google Scholar] [CrossRef] [PubMed]
- Seigers, R.; Schagen, S.B.; Coppens, C.M.; van der Most, P.J.; van Dam, F.S.A.M.; Koolhaas, J.M.; Buwalda, B. Methotrexate decreases hippocampal cell proliferation and induces memory deficits in rats. Behav. Brain Res. 2009, 201, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E. Cells of origin in cancer. Nature 2011, 469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Zhang, P.-Y. Combinations in multimodality treatments and clinical outcomes during cancer. Oncol. Lett. 2016, 12, 4301–4304. [Google Scholar] [CrossRef] [PubMed]
- Kruijtzer, C.M.; Beijnen, J.H.; Rosing, H.; ten Bokkel Huinink, W.W.; Schot, M.; Jewell, R.C.; Paul, E.M.; Schellens, J.H. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and p-glycoprotein inhibitor GF120918. J. Clin. Oncol. 2002, 20, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Kuppens, I.E.L.M.; Witteveen, E.O.; Jewell, R.C.; Radema, S.A.; Paul, E.M.; Mangum, S.G.; Beijnen, J.H.; Voest, E.E.; Schellens, J.H.M. A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (GF120918) and oral topotecan in cancer patients. Clin. Cancer Res. 2007, 13, 3276–3285. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.J.; Haber, M.; Porro, A.; Munoz, M.A.; Iraci, N.; Xue, C.; Murray, J.; Flemming, C.L.; Smith, J.; Fletcher, J.I.; et al. ABCC multidrug transporters in childhood neuroblastoma: Clinical and biological effects independent of cytotoxic drug efflux. J. Natl. Cancer Inst. 2011, 103, 1236–1251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Shen, B.; Peng, C.; Zheng, M. The ABCC4 gene is a promising target for pancreatic cancer therapy. Gene 2012, 491, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Chen, Z.-S.; Ambudkar, S.V. Tyrosine kinase inhibitors as modulators of ABC transporter-mediated drug resistance. Drug Resist. Updat. 2012, 15, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Sodani, K.; Wang, S.-R.; Kuang, Y.-H.; Ashby, C.R.; Chen, X.; Chen, Z.-S. Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem. Pharmacol. 2009, 78, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.A.; Szakács, G.; Martin, S.E.; Chu, B.F.; Cardarelli, C.; Sauna, Z.E.; Caplen, N.J.; Fales, H.M.; Ambudkar, S.V.; Weinstein, J.N.; et al. Selective toxicity of NSC73306 in MDR1-positive cells as a new strategy to circumvent multidrug resistance in cancer. Cancer Res. 2006, 66, 4808–4815. [Google Scholar] [CrossRef] [PubMed]
- Heffeter, P.; Jakupec, M.A.; Körner, W.; Wild, S.; von Keyserlingk, N.G.; Elbling, L.; Zorbas, H.; Korynevska, A.; Knasmüller, S.; Sutterlüty, H.; et al. Anticancer activity of the lanthanum compound [tris(1,10-phenanthroline)lanthanum(III)]trithiocyanate (KP772; FFC24). Biochem. Pharmacol. 2006, 71, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Heffeter, P.; Jakupec, M.A.; Körner, W.; Chiba, P.; Pirker, C.; Dornetshuber, R.; Elbling, L.; Sutterlüty, H.; Micksche, M.; Keppler, B.K.; et al. Multidrug-resistant cancer cells are preferential targets of the new antineoplastic lanthanum compound KP772 (FFC24). Biochem. Pharmacol. 2007, 73, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Kovalev, A.A.; Tsvetaeva, D.A.; Grudinskaja, T.V. Role of ABC-cassette transporters (MDR1, MRP1, BCRP) in the development of primary and acquired multiple drug resistance in patients with early and metastatic breast cancer. Exp. Oncol. 2013, 35, 287–290. [Google Scholar] [PubMed]
