MicroRNA-125a-5p Mediates 3T3-L1 Preadipocyte Proliferation and Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Cell Culture and Transfection
2.3. Quantitative RT-PCR
2.4. CCK-8 and EdU Assays
2.5. Immunocytochemical Analysis
2.6. Oil Red O Staining and Triglyceride Assay
2.7. Luciferase Reporter Assay
2.8. Statistical Analysis
3. Results and Discussion
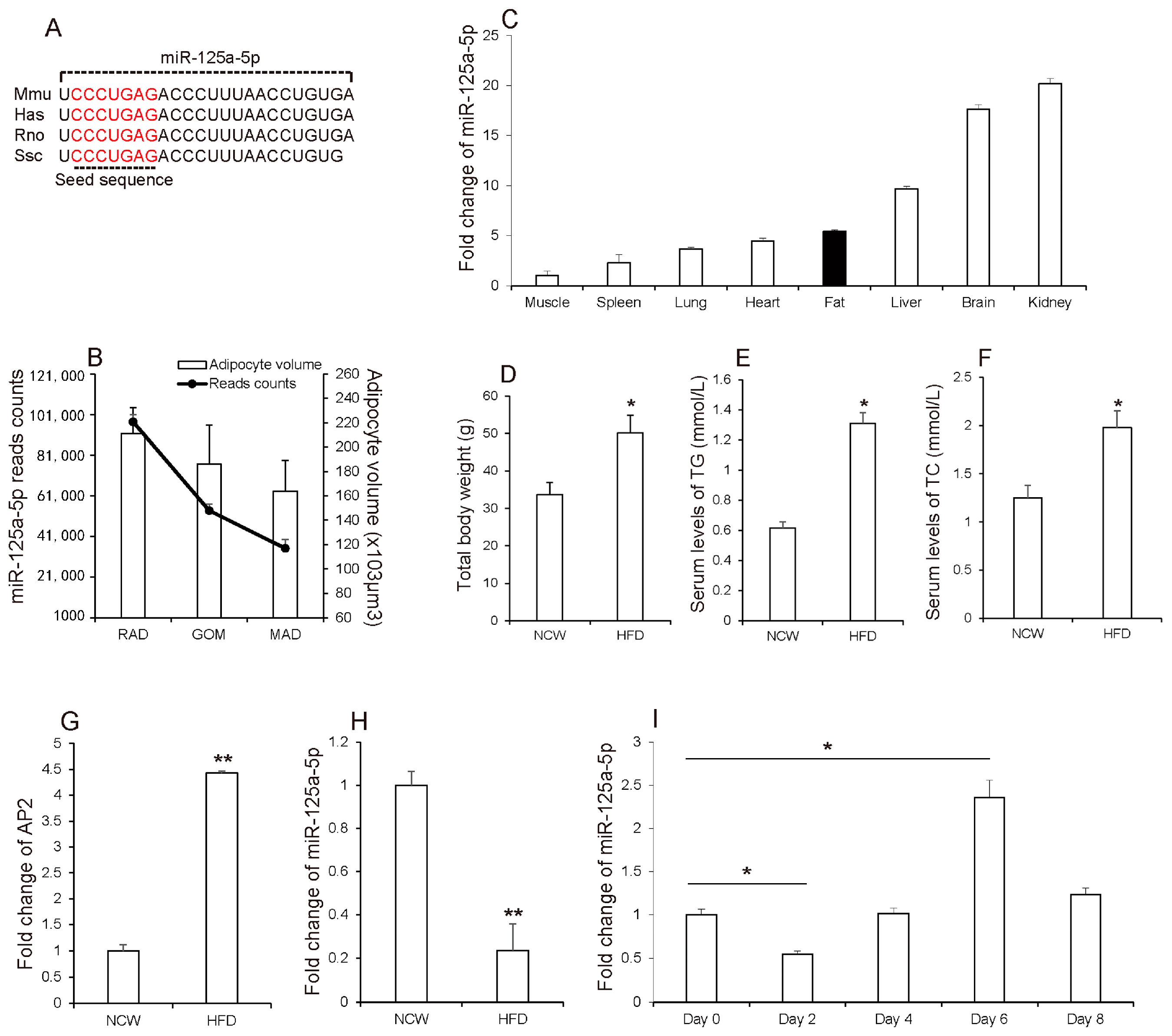
3.1. MiR-125a-5p Is Involved in Adipogenesis
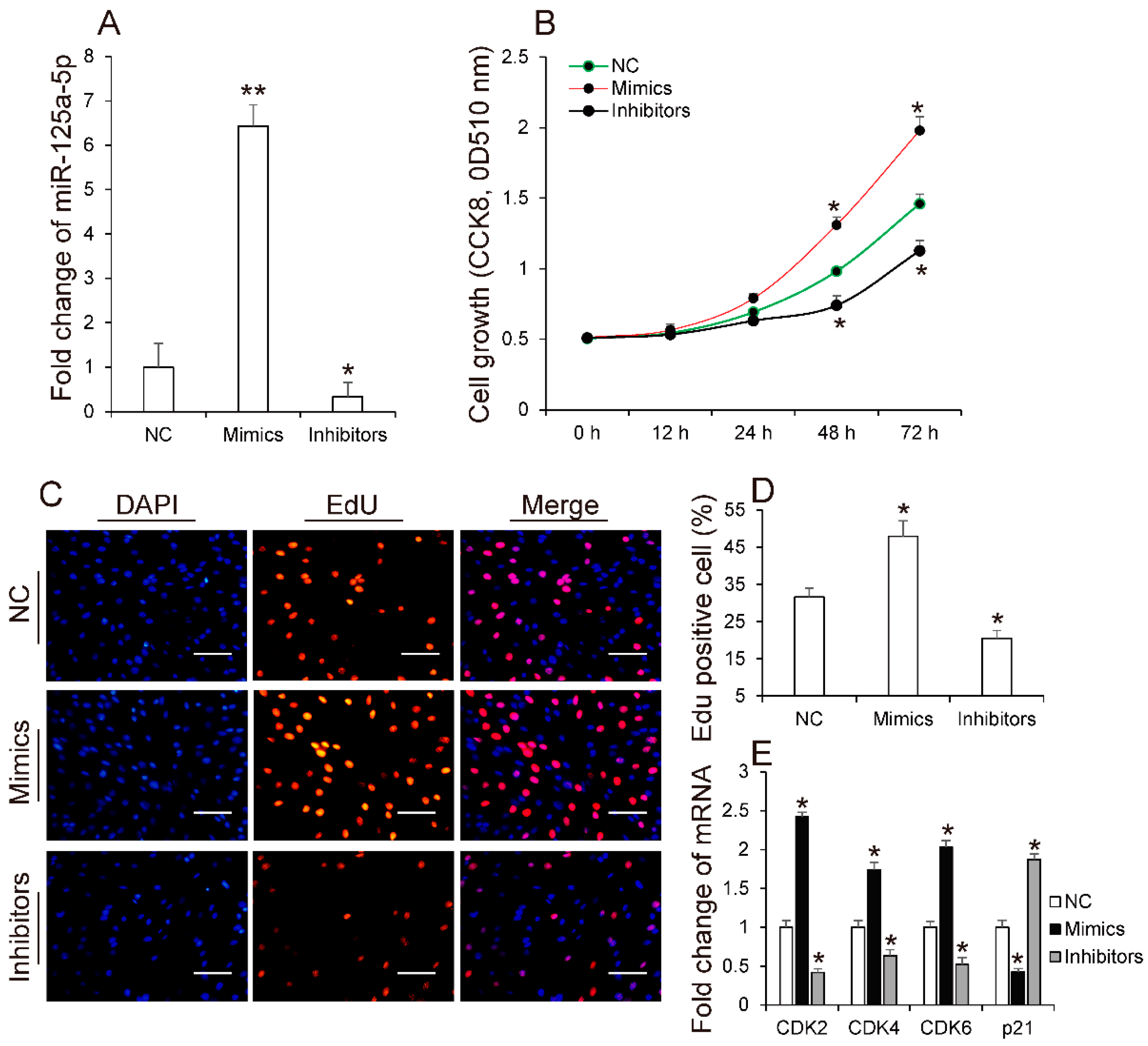
3.2. MiR-125a-5p Promotes 3T3-L1 Preadipocyte Proliferation
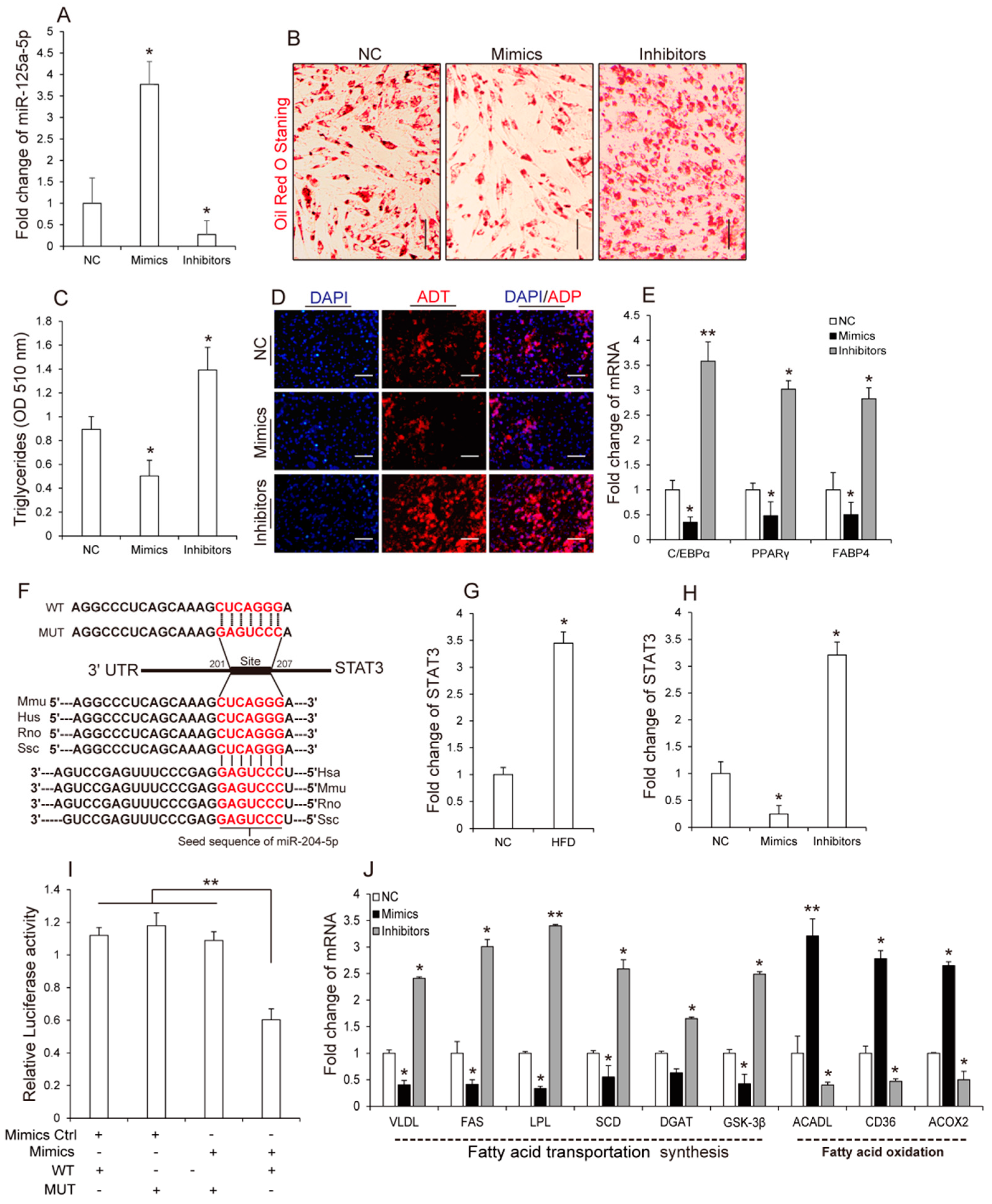
3.3. MiR-125a-5p Inhibits 3T3-L1 Preadipocyte Differentiation by Targeting STAT3
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Polikandrioti, M.; Stefanou, E. Obesity disease. Health Sci. J. 2009, 3, 132–134. [Google Scholar]
- Rössner, S. Obesity: The disease of the twenty-first century. Int. J. Pediatr. Obes. 2002, 26, S2–S4. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kapil, U.; Singh, P. Pattern of chronic diseases amongst adolescent obese children in developing countries. Curr. Dir. Psychol. Sci. 2005, 88, 1052–1056. [Google Scholar]
- Fransson, P.A. Analysis of Adaptation in Human Postural Control; Lund University: Lund, Sweden, 2009; Volume 23, p. 112. [Google Scholar]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Collier, A.; Ghosh, S.; Mcglynn, B.; Hollins, G. Prostate cancer, androgen deprivation therapy, obesity, the metabolic syndrome, type 2 diabetes, and cardiovascular disease: A review. Am. J. Clin. Oncol. 2012, 35, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Paul Angulo, M.D. Obesity and nonalcoholic fatty liver disease. Nutr. Rev. 2010, 65, S57–S63. [Google Scholar] [CrossRef]
- Renehan, D.A.G.; Roberts, D.L.; Dive, C. Obesity and cancer: Pathophysiological and biological mechanisms. Arch. Physiol. Biochem. 2008, 114, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Tordjman, J.; Clément, K.; Scherer, P. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Leyvraz, C.; Verdumo, C.; Giusti, V. Localization of adipose tissue: Clinical implications. Revue Médicale Suisse 2008, 151, 844–847. [Google Scholar]
