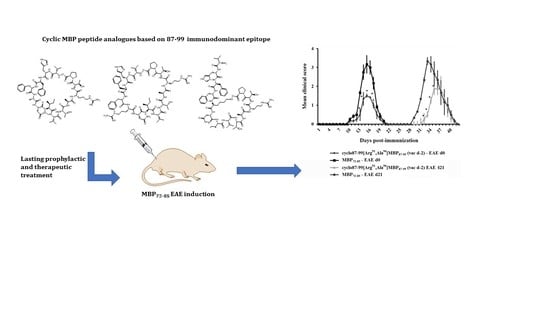
A Cyclic Altered Peptide Analogue Based on Myelin Basic Protein 87–99 Provides Lasting Prophylactic and Therapeutic Protection Against Acute Experimental Autoimmune Encephalomyelitis
Abstract
1. Introduction
2. Results
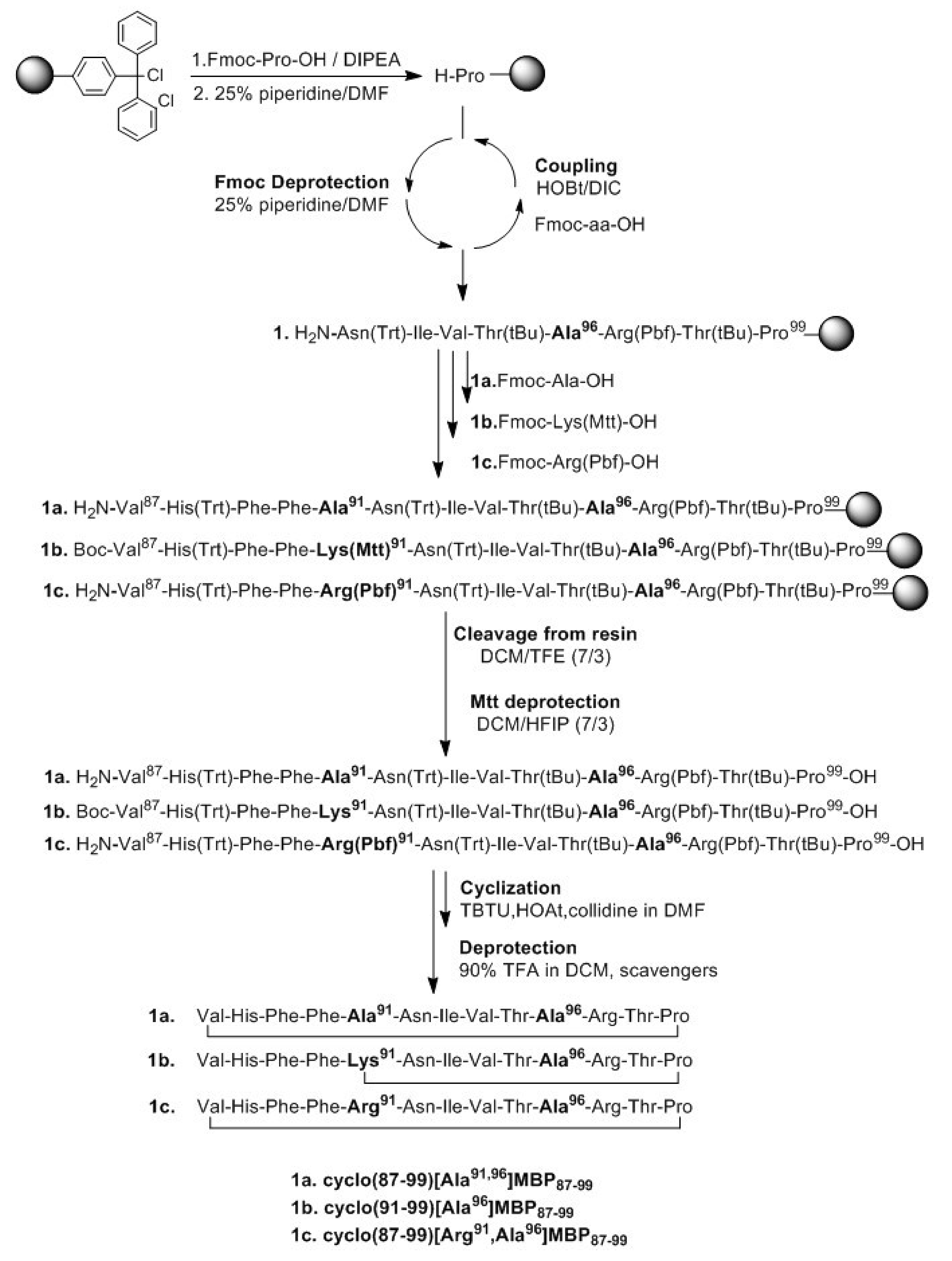
2.1. Solid-Phase Peptide Synthesis and Cyclization of the Mutated Peptide Analogues
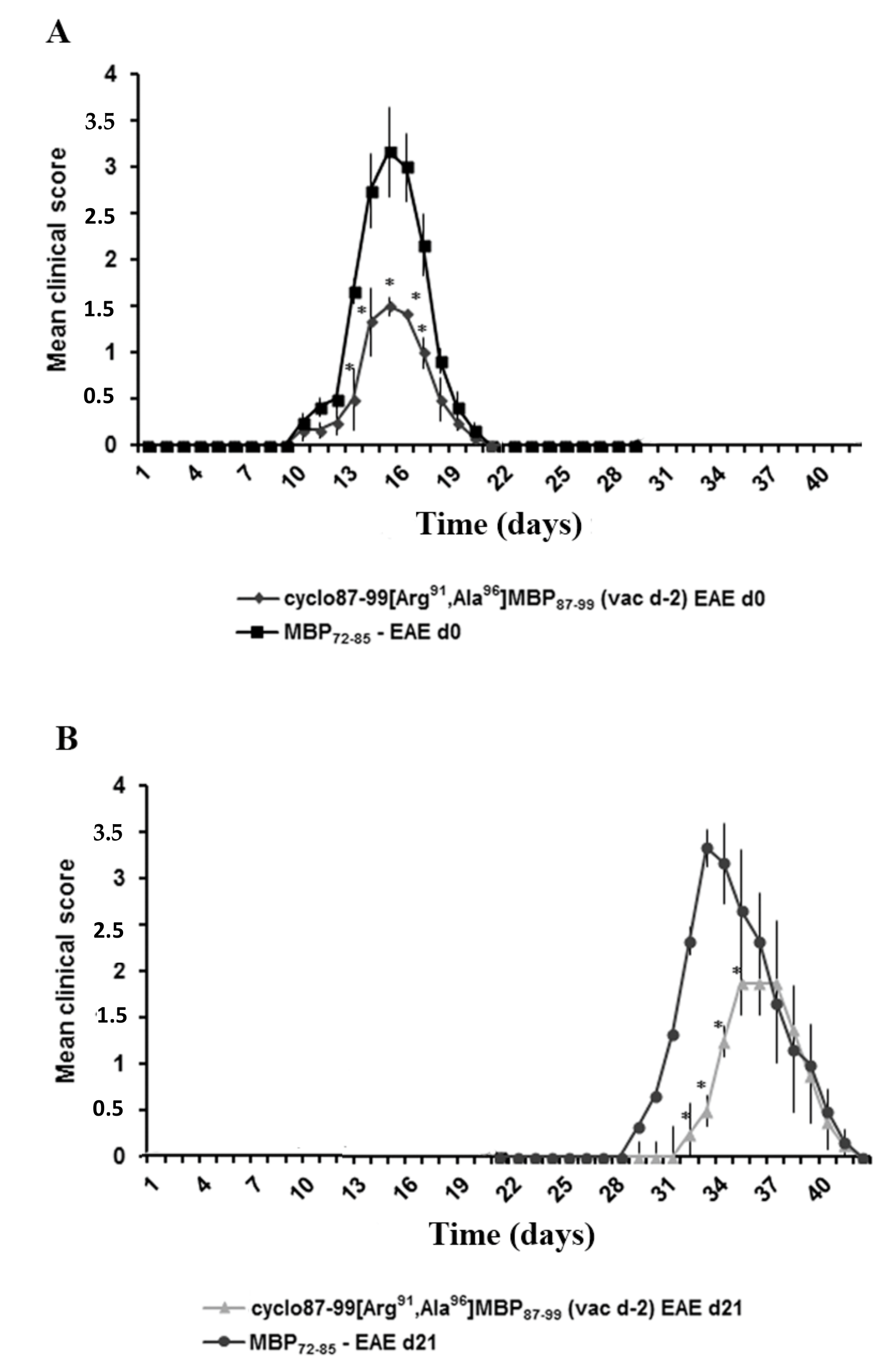
2.2. Prophylactic or Early Therapeutic Delivery of Cyclic Mutated MBP87–99 Analogues Ameliorates MBP72–85-EAE
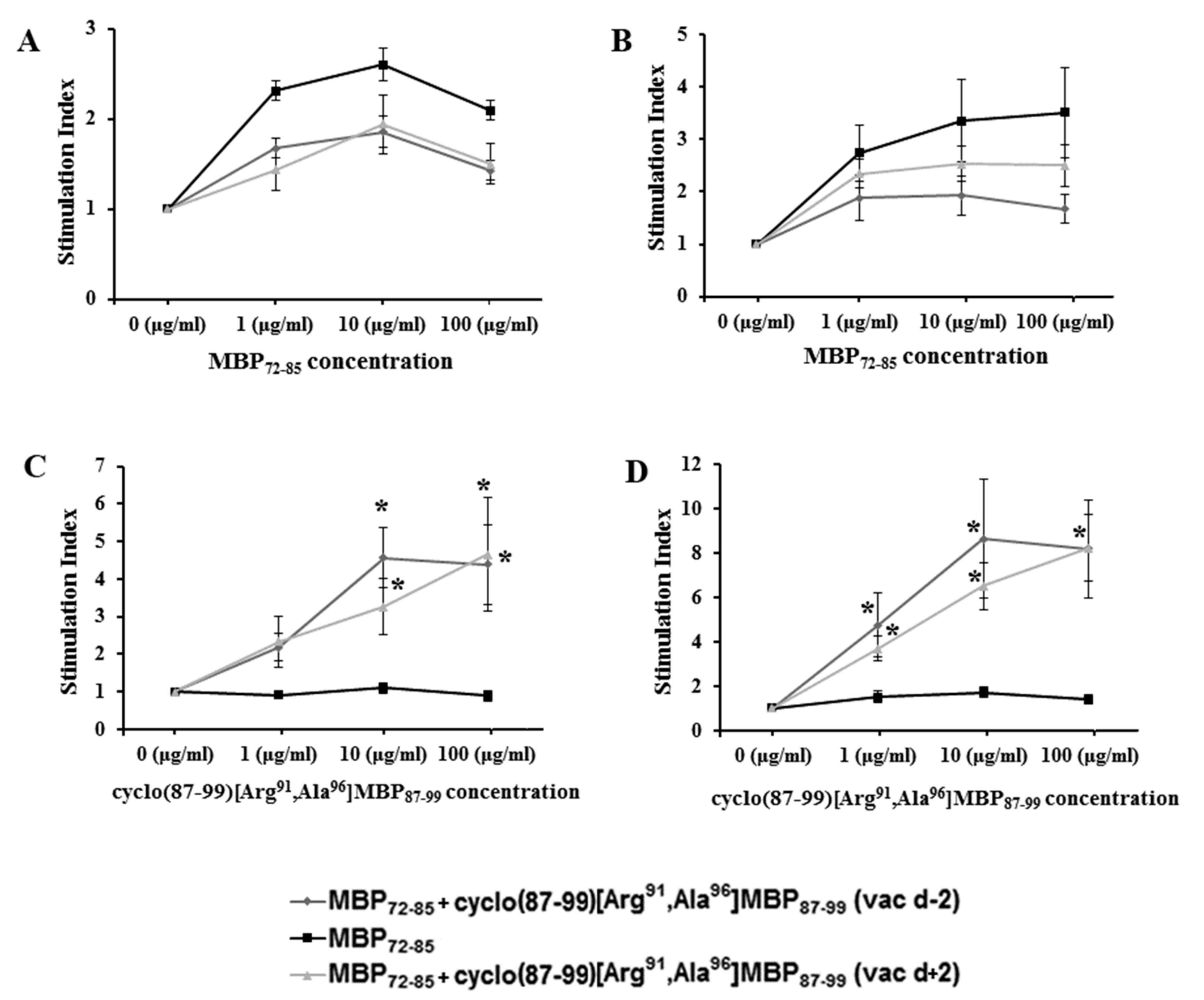
2.3. Cyclic Altered MBP87–99 Analogues Induce T Cell Proliferation Responses
2.4. Prophylactic Treatment with cyclo(87–99)[Arg91, Ala96]MBP87–99 Provides Lasting Protection Against MBP72–85-Induced EAE
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis, Cyclization and Analysis
4.2. EAE Induction Evaluation and Tissue Sampling
4.3. In Vivo Treatment with Cyclic Peptide Analogues
4.4. T Cell Proliferation Assay
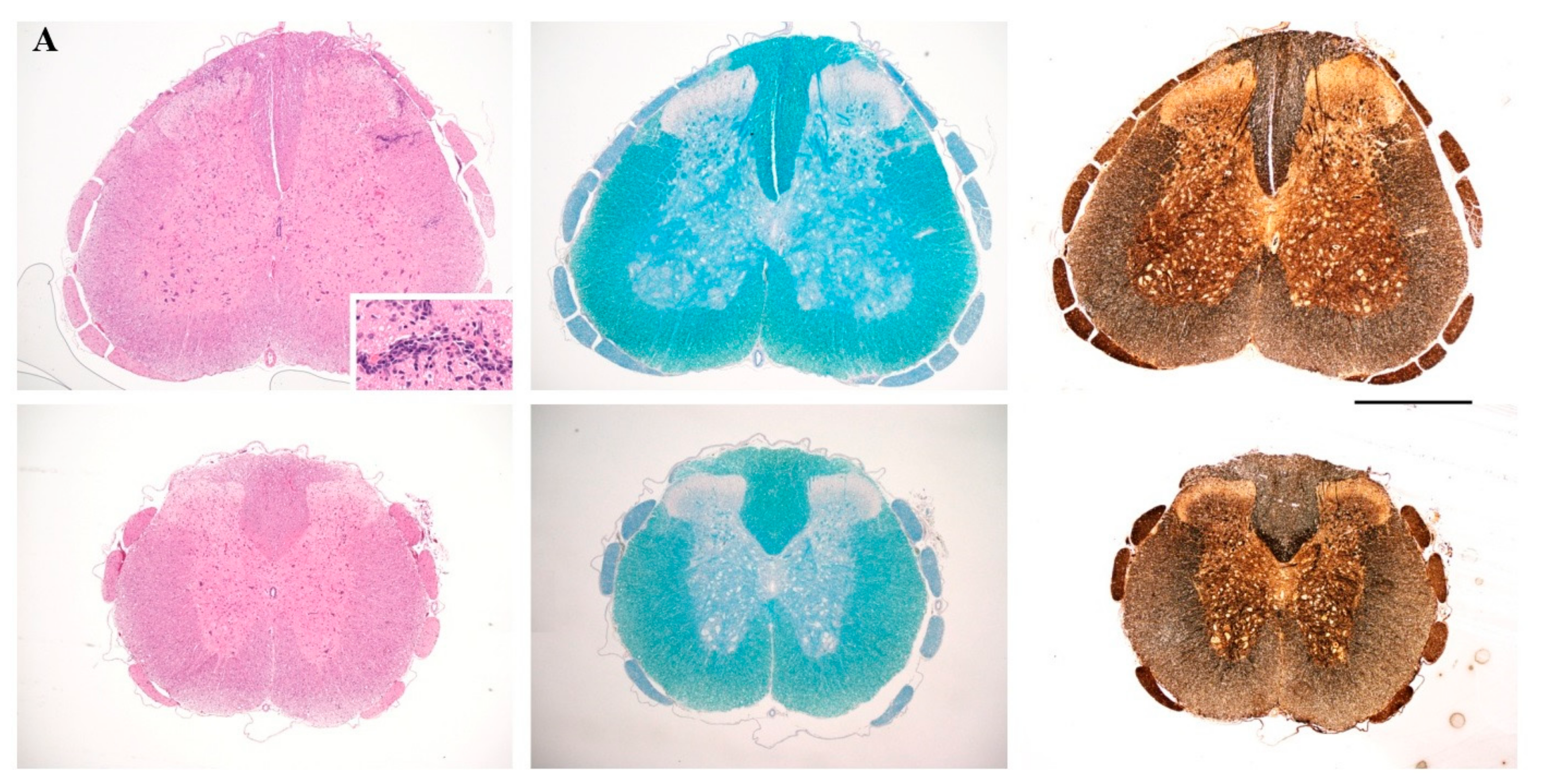
4.5. Histopathology
4.6. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lassmann, H. Mechanisms of white matter damage in multiple sclerosis. Glia 2014, 62, 1816–1830. [Google Scholar] [CrossRef] [PubMed]
- Rangachari, M.; Kuchroo, V.K. Using eae to better understand principles of immune function and autoimmune pathology. J. Autoimmun. 2013, 45, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Diego, R.S.; Weiner, H.L. Novel therapeutic strategies for multiple sclerosis—A multifaceted adversary. Nat. Rev. Drug Discov. 2008, 7, 909–925. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Goods, B.A.; Raddassi, K.; Nepom, G.T.; Kwok, W.W.; Love, J.C.; Hafler, D.A. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci. Transl. Med. 2015, 7, 287ra274. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Jaraquemada, D.; Flerlage, M.; Richert, J.; Whitaker, J.; Long, E.O.; McFarlin, D.E.; McFarland, H.F. Fine specificity and HLA restriction of myelin basic protein-specific cytotoxic T cell lines from multiple sclerosis patients and healthy individuals. J. Immunol. 1990, 145, 540–548. [Google Scholar] [PubMed]
- Pette, M.; Fujita, K.; Kitze, B.; Whitaker, J.N.; Albert, E.; Kappos, L.; Wekerle, H. Myelin basic protein-specific T lymphocyte lines from MS patients and healthy individuals. Neurology 1990, 40, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.; Matsui, M.; Milford, E.L.; Mackin, G.A.; Weiner, H.L.; Hafler, D.A. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature 1990, 346, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Bielekova, B.; Sung, M.H.; Kadom, N.; Simon, R.; McFarland, H.; Martin, R. Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J. Immunol. 2004, 172, 3893–3904. [Google Scholar] [CrossRef] [PubMed]
- Wucherpfennig, K.W.; Catz, I.; Hausmann, S.; Strominger, J.L.; Steinman, L.; Warren, K.G. Recognition of the immunodominant myelin basic protein peptide by autoantibodies and HLA-DR2-restricted T cell clones from multiple sclerosis patients. Identity of key contact residues in the B-cell and T-cell epitopes. J. Clin. Investig. 1997, 100, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Lutterotti, A.; Yousef, S.; Sputtek, A.; Sturner, K.H.; Stellmann, J.P.; Breiden, P.; Reinhardt, S.; Schulze, C.; Bester, M.; Heesen, C.; et al. Antigen-specific tolerance by autologous myelin peptide-coupled cells: A phase 1 trial in multiple sclerosis. Sci. Transl. Med. 2013, 5, 188ra175. [Google Scholar] [CrossRef] [PubMed]