- Choi, Y.H.; Yu, A.-M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Cronin-Fenton, D.P.; Damkier, P.; Lash, T.L. Metabolism and transport of tamoxifen in relation to its effectiveness: New perspectives on an ongoing controversy. Future Oncol. 2014, 10, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Sakunrangsit, N.; Kalpongnukul, N.; Pisitkun, T.; Ketchart, W. Plumbagin enhances tamoxifen sensitivity and inhibits tumor invasion in endocrine resistant breast cancer through EMT regulation. Phytother. Res. 2016, 30, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, D.D.; Yang, M.; Chen, D.; Pang, L.; Guo, S.; Cai, J.; Wery, J.-P.; Li, L.; Li, H.Q.; et al. Comprehensive characterization of chemotherapeutic efficacy on metastases in the established gastric neuroendocrine cancer patient derived xenograft model. Oncotarget 2015, 6, 15639–15651. [Google Scholar] [CrossRef] [PubMed]
- Kryczka, J.; Przygodzka, P.; Bogusz, H.; Boncela, J. HMEC-1 adopt the mixed amoeboid-mesenchymal migration type during EndMT. Eur. J. Cell Biol. 2017, 96, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Sheetz, M.P.; Felsenfeld, D.; Galbraith, C.G.; Choquet, D. Cell migration as a five-step cycle. Biochem. Soc. Symp. 1999, 65, 233–243. [Google Scholar] [PubMed]
- Wiesner, C.; Le-Cabec, V.; El Azzouzi, K.; Maridonneau-Parini, I.; Linder, S. Podosomes in space: Macrophage migration and matrix degradation in 2D and 3D settings. Cell Adh. Migr. 2014, 8, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Lämmermann, T.; Sixt, M. Mechanical modes of “amoeboid” cell migration. Curr. Opin. Cell Biol. 2009, 21, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Kanthou, C.; Dachs, G.U.; Lefley, D.V.; Steele, A.J.; Coralli-Foxon, C.; Harris, S.; Greco, O.; Dos Santos, S.A.; Reyes-Aldasoro, C.C.; English, W.R.; et al. Tumour cells expressing single VEGF isoforms display distinct growth, survival and migration characteristics. PLoS ONE 2014, 9, e104015. [Google Scholar] [CrossRef] [PubMed]
- Kryczka, J.; Boncela, J. Proteases Revisited: Roles and Therapeutic Implications in Fibrosis. Mediators Inflamm. 2017, 2017, 2570154. [Google Scholar] [CrossRef] [PubMed]
- Copsel, S.; Garcia, C.; Diez, F.; Vermeulem, M.; Baldi, A.; Bianciotti, L.G.; Russel, F.G.M.; Shayo, C.; Davio, C. Multidrug resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels and controls human leukemia cell proliferation and differentiation. J. Biol. Chem. 2011, 286, 6979–6988. [Google Scholar] [CrossRef] [PubMed]
- Sinha, C.; Ren, A.; Arora, K.; Moon, C.-S.; Yarlagadda, S.; Zhang, W.; Cheepala, S.B.; Schuetz, J.D.; Naren, A.P. Multi-drug resistance protein 4 (MRP4)-mediated regulation of fibroblast cell migration reflects a dichotomous role of intracellular cyclic nucleotides. J. Biol. Chem. 2013, 288, 3786–3794. [Google Scholar] [CrossRef] [PubMed]
- Elferink, J.G.; VanUffelen, B.E. The role of cyclic nucleotides in neutrophil migration. Gen. Pharmacol. 1996, 27, 387–393. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.J.; Huang, X.-Y. cAMP inhibits cell migration by interfering with Rac-induced lamellipodium formation. J. Biol. Chem. 2008, 283, 13799–13805. [Google Scholar] [CrossRef] [PubMed]
- Sinha, C.; Ren, A.; Arora, K.; Moon, C.S.; Yarlagadda, S.; Woodrooffe, K.; Lin, S.; Schuetz, J.D.; Ziady, A.G.; Naren, A.P. PKA and actin play critical roles as downstream effectors in MRP4-mediated regulation of fibroblast migration. Cell. Signal. 2015, 27, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Negash, S.; Narasimhan, S.R.; Zhou, W.; Liu, J.; Wei, F.L.; Tian, J.; Raj, J.U. Role of cGMP-dependent protein kinase in regulation of pulmonary vascular smooth muscle cell adhesion and migration: effect of hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H304–H312. [Google Scholar] [CrossRef] [PubMed]
