Synthesis of Scutellarein Derivatives with a Long Aliphatic Chain and Their Biological Evaluation against Human Cancer Cells
Abstract
:1. Introduction
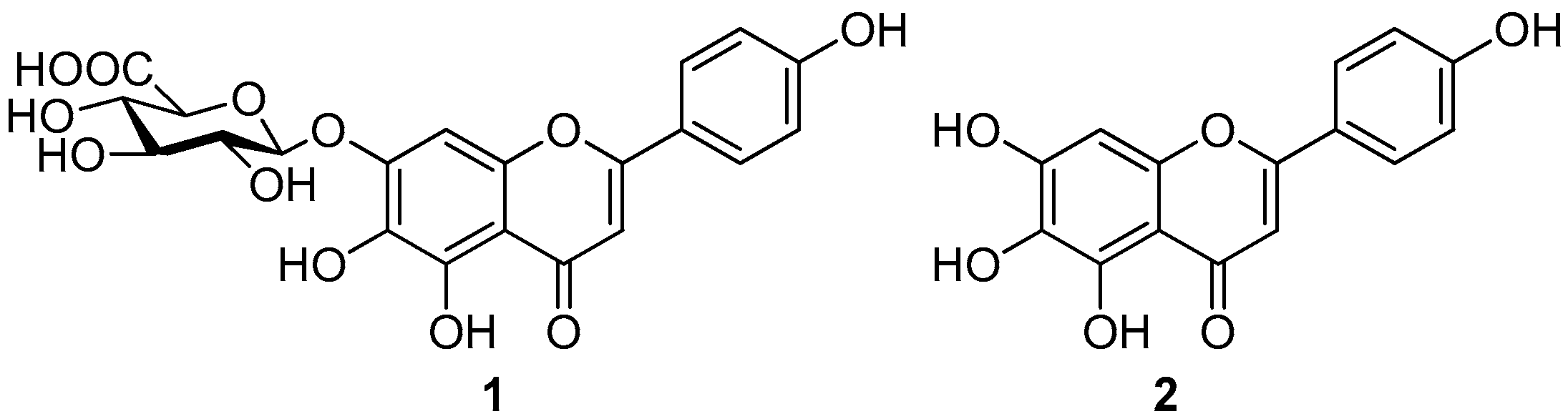
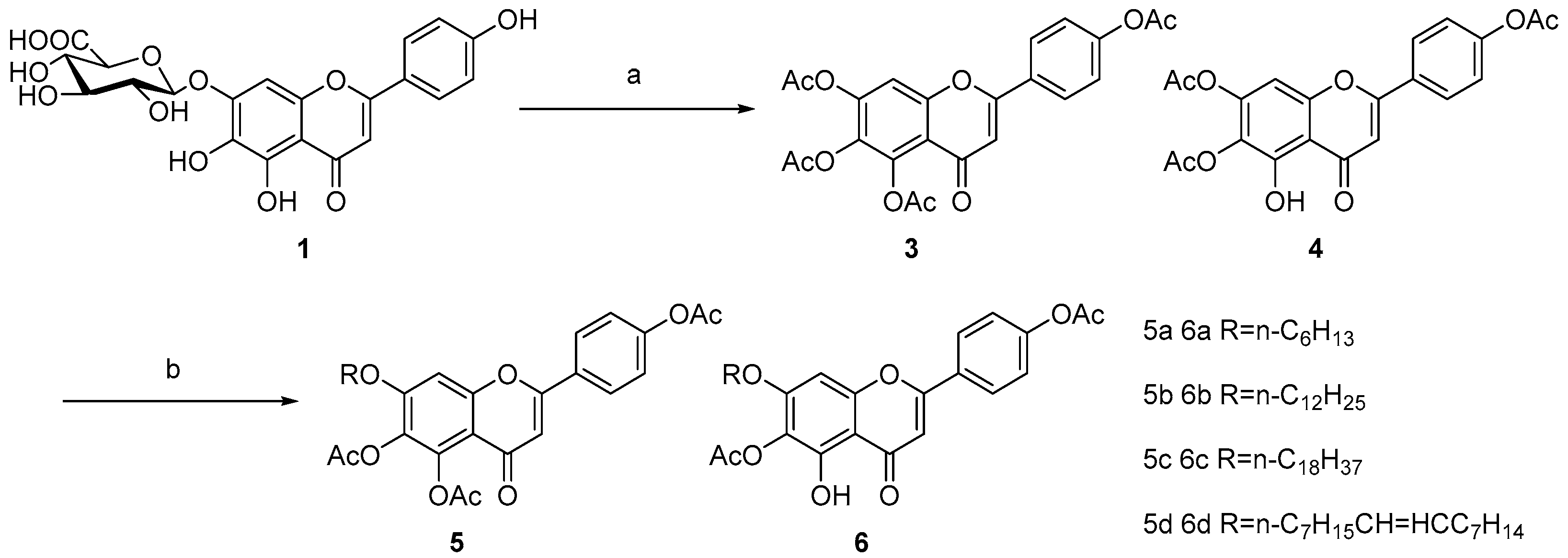
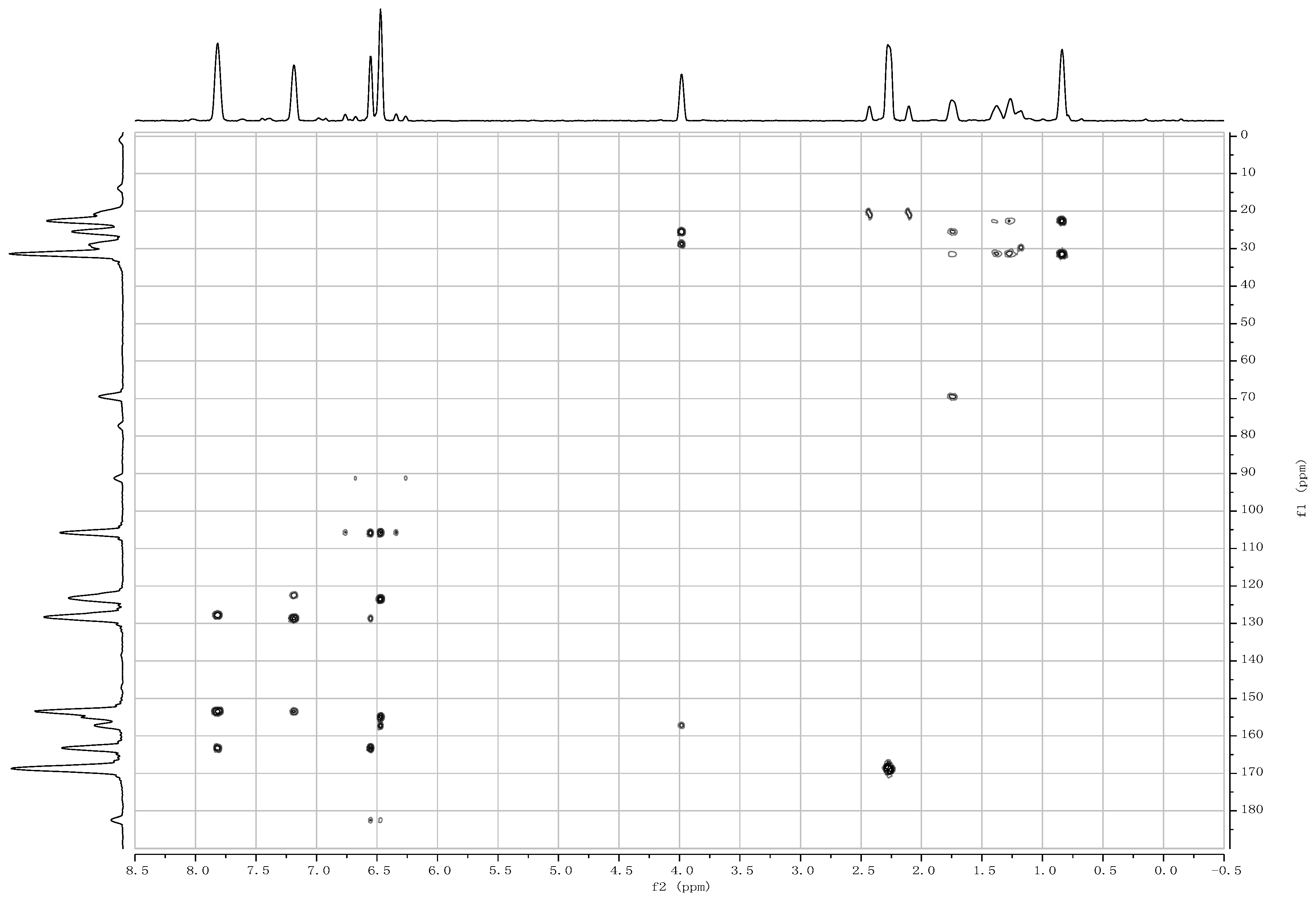
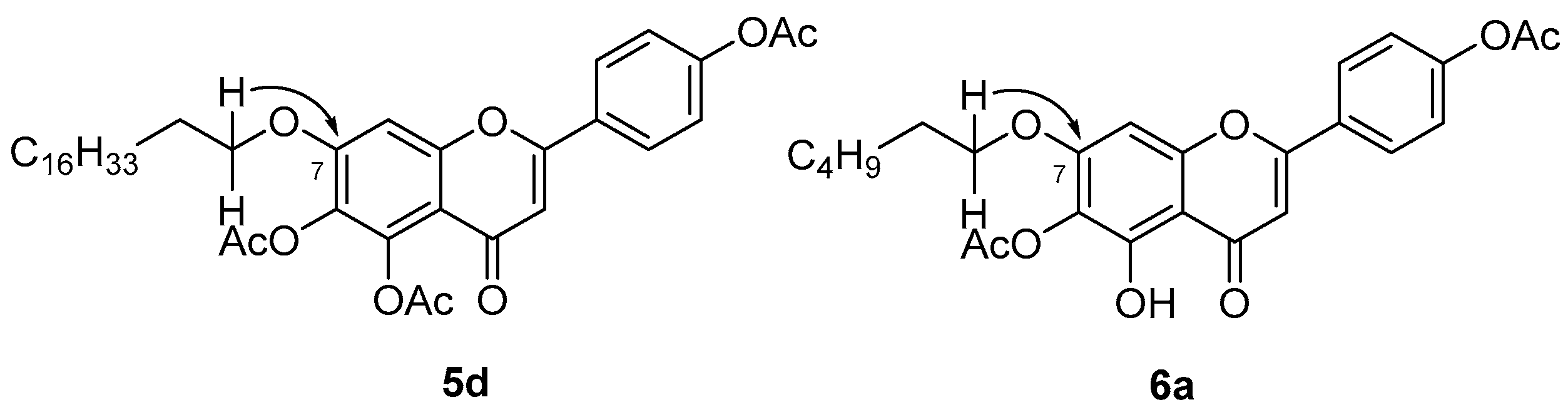
2. Results and Discussion
3. Materials and Methods
Cell Culture and Treatment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hsiao, W.; Liu, L. The role of traditional Chinese herbal medicines in cancer therapy–from TCM theory to mechanistic insights. Planta Med. 2010, 76, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Sucher, N.J. The application of Chinese medicine to novel drug discovery. Expert Opin. Drug Discov. 2013, 8, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Li, P.C.; Konkimalla, V.S.; Kaina, B. From traditional Chinese medicine to rational cancer therapy. Trends Mol. Med. 2007, 13, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, G.; He, H.; Liu, C.; Xiong, X.; Li, J.; Wang, J. Therapeutic Effects of Breviscapine in Cardiovascular Diseases: A Review. Front. Pharmacol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Xia, H.; Zhang, T.; Di, X.; Qu, G.; Yao, X. Two major urinary metabolites of scutellarin in rats. Planta Med. 2007, 73, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.; Tan, B.K.H.; Lee, S.C. Scutellarin Sensitizes Drug-evoked Colon Cancer Cell Apoptosis through Enhanced Caspase-6 Activation. Anticancer Res. 2009, 29, 3043–3047. [Google Scholar] [PubMed]