- Hwang, H.W.; Mendell, J.T. Micrornas in cell proliferation, cell death, and tumorigenesis. Br. J. Cancer 2006, 94, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Carlsbecker, A.; Lee, J.Y.; Roberts, C.J.; Dettmer, J.; Lehesranta, S.; Zhou, J.; Lindgren, O.; Moreno-Risueno, M.A.; Vatén, A.; Thitamadee, S. Cell signaling by microrna165/6 directs gene dose-dependent root cell fate. Nature 2010, 465, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandezcarretero, A.; Fu, W. Adipose tissue macrophage-derived exosomal mirnas can modulate in vivo and in vitro insulin sensitivity. Cell 2017, 171, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Nucera, S.; Giustacchini, A.; Boccalatte, F.; Calabria, A.; Fanciullo, C.; Plati, T.; Ranghetti, A.; Garciamanteiga, J.; Cittaro, D.; Benedicenti, F. Mirna-126 orchestrates an oncogenic program in b cell precursor acute lymphoblastic leukemia. Cancer Cell 2016, 29, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Siegel, F.; Kipschull, S.; Haas, B.; Fröhlich, H.; Meister, G.; Pfeifer, A. MiR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat. Commun. 2013, 4, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; He, H.; Xie, Y.; Zhao, L.; Zhao, S.; Wan, X.; Yang, W.; Mo, Z. MiR-125a-3p and miR-483-5p promote adipogenesis via suppressing the Rhoa/ROCK1/ERK1/2 pathway in multiple symmetric lipomatosis. Sci. Rep. 2015, 5, 11909–11923. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.; Vallespinosserrano, M.; Trabulo, S.M.; Fernandezmarcos, P.J.; Firment, A.N.; Vazquez, B.N.; Vieira, C.R.; Mulero, F.; Camara, J.A.; Cronin, U.P. MiR-93 controls adiposity via inhibition of Sirt7 and Tbx3. Cell Rep. 2015, 12, 1594–1605. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cheng, X.; Shen, L.; Tan, Z.; Luo, J.; Wu, X.; Liu, C.; Yang, Q.; Jiang, Y.; Tang, G. Methylation of miR-145a-5p promoter mediates adipocytes differentiation. Biochem. Biophys. Res. Commun. 2016, 475, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, H.; Luo, Z.; Xia, Y.; Guan, J.; Wang, T.; Gu, Y.; Chen, L.; Zhang, K.; Ma, J. An atlas of DNA methylomes in porcine adipose and muscle tissues. Nat. Commun. 2012, 3, 850–871. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.L.; Song, C.C.; Li, Y.F.; He, J.J.; Li, Y.L.; Zheng, X.L.; Yang, G.S. Mir-125a inhibits porcine preadipocyte differentiation by targeting errα. Mol. Cell. Biochem. 2014, 395, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Du, J.; Shen, L.; Liu, C.; Ma, J.; Bai, L.; Jiang, Y.; Tang, G.; Li, M.; Li, X. MiR-199a-3p affects adipocytes differentiation and fatty acid composition through targeting SCD. Biochem. Biophys. Res. Commun. 2017, 492, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Kawada, T.; Yoo, H.; Kwon, B.S.; Yu, R. Macrophage inflammatory protein-related protein-2, a novel cc chemokine, can regulate preadipocyte migration and adipocyte differentiation. FEBS Lett. 2003, 548, 125–130. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Johnson, R.S.; Distel, R.J.; Ellis, R.; Papaioannou, V.E.; Spiegelman, B.M. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science 1996, 274, 1377–1379. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; Pekala, P.H.; Lane, M.D.; Cerami, A. Lipoprotein lipase suppression in 3T3-L1 cells by an endotoxin-induced mediator from exudate cell. Proc. Natl. Acad. Sci. USA 1982, 79, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Xiang, H.; Chen, C.; Zheng, R.; Chai, J.; Peng, J.; Jiang, S. MiR-224 impairs adipocyte early differentiation and regulates fatty acid metabolism. Int. J. Biochem. Cell Biol. 2013, 45, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Macdougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Graña, X.; Reddy, E.P. Cell cycle control in mammalian cells: Role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene 1995, 11, 211–219. [Google Scholar] [PubMed]