- Vergelli, M.; Hemmer, B.; Utz, U.; Vogt, A.; Kalbus, M.; Tranquill, L.; Conlon, P.; Ling, N.; Steinman, L.; McFarland, H.F.; et al. Differential activation of human autoreactive T cell clones by altered peptide ligands derived from myelin basic protein peptide (87–99). Eur. J. Immunol. 1996, 26, 2624–2634. [Google Scholar] [CrossRef] [PubMed]
- Bielekova, B.; Goodwin, B.; Richert, N.; Cortese, I.; Kondo, T.; Afshar, G.; Gran, B.; Eaton, J.; Antel, J.; Frank, J.A.; et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: Results of a phase II clinical trial with an altered peptide ligand. Nat. Med. 2000, 6, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Comi, G.; Panitch, H.; Oger, J.; Antel, J.; Conlon, P.; Steinman, L. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The altered peptide ligand in relapsing ms study group. Nat. Med. 2000, 6, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Warren, K.G.; Catz, I.; Ferenczi, L.Z.; Krantz, M.J. Intravenous synthetic peptide mbp8298 delayed disease progression in an hla class II-defined cohort of patients with progressive multiple sclerosis: Results of a 24-month double-blind placebo-controlled clinical trial and 5 years of follow-up treatment. Eur. J. Neurol. 2006, 13, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Gaur, A.; Boehme, S.A.; Chalmers, D.; Crowe, P.D.; Pahuja, A.; Ling, N.; Brocke, S.; Steinman, L.; Conlon, P.J. Amelioration of relapsing experimental autoimmune encephalomyelitis with altered myelin basic protein peptides involves different cellular mechanisms. J. Neuroimmunol. 1997, 74, 149–158. [Google Scholar] [CrossRef]
