Nine New Gingerols from the Rhizoma of Zingiber officinale and Their Cytotoxic Activities
Abstract
:1. Introduction
2. Results and Discussion
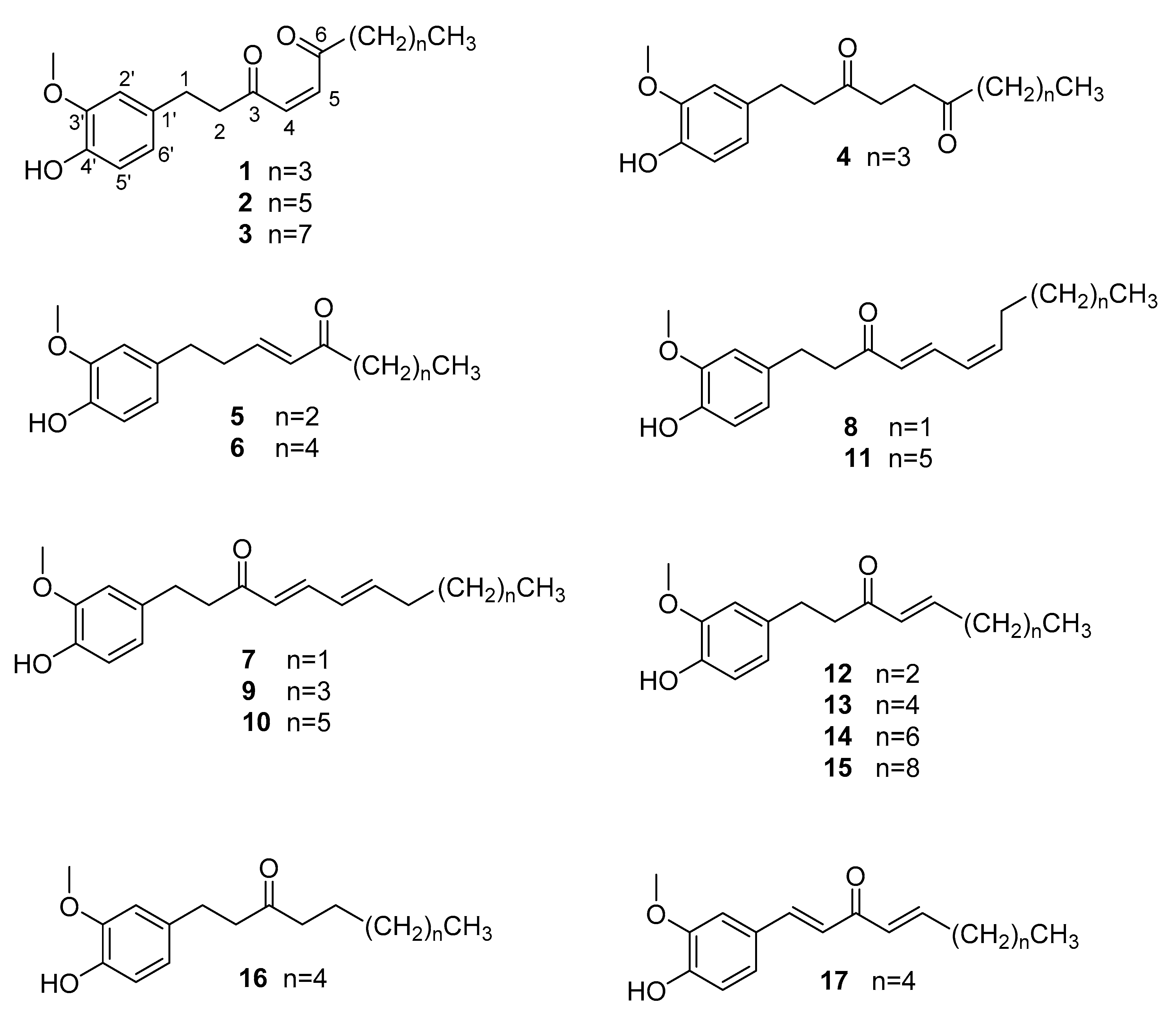
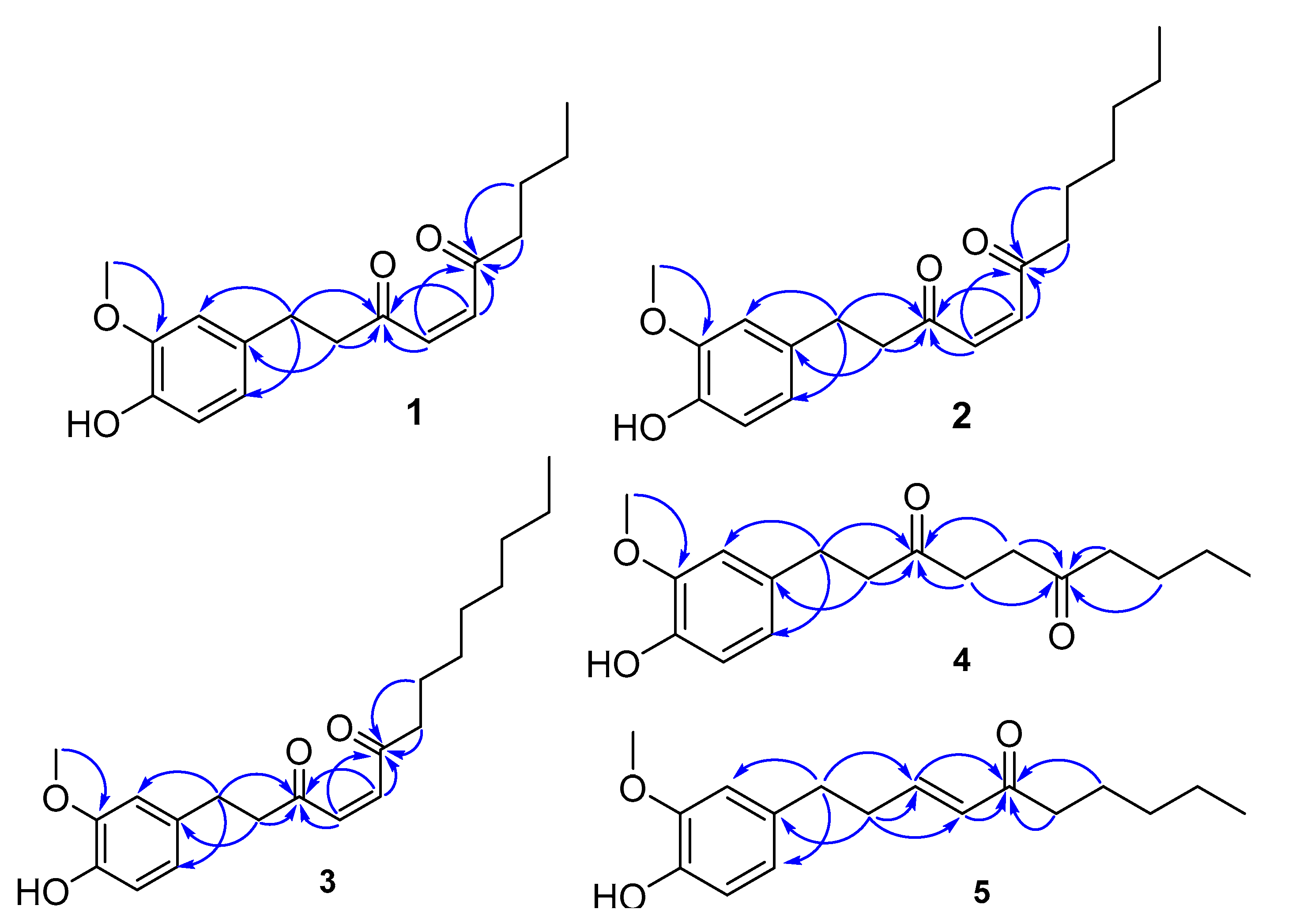
Structure Elucidation of the Compounds
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectroscopic and Physical Data
3.5. Cytotoxic Assay
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chang, T.T.; Chen, K.C.; Chang, K.W.; Chen, H.Y.; Tsai, F.J.; Sun, M.F.; Chen, C.Y. In silico pharmacology suggests ginger extracts may reduce stroke risks. Mol. Biosyst. 2011, 7, 2702–2710. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Cai, P.W.; Fu, M.W.; Ying, P. Combined administration of the mixture of honokiol and magnolol and ginger oil evokes antidepressant-like synergism in rats. Arch. Pharm. Res. 2009, 32, 1281–1292. [Google Scholar]
- Therkleson, T. Ginger compress therapy for adults with osteoarthritis. J. Adv. Nurs. 2010, 66, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Hisamoto, M.; Nakatani, N. Antioxidant properties of gingerol related compounds from ginger. Biofactors 2004, 21, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Yang, S.W.; Li, B.; Zhang, K.; Li, Y.; Hu, Y.N. Analysis of antioxidant activity of gingerol from ginger by supercritical CO2 fluid extraction. China Food Addit. 2014, 8, 96–101. [Google Scholar]
- Chrubasik, S.; Pittler, M.H.; Roufogalis, B.D. Zingiber is rhizoma: A comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005, 12, 684. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.J.; Wu, C.F.; Huang, H.W.; Ho, C.T.; Yen, G.C. Anti-invasion effects of 6-shogaol and 6-gingerol, two active components in ginger, on human hepatocarcinoma cells. Mol. Nutr. Food Res. 2010, 54, 1618. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.J.; Zheng, Z.X.; Hyun, J.K.; Steven, P.M.; Barbara, N.T.; David, R.G. Metabolic profiling and phylogenetic analysis of medicinal Zingiber species: Tools for authentication of ginger (Zingiber officinale Rosc.). Phytochemistry 2006, 67, 1673–1685. [Google Scholar]