- Cheng, C.-Y.; Hu, C.C.; Yang, H.J.; Lee, M.C.; Kao, E.S. Inhibitory Effects of Scutellarein on Proliferation of Human Lung Cancer A549 Cells through ERK and NFκB Mediated by the EGFR Pathway. Chin. J. Physiol. 2014, 57, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Huang, D.Y.; Gao, Z.X.Z.; Chen, Y.; Zhang, L.N.; Zheng, J.H. Scutellarin inhibits the growth and invasion of human tongue squamous carcinoma through the inhibition of matrix metalloproteinase-2 and -9 and αvβ6 integrin. Int. J. Oncol. 2013, 42, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Li, J.; Xue, J.; Li, H.; Xu, F.; Cheng, K.; Li, D.; Li, Z.; Gao, M.; Hua, H. Scutellarin derivatives as apoptosis inducers: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2017, 135, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.H.; Li, N.G.; Shi, Q.P.; Zhang, W.; Dong, Z.X.; Tang, Y.P.; Zhang, P.X.; Gu, T.; Wu, W.Y.; Fang, F.; et al. Synthesis of scutellarein derivatives to increase biological activity and water solubility. Bioorg. Med. Chem. 2015, 23, 6875–6884. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.X.; Shi, Z.H.; Li, N.G.; Zhang, W.; Gu, T.; Zhang, P.X.; Wu, W.Y.; Tang, Y.P.; Fang, F.; Xue, X.; et al. Design, Synthesis, and Biological Evaluation of Scutellarein Derivatives Based on Scutellarin Metabolic MechanismIn Vivo. Chem. Biol. Drug Des. 2016, 87, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Li, N.; Tang, Y.; Wang, Z.; Qian, L.; Tang, H.; Duan, J. Design, Synthesis and Biological Evaluation of Scutellarein Derivatives as Potential Anti-Alzheimer’s Disease Candidates Based on Metabolic Mechanism. Lett. Drug Des. Discov. 2012, 9, 78–83. [Google Scholar] [CrossRef]
- Zhanga, W.; Dong, Z.X.; Gua, T.; Li, N.G.; Wu, W.Y.; Zhang, P.X.; Tang, Y.P.; Yang, J.P.; Fanga, F.; Li, H.M.; et al. An improved synthesis of 6-O-methyl-scutellarein through selective benzylation. J. Chem. Res. 2015, 39, 674–676. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, W.; Dong, Z.X.; Gu, T.; Li, N.G.; Shi, Z.H.; Kai, J.; Qu, C.; Shang, G.X.; Tang, Y.P.; et al. A New and Practical Synthetic Method for the Synthesis of 6-O-Methyl-scutellarein: One Metabolite of Scutellarin In Vivo. Int. J. Mol. Sci. 2015, 16, 7587–7594. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.H.; Li, N.G.; Wang, Z.J.; Tang, Y.P.; Dong, Z.X.; Zhang, W.; Zhang, P.X.; Gu, T.; Wu, W.Y.; Yang, J.P.; et al. Synthesis and biological evaluation of methylated scutellarein analogs based on metabolic mechanism of scutellarin in vivo. Eur. J. Med. Chem. 2015, 106, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Mo, T.; Liu, X.; Liu, Y.; Wang, X.; Zhang, L.; Wang, J.; Zhang, Z.; Shi, S.; Tu, P. Expanded investigations of the aglycon promiscuity and catalysis characteristic of flavonol 3-O-rhamnosyltransferase from Arabidopsis thaliana. RSC Adv. 2016, 6, 84616–84626. [Google Scholar] [CrossRef]
- Kong, L.R.; Chua, K.N.; Sim, W.J.; Ng, H.C.; Bi, C.; Ho, J.; Nga, M.E.; Pang, Y.H.; Ong, W.R.; Soo, R.A.; et al. MEK Inhibition Overcomes Cisplatin Resistance Conferred by SOS/MAPK Pathway Activation in Squamous Cell Carcinoma. Mol. Cancer Ther. 2015, 14, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, L.; Huang, Y.; Liu, B.; Chi, H.; Shi, L.; Zhang, W.; Li, G.; Niu, Y.; Zhu, X. Synergistic therapy of chemotherapeutic drugs and MTH1 inhibitors using a pH-sensitive polymeric delivery system for oral squamous cell carcinoma. Biomater. Sci. 2017, 5, 2068–2078. [Google Scholar] [CrossRef] [PubMed]
- Dilda, P.J.; Hogg, P.J. Arsenical-based cancer drugs. Cancer Treat. Rev. 2007, 33, 542–564. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–6 in this article are available from the authors. |

| Compound | IC50 Values (μM) | ||
|---|---|---|---|
| Jurkat | HCT-116 | MDA-MB-231 | |
| NaAsO2 | 4.70 ± 0.44 | 31.20 ± 3.35 | 8.57 ± 1.13 |
| 1 | 156.02 ± 7.21 | >200 | >200 |
| 2 | 95.77 ± 2.56 | >200 | >200 |
| 3 | 181.06 ± 6.21 | >200 | >200 |
| 4 | >200 | >200 | >200 |