- Tagami, M.; Kusuhara, S.; Imai, H.; Uemura, A.; Honda, S.; Tsukahara, Y.; Negi, A. MRP4 knockdown enhances migration, suppresses apoptosis, and produces aggregated morphology in human retinal vascular endothelial cells. Biochem. Biophys. Res. Commun. 2010, 400, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Sinha, C.; Arora, K.; Naren, A.P. Methods to study MRP4-containing macromolecular complexes in the regulation of fibroblast migration. J. Vis. Exp. 2016, 53973. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Donthireddy, L.; Marvel, D.; Condamine, T.; Wang, F.; Lavilla-Alonso, S.; Hashimoto, A.; Vonteddu, P.; Behera, R.; Goins, M.A.; et al. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell 2017, 32, 654–668.e5. [Google Scholar] [CrossRef] [PubMed]
- Bremnes, R.M.; Dønnem, T.; Al-Saad, S.; Al-Shibli, K.; Andersen, S.; Sirera, R.; Camps, C.; Marinez, I.; Busund, L.-T. The role of tumor stroma in cancer progression and prognosis: Emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Sakai, R. Direct Interaction between carcinoma cells and cancer associated fibroblasts for the regulation of cancer invasion. Cancers 2015, 7, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.; Ao, M.; White, L.M.; Means, A.L.; Brewer, B.M.; Yang, L.; Washington, M.K.; Shi, C.; Franco, O.E.; Weaver, A.M.; et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J. Cell Biol. 2017, 216, 3799–3816. [Google Scholar] [CrossRef] [PubMed]
- Um, E.; Oh, J.M.; Granick, S.; Cho, Y.-K. Cell migration in microengineered tumor environments. Lab. Chip 2017, 17, 4171–4185. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, G.S.; Poutahidis, T.; Erdman, S.E.; Kirsch, R.; Riddell, R.H.; Diamandis, E.P. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol. Cancer Res. 2012, 10, 1403–1418. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Hu, S.-Q.; Xiao, L. The cancer-associated fibroblasts and drug resistance. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2112–2119. [Google Scholar] [PubMed]
- Bello, I.O.; Vered, M.; Dayan, D.; Dobriyan, A.; Yahalom, R.; Alanen, K.; Nieminen, P.; Kantola, S.; Läärä, E.; Salo, T. Cancer-associated fibroblasts, a parameter of the tumor microenvironment, overcomes carcinoma-associated parameters in the prognosis of patients with mobile tongue cancer. Oral Oncol. 2011, 47, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Vered, M.; Dayan, D.; Yahalom, R.; Dobriyan, A.; Barshack, I.; Bello, I.O.; Kantola, S.; Salo, T. Cancer-associated fibroblasts and epithelial-mesenchymal transition in metastatic oral tongue squamous cell carcinoma. Int. J. Cancer 2010, 127, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Gaggioli, C.; Hooper, S.; Hidalgo-Carcedo, C.; Grosse, R.; Marshall, J.F.; Harrington, K.; Sahai, E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 2007, 9, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Dimanche-Boitrel, M.T.; Vakaet, L.; Pujuguet, P.; Chauffert, B.; Martin, M.S.; Hammann, A.; van Roy, F.; Mareel, M.; Martin, F. In vivo and in vitro invasiveness of a rat colon-cancer cell line maintaining E-cadherin expression: An enhancing role of tumor-associated myofibroblasts. Int. J. Cancer 1994, 56, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, S.V.; Pasapera, A.M.; Sabass, B.; Waterman, C.M. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 2012, 151, 1513–1527. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shi, N.; Lu, H.; Zhang, J.; Ma, Y.; Qiao, Y.; Mao, Y.; Jia, K.; Han, L.; Liu, F.; et al. ABCC4 copy number variation is associated with susceptibility to esophageal squamous cell carcinoma. Carcinogenesis 2014, 35, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