- Karin, N.; Mitchell, D.J.; Brocke, S.; Ling, N.; Steinman, L. Reversal of experimental autoimmune encephalomyelitis by a soluble peptide variant of a myelin basic protein epitope: T cell receptor antagonism and reduction of interferon gamma and tumor necrosis factor alpha production. J. Exp. Med. 1994, 180, 2227–2237. [Google Scholar] [CrossRef] [PubMed]
- De Magistris, M.T.; Alexander, J.; Coggeshall, M.; Altman, A.; Gaeta, F.C.; Grey, H.M.; Sette, A. Antigen analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell 1992, 68, 625–634. [Google Scholar] [CrossRef]
- Sloan-Lancaster, J.; Evavold, B.D.; Allen, P.M. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature 1993, 363, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Evavold, B.D.; Allen, P.M. Separation of IL-4 production from th cell proliferation by an altered T cell receptor ligand. Science 1991, 252, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Brocke, S.; Gijbels, K.; Allegretta, M.; Ferber, I.; Piercy, C.; Blankenstein, T.; Martin, R.; Utz, U.; Karin, N.; Mitchell, D.; et al. Treatment of experimental encephalomyelitis with a peptide analogue of myelin basic protein. Nature 1996, 379, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, L.B.; Kuchroo, V.K. Manipulation of the Th1/Th2 balance in autoimmune disease. Curr. Opin. Immunol. 1996, 8, 837–842. [Google Scholar] [CrossRef]