| 5a | 14.79 ± 2.69 | 22.02 ± 2.27 | 59.93 ± 4.46 |
| 5b | 22.47 ± 1.70 | 55.56 ± 7.21 | 68.54 ± 9.18 |
| 5c | >200 | >200 | >200 |
| 5d | 76.23 ± 9.85 | 184.06 ± 7.31 | >200 |
| 6a | 1.80 ± 0.49 | 11.50 ± 2.00 | 53.91 ± 4.04 |
| 6b | 19.12 ± 0.70 | 53.53 ± 8.25 | 145.01 ± 10.03 |
| 6c | >200 | >200 | >200 |
| 6d | >200 | >200 | >200 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, G.; Tang, Y.; Li, M.; He, Y.; Rao, G. Synthesis of Scutellarein Derivatives with a Long Aliphatic Chain and Their Biological Evaluation against Human Cancer Cells. Molecules 2018, 23, 310. https://doi.org/10.3390/molecules23020310
Ni G, Tang Y, Li M, He Y, Rao G. Synthesis of Scutellarein Derivatives with a Long Aliphatic Chain and Their Biological Evaluation against Human Cancer Cells. Molecules. 2018; 23(2):310. https://doi.org/10.3390/molecules23020310
Chicago/Turabian StyleNi, Guanghui, Yanling Tang, Minxin Li, Yuefeng He, and Gaoxiong Rao. 2018. "Synthesis of Scutellarein Derivatives with a Long Aliphatic Chain and Their Biological Evaluation against Human Cancer Cells" Molecules 23, no. 2: 310. https://doi.org/10.3390/molecules23020310
APA StyleNi, G., Tang, Y., Li, M., He, Y., & Rao, G. (2018). Synthesis of Scutellarein Derivatives with a Long Aliphatic Chain and Their Biological Evaluation against Human Cancer Cells. Molecules, 23(2), 310. https://doi.org/10.3390/molecules23020310