- Gartel, A.L.; Serfas, M.S.; Tyner, A.L. P21—Negative regulator of the cell cycle. Proc. Soc. Exp. Biol. Med. 1996, 213, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dai, Y.M.; Ji, C.B.; Yang, L.; Shi, C.M.; Xu, G.F.; Pang, L.X.; Huang, F.Y.; Zhang, C.M.; Guo, X.R. MiR-146b is a regulator of human visceral preadipocyte proliferation and differentiation and its expression is altered in human obesity. Mol. Cell. Endocrinol. 2014, 393, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, L.; Hematology, D.O.; Hosiptal, T. The research progress of the relationship between miR-21 through regulate its target gene PTEN and tumor resistance. J. Mod. Oncol. 2015, 13, 143–146. [Google Scholar]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M. MiR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Calado, D.P.; Galler, G.; Thai, T.H.; Patterson, H.C.; Wang, J.; Rajewsky, N.; Bender, T.P.; Rajewsky, K. MiR-150 controls b cell differentiation by targeting the transcription factor c-Myb. Cell 2007, 13, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Sun, T.; Wang, H.; Chen, Z.; Wang, S.; Yuan, L.; Liu, T.; Li, H.R.; Wang, P.; Feng, Y. MiR-215 is induced post-transcriptionally via hif-drosha complex and mediates glioma-initiating cell adaptation to hypoxia by targeting KDM1B. Cancer Cell 2016, 29, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Yukyoung, P.; Jinho, L.; Victor, S.H.; Jongsoon, C.; Taeyoon, L.; Byeongchurl, J. Effect of KMU-3 or AG490 on lipid accumulation and/or stat-3 phosphorylation during 3T3-L1 adipocyte differentiation. PLoS ONE 2014, 9, e109344. [Google Scholar]
- Cernkovich, E.R.; Deng, J.; Hua, K.; Harp, J.B. Midkine is an autocrine activator of stat3 in 3T3-L1 cells. Endocrinology 2007, 1106, 1–34. [Google Scholar]