- Shukla, Y.; Singh, M. Cancer preventive properties of ginger: A brief review. Food Chem. Toxicol. 2007, 45, 683. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, W.P.; Li, Y.L.; Yang, M.; Lei, Z.X. Effects of dried ginger decoction on plasma angiotensin II, serum tumor necrosis factor alpha, malondialdehyde and nitric oxide in rats with acute myocardial ischemia. Lishizhen Med. Mater. Med. Res. 2014, 25, 288–290. [Google Scholar]
- Nafiseh, S.M.; Reza, G.; Gholamreza, A.; Mitra, H.; Leila, D.; Mohammad, R.M. Anti-oxidative and anti-inflammatory effects of ginger in health and physical activity: Review of current evidence. Int. J. Prev. Med. 2013, 4, 36. [Google Scholar]
- Jiang, S.Z.; Liao, K. Effect of alcohol extract from dried ginger on experimental gastric ulcer. Chin. J. Ethnomed. Ethnopharm. 2010, 19, 79–80. [Google Scholar]
- Yu, S.J.; Qin, H.L.; Yong, Z.; Yi, Q.L.; Jian, B.L. Transcriptome analysis reveals the genetic basis underlying the biosynthesis of volatile oil, gingerols, and diarylheptanoids in ginger (Zingiber officinale Rosc.). Bot. Stud. 2017, 58, 41. [Google Scholar] [CrossRef]
- Roberto, B.; Giovanna, B. A New Stereoselective Synthesis of (E)-α,β-Unsaturated-γ-dicarbonyl Compounds by the Henry Reaction. J. Org. Chem. 1994, 59, 5466–5467. [Google Scholar]
- Hung, C.S.; Ching, Y.C.; Ping, C.K.; You, C.W.; Yu, Y.C. Synthesis of analogues of gingerol and shogaol, the active pungent principles from the rhizomes of Zingiber officinale and evaluation of their anti-platelet aggregation effects. Int. J. Mol. Sci. 2014, 15, 3926–3951. [Google Scholar]
- Christopher, J.H.; Steven, V.L.; Edward, A. 1,5-Asymmetric induction of chirality using π-allyltricarbonyliron lactone complexes: Highly diastereoselective synthesis of π-functionalised carbonyl compounds. Org. Biomol. Chem. 2003, 1, 3208–3216. [Google Scholar]