- Tapeinou, A.; Matsoukas, M.T.; Simal, C.; Tselios, T. Review cyclic peptides on a merry-go-round; towards drug design. Biopolymers 2015, 104, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Tselios, T.; Daliani, I.; Deraos, S.; Thymianou, S.; Matsoukas, E.; Troganis, A.; Gerothanassis, I.; Mouzaki, A.; Mavromoustakos, T.; Probert, L.; et al. Treatment of experimental allergic encephalomyelitis (EAE) by a rationally designed cyclic analogue of myelin basic protein (MBP) epitope 72–85. Bioorg. Med. Chem. Lett. 2000, 10, 2713–2717. [Google Scholar] [CrossRef]
- Tselios, T.; Apostolopoulos, V.; Daliani, I.; Deraos, S.; Grdadolnik, S.; Mavromoustakos, T.; Melachrinou, M.; Thymianou, S.; Probert, L.; Mouzaki, A.; et al. Antagonistic effects of human cyclic mbp(87–99) altered peptide ligands in experimental allergic encephalomyelitis and human T-cell proliferation. J. Med. Chem. 2002, 45, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Matsoukas, J.; Apostolopoulos, V.; Kalbacher, H.; Papini, A.M.; Tselios, T.; Chatzantoni, K.; Biagioli, T.; Lolli, F.; Deraos, S.; Papathanassopoulos, P.; et al. Design and synthesis of a novel potent myelin basic protein epitope 87–99 cyclic analogue: Enhanced stability and biological properties of mimics render them a potentially new class of immunomodulators. J. Med. Chem. 2005, 48, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Deraos, G.; Rodi, M.; Kalbacher, H.; Chatzantoni, K.; Karagiannis, F.; Synodinos, L.; Plotas, P.; Papalois, A.; Dimisianos, N.; Papathanasopoulos, P.; et al. Properties of myelin altered peptide ligand cyclo(87–99)(Ala91,Ala96)MBP87–99 render it a promising drug lead for immunotherapy of multiple