- Hems, D.A.; Rath, E.A.; Verrinder, T.R. Fatty acid synthesis in liver and adipose tissue of normal and genetically obese (ob/ob) mice during the 24-hour cycle. Biochem. J. 1975, 150, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef] [PubMed]
- Rozovski, U.; Grgurevic, S.; Buesoramos, C.; Harris, D.M.; Ping, L.; Liu, Z.; Ji, Y.W.; Jain, P.; Wierda, W.; Burger, J. Aberrant LPL expression, driven by stat3, mediates free fatty acid metabolism in CLL cells. Mol. Cancer Res. 2015, 13, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Niso-Santano, M.; Shen, S.; Adjemian, S.; Malik, S.A.; Mariño, G.; Lachkar, S.; Senovilla, L.; Kepp, O.; Galluzzi, L.; Maiuri, M.C. Direct interaction between stat3 and EIF2AK2 controls fatty acid-induced autophagy. Autophagy 2013, 9, 415–417. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Du, J.; Zhang, P.; Zhao, X.; Li, Q.; Jiang, A.; Jiang, D.; Tang, G.; Jiang, Y.; Wang, J.; et al. MicroRNA-125a-5p Mediates 3T3-L1 Preadipocyte Proliferation and Differentiation. Molecules 2018, 23, 317. https://doi.org/10.3390/molecules23020317
Xu Y, Du J, Zhang P, Zhao X, Li Q, Jiang A, Jiang D, Tang G, Jiang Y, Wang J, et al. MicroRNA-125a-5p Mediates 3T3-L1 Preadipocyte Proliferation and Differentiation. Molecules. 2018; 23(2):317. https://doi.org/10.3390/molecules23020317
Chicago/Turabian StyleXu, Yan, Jingjing Du, Peiwen Zhang, Xue Zhao, Qiang Li, Anan Jiang, Dongmei Jiang, Guoqing Tang, Yanzhi Jiang, Jinyong Wang, and et al. 2018. "MicroRNA-125a-5p Mediates 3T3-L1 Preadipocyte Proliferation and Differentiation" Molecules 23, no. 2: 317. https://doi.org/10.3390/molecules23020317
APA StyleXu, Y., Du, J., Zhang, P., Zhao, X., Li, Q., Jiang, A., Jiang, D., Tang, G., Jiang, Y., Wang, J., Li, X., Zhang, S., & Zhu, L. (2018). MicroRNA-125a-5p Mediates 3T3-L1 Preadipocyte Proliferation and Differentiation. Molecules, 23(2), 317. https://doi.org/10.3390/molecules23020317