- Alberto, A.; Rosanna, C.; Gianluca, N.; Orso, V.P.; Sergio, Q. A new strain of streptomyces: An anthracycline containing a C-Glucoside moiety and a chiral decanol. Phytochemistry 1988, 27, 3611–3617. [Google Scholar]
- Guillermo, T.; Carmen, S.; Pilar, R.; Marta, P.; Librada, M.C.; Carmen, C. Streptenols F-I isolated from the marine-derived Streptomyces misionensis BAT-10-03-023. J. Nat. Prod. 2017, 80, 1034–1038. [Google Scholar]
- Chen, C.C.; Rosen, R.T.; Ho, C.T. Chromatographic analyses of isomeric shogaol compounds derived from isolated gingerol compounds of ginger (Zingiber Officinale, roscoe). J. Chromatogr. A 1986, 360, 175–184. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 4, 8–15 are available from the authors. |

| 1 | 2 | 3 | 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Position | δC | δH | δC | δH | δC | δH | δC | δH |
| 1′ | 132.4 | 132.4 | 132.4 | 132.9 | ||||
| 2′ | 111.0 | 6.67, d (1.5) | 111.0 | 6.67, d (1.5) | 111.0 | 6.67, s | 111.0 | 6.66, d (1.9) |
| 3′ | 146.4 | 146.4 | 146.4 | 146.4 | ||||
| 4′ | 144.0 | 144.0 | 144.0 | 143.9 | ||||
| 5′ | 114.4 | 6.81, d (8.0) | 114.4 | 6.81, d (8.0) | 114.4 | 6.80, d (8.0) | 114.2 | 6.79, d (8.0) |
| 6′ | 120.8 | 6.65, dd (8.0,1.5) | 120.8 | 6.66, dd (8.0,1.5) | 120.8 | 6.65, d (8.0) | 120.7 | 6.62, dd (8.0,1.9) |
| 1 | 29.3 | 2.86, m | 29.3 | 2.86, m | 29.3 | 2.88, m | 29.4 | 2.73, m |
| 2 | 43.4 | 2.92, m | 43.5 | 2.94, m | 43.5 | 2.92, m | 44.6 | 2.80, m |
| 3 | 200.7 | 200.7 | 200.7 | 208.8 | ||||
| 4 | 136.4 | 6.82, s | 136.4 | 6.83, s | 136.4 | 6.83, s | 36.1 | 2.65, m |
| 5 | 136.0 | 6.82, s | 136.1 | 6.83, s | 136.0 | 6.83, s | 35.9 | 2.62, m |
| 6 | 199.8 | 199.8 | 199.8 | 209.7 | ||||
| 7 | 41.3 | 2.59, t (7.4) | 41.7 | 2.59, t (7.4) | 41.6 | 2.58, t (7.4) | 42.5 | 2.42, t (7.4) |