sclerosis. Eur. J. Med. Chem. 2015, 101, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Katsara, M.; Deraos, G.; Tselios, T.; Matsoukas, M.T.; Friligou, I.; Matsoukas, J.; Apostolopoulos, V. Design and synthesis of a cyclic double mutant peptide (cyclo(87–99)[A91,A96]MBP87–99) induces altered responses in mice after conjugation to mannan: Implications in the immunotherapy of multiple sclerosis. J. Med. Chem. 2009, 52, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Katsara, M.; Deraos, G.; Tselios, T.; Matsoukas, J.; Apostolopoulos, V. Design of novel cyclic altered peptide ligands of myelin basic protein MBP83–99 that modulate immune responses in SJL/J mice. J. Med. Chem. 2008, 51, 3971–3978. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, E.D.; Platts, J.A.; Brancale, A.; Mavromoustakos, T.M.; Tselios, T.V. Molecular dynamics at the receptor level of immunodominant myelin basic protein epitope 87–99 implicated in multiple sclerosis and its antagonists altered peptide ligands: Triggering of immune response. J. Mol. Graph. Model. 2007, 26, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, E.D.; Mavromoustakos, T.M.; Platts, J.A.; Matsoukas, J.M.; Tselios, T.V. Structural requirements for binding of myelin basic protein (MBP) peptides to MHC II: Effects on immune regulation. Curr. Med. Chem. 2005, 12, 1521–1535. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations (*). Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef] [PubMed]
- Cua, D.J.; Sherlock, J.; Chen, Y.; Murphy, C.A.; Joyce, B.; Seymour, B.; Lucian, L.; To, W.; Kwan, S.; Churakova, T.; et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003, 421, 744–748. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A.; Arai, N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 2000, 10, 542–550. [Google Scholar] [CrossRef]
- Lee, Y.; Awasthi, A.; Yosef, N.; Quintana, F.J.; Xiao, S.; Peters, A.; Wu, C.; Kleinewietfeld, M.; Kunder, S.; Hafler, D.A.; et al. Induction and molecular signature of pathogenic Th17 cells. Nat. Immunol. 2012, 13, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, J.; Wegner, A.; Wraith, D.C. Extra-thymically induced T regulatory cell subsets: The optimal target for antigen-specific immunotherapy. Immunology 2015, 145, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Friligou, I.; Papadimitriou, E.; Gatos, D.; Matsoukas, J.; Tselios, T. Microwave-assisted solid-phase peptide synthesis of the 60-110 domain of human pleiotrophin on 2-chlorotrityl resin. Amino acids 2011, 40, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Friligou, I.; Rizzolo, F.; Nuti, F.; Tselios, T.; Evangelidou, M.; Emmanouil, M.; Karamita, M.; Matsoukas, J.; Chelli, M.; Rovero, P.; et al. Divergent and convergent synthesis of polymannosylated dibranched antigenic peptide of the immunodominant epitope MBP(83–99). Bioorg. Med. Chem. 2013, 21, 6718–6725. [Google Scholar] [CrossRef] [PubMed]
- Ieronymaki, M.; Androutsou, M.E.; Pantelia, A.; Friligou, I.; Crisp, M.; High, K.; Penkman, K.; Gatos, D.; Tselios, T. Use of the 2-chlorotrityl chloride resin for microwave-assisted solid phase peptide synthesis. Biopolymers 2015, 104, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Nicolussi, E.M.; Huck, S.; Lassmann, H.; Bradl, M. The cholinergic anti-inflammatory system limits T cell infiltration into the neurodegenerative CNS, but cannot counteract complex CNS inflammation. Neurobiol. Dis. 2009, 35, 24–31. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all the synthesized peptides described in this manuscript are freely available from the authors. |


| Rats | Incidence | Mean Day of Onset | Mean Maximal Score |
|---|---|---|---|
| Expt1 | |||
| Control | 3/3 (100%) | 11.00 ± 1.08 | 2.60 ± 0.27 |
| Prophylactic vac d − 2 | 1/3 (33.3%) | 15.00 | 1.00 ± 0.27 |
| Therapeutic vac d + 2 | 1/3 (33.3%) | 12.00 | 1.00 ± 0.27 |
| Expt2 | |||
| Control | 6/6 (100%) | 8.16 ± 0.15 | 3.00 ± 0.35 |
| Prophylactic vac d − 2 | 4/6 (66.6%) | 9.25 ± 0.45 | 0.41 ± 0.14 |
| Therapeutic vac d + 2 | 5/6 (83.4%) | 10.40 ± 1.25 | 1.25 ± 0.32 |
| Expt3 | |||
| Control | 6/6 (100%) | 11.30 ± 0.45 | 2.60 ± 0.34 |
| Prophylactic vac d − 2 | 5/6 (83.4%) | 12.00 ± 0.32 | 1.58 ± 0.41 |
| Expt4 | |||
| Control | 4/4 (100%) | 9.30 ± 0.27 | 3.33 ± 0.13 |
| Prophylactic vac d − 23 | 4/4 (100%) | 12.75 ± 0.41 | 1.87 ± 0.44 |
| a/a | Peptide Sequence | Short Name |
|---|---|---|
| 1 | L72PQKSQRSQDENPV85 (guinea pig) | MBP72–85 |
| 2 | V87HFFKNIVTPRTP99 | MBP87–99 |
| 3 | V87HFFA91NIVTA96RTP99 | cyclo(87–99)[Ala91,96]MBP87–99 |
| 4 | V87HFFKNIVTA96RTP99 | cyclo(91–99)[Ala96]MBP87–99 |
| 5 | V87HFFR91NIVTA96RTP99 | cyclo(87–99)[Arg91, Ala96]MBP87–99 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emmanouil, M.; Tseveleki, V.; Triantafyllakou, I.; Nteli, A.; Tselios, T.; Probert, L. A Cyclic Altered Peptide Analogue Based on Myelin Basic Protein 87–99 Provides Lasting Prophylactic and Therapeutic