| ABC Gene | Alternative Names |
|---|---|
| ABCB1 | MDR1 P-gp (P-glycoprotein 1) |
| ABCC1 | MRP1 |
| ABCC2 | MRP2 cMOAT |
| ABCC3 | MRP3 cMOAT-2 |
| ABCC4 | MRP4 MOAT-B |
| ABCC5 | MRP5 MOAT-C |
| ABCC6 | MRP6 |
| ABCC10 | MRP7 |
| ABCC11 | MRP8 |
| ABCC12 | MRP9 |
| ABCG2 | BCRP1 MXR1 |
| ABC Protein Activity Modulator | Target | Effect |
|---|---|---|
| Biricodar | ABCB1 ABCC1 ABCG2 | direct interaction [65] |
| Celecoxib | ABCC1 | COX-2 inhibitor [66] |
| Curcumin | ABCB1 ABCC1 ABCG2 | interacts directly with drug binding site of the transporter [67] |
| Cyclosporine A | ABCB1 ABCC1 ABCC2 ABCC10 ABCG2 | interacts directly with drug binding site of the transporter [65,68] |
| Dexaverapamil | ABCB1 | interacts directly with drug binding site of the transporter [69] |
| Dipiridamole | ABCB1 ABCC1 ABCC4 | phosphodiesterase inhibitor [70] |
| Dofequidar | ABCB1 ABCC1 ABCG2 | direct interaction [71] |
| Elacradir | ABCB1 ABCG2 | direct interaction [65] |
| Indomethacin | ABCC1 ABCC2 | COX and glutathione-S-transferase inhibitor, direct ABC protein inhibition [72] |
| Losartan | ABCB1 ABCC4 | direct interaction [73,74] |
| MK571 | ABCC family ABCG2 | LTC4 receptor antagonist [55,75] |
| MRK-16 | ABCB1 | Antibody [44,76] |
| Ontogen | ABCB1 | direct interaction [49] |
| Piperine | ABCB1 ABCC1 ABCG2 | reduces ATPase activity of ABCB1 at high concentration and stimulates it at low concentration, decreases the expression level of ABCB1, ABCC1 and ABCG2 genes [77] |
| Probenecid | ABCC family | an organic anion transport inhibitor [56,78] |
| Quercetin | ABCC family | Interact with ATP binding site (NBD) [79] |
| Reversan | ABCB1 ABCC1 | small molecule inhibitor [80] |
| Sildenafil | ABCB1 ABCC4 ABCG2 | PDE5 inhiitor [70,81] |
| Sorafenib | ABCB1 ABCC1-3 | multi-kinase inhibitor, downregulates ABC mRNA [58] |
| Tariquidar | ABCB1 ABCC1 ABCC10 ABCG2 | interacts the transporter but not with drug binding site [51,52,82] |
| Valspodar | ABCB1 ABCC2 | interacts directly with drug binding site of the transporter [75,83] |
| Verapamil | ABCB1 ABCC1 | interacts directly with drug binding site of the transporter [65] |
| Zosuquidar | ABCB1 | direct interaction [50] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryczka, J.; Boncela, J. Cell Migration Related to MDR—Another Impediment to Effective Chemotherapy? Molecules 2018, 23, 331. https://doi.org/10.3390/molecules23020331
Kryczka J, Boncela J. Cell Migration Related to MDR—Another Impediment to Effective Chemotherapy? Molecules. 2018; 23(2):331. https://doi.org/10.3390/molecules23020331
Chicago/Turabian StyleKryczka, Jakub, and Joanna Boncela. 2018. "Cell Migration Related to MDR—Another Impediment to Effective Chemotherapy?" Molecules 23, no. 2: 331. https://doi.org/10.3390/molecules23020331
APA StyleKryczka, J., & Boncela, J. (2018). Cell Migration Related to MDR—Another Impediment to Effective Chemotherapy? Molecules, 23(2), 331. https://doi.org/10.3390/molecules23020331