| 8 | 25.7 | 1.58, m | 23.7 | 1.60, m | 23.7 | 1.59, m | 25.9 | 1.54, m |
| 9 | 22.1 | 1.31, m | 28.8 | 1.28, m | 29.0 | 1.24, m | 22. | 1.27, m |
| 10 | 13.7 | 0.88, t (7.3) | 31.5 | 1.28, m | 29.3 | 1.24, m | 13.8 | 0.87, t (7.4) |
| 11 | 22.4 | 1.28, m | 29.3 | 1.24, m | ||||
| 12 | 14.0 | 0.85, t (6.4) | 31.7 | 1.24, m | ||||
| 13 | 22.3 | 1.24, m | ||||||
| 14 | 14.0 | 0.85, t (6.4) | ||||||
| 3′-OCH3 | 55.8 | 3.85, s | 55.9 | 3.85, s | 55.8 | 3.85, s | 55.8 | 3.85, s |
| 5 | 8 | 9 | 10 | 11 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Position | δC | δH | δC | δH | δC | δH | δC | δH | δC | δH |
| 1′ | 132.7 | 133.1 | 133.2 | 133.2 | 133.2 | |||||
| 2′ | 110.9 | 6.64, s | 111.1 | 6.69, d(1.5) | 111.1 | 6.68, d(1.5) | 111.1 | 6.69, d(1.6) | 111.1 | 6.68, d (1.5) |
| 3′ | 146.4 | 146.4 | 146.4 | 146.4 | 146.4 | |||||
| 4′ | 143.9 | 143.8 | 143.8 | 143.8 | 143.9 | |||||
| 5′ | 114.3 | 6.82, d (8.5) | 114.3 | 6.81, d (8.0) | 114.3 | 6.80, d (8.0) | 114.3 | 6.80, d (8.0) | 114.3 | 6.81, d (8.0) |
| 6′ | 120.9 | 6.65, dd (8.5, 1.9) | 120.7 | 6.66, dd (8.0, 1.5) | 120.8 | 6.66, dd (8.0, 1.5) | 120.7 | 6.66, dd (8.0, 1.6) | 120.8 | 6.66, dd (8.0, 1.5) |
| 1 | 34.2 | 2.70, t (7.2) | 29.9 | 2.83, m | 29.9 | 2.82, m | 29.9 | 2.82, m | 29.9 | 2.83, m |
| 2 | 34.5 | 2.48, t (7.2) | 42.8 | 2.85, m | 42.4 | 2.85, m | 42.3 | 2.85, m | 42.90 | 2.85, m |
| 3 | 145.9 | 6.81, m | 200.0 | 199.9 | 199.9 | 199.9 | ||||
| 4 | 130.7 | 6.10, dd (16.0,1.5) | 129.3 | 6.14, d (15.3) | 127.7 | 6.05, d (15.5) | 127.7 | 6.05, d (15.5) | 129.3 | 6.14, d (15.5) |
| 5 | 200.7 | 137.4 | 7.48, m | 143.3 | 7.10, m | 143.4 | 7.10, m | 137.4 | 7.45, m | |
| 6 | 42.0 | 2.47, t (7.0) | 127.0 | 6.11, q (11.2) | 128.8 | 6.14, m | 128.7 | 6.14, m | 126.8 | 6.09, q (11.0) |
| 7 | 17.5 | 1.61, m | 142.6 | 5.89, m | 146.0 | 6.13, m | 146.0 | 6.13, m | 143.0 | 5.88, m |
| 8 | 13.8 | 0.83, t (7.4) | 30.3 | 2.25, m | 33.1 | 2.15, m | 33.1 | 2.15, m | 29.3 | 2.26, q (7.5) |
| 9 | 22.5 | 1.42, m | 28.3 | 1.40, m | 28.6 | 1.40, m | 28.3 | 1.38, m | ||
| 10 | 13.6 | 0.90, t (7.4) | 31.3 | 1.27, m | 29.1 | 1.25, m | 29.1 | 1.25, m | ||
| 11 | 22.4 | 1.27, m | 29.0 | 1.25, m | 29.0 | 1.25, m | ||||
| 12 | 14.0 | 0.86, t (7.0) | 31.6 | 1.25, m | 31.7 | 1.25, m | ||||
| 13 | 22.6 | 1.25, m | 22.6 | 1.25, m | ||||||
| 14 | 14.0 | 0.86, t (6.7) | 14.1 | 0.85, t (6.1) | ||||||
| 3′-OCH3 | 55.85 | 3.85 | 55.81 | 3.85, s | 55.83 | 3.8, s | 55.8 | 3.85, s | 55.85 | 3.85, s |
| Compound | IC50 (μM) | ||
|---|---|---|---|
| HepG-2 | Mcf-7 | KYSE-150 | |
| 1 | 8.92 ± 0.34 | 6.27 ± 0.21 | >50 |
| 2 | 45.14 ± 1.69 | 47.22 ± 2.31 | >50 |
| 3 | >50 | >50 | >50 |
| 4 | >50 | >50 | >50 |
| 5 | 14.87 ± 0.57 | >50 | >50 |
| 13 | 21.56 ± 1.47 | 22.85 ± 1.01 | 20.41 ± 0.53 |
| 14 | >50 | >50 | >50 |
| 15 | >50 | >50 | >50 |
| 16 | >50 | >50 | >50 |
| 5-Fluorouracil | 8.18 ± 0.53 | 7.35 ± 0.37 | 13.26 ± 0.47 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, Y.; Gao, M.; Cui, W.; Zeng, M.; Cheng, Y.; Li, J. Nine New Gingerols from the Rhizoma of Zingiber officinale and Their Cytotoxic Activities. Molecules 2018, 23, 315. https://doi.org/10.3390/molecules23020315
Li Z, Wang Y, Gao M, Cui W, Zeng M, Cheng Y, Li J. Nine New Gingerols from the Rhizoma of Zingiber officinale and Their Cytotoxic Activities. Molecules. 2018; 23(2):315. https://doi.org/10.3390/molecules23020315
Chicago/Turabian StyleLi, Zezhi, Yanzhi Wang, MeiLing Gao, Wanhua Cui, Mengnan Zeng, Yongxian Cheng, and Juan Li. 2018. "Nine New Gingerols from the Rhizoma of Zingiber officinale and Their Cytotoxic Activities" Molecules 23, no. 2: 315. https://doi.org/10.3390/molecules23020315
APA StyleLi, Z., Wang, Y., Gao, M., Cui, W., Zeng, M., Cheng, Y., & Li, J. (2018). Nine New Gingerols from the Rhizoma of Zingiber officinale and Their Cytotoxic Activities. Molecules, 23(2), 315. https://doi.org/10.3390/molecules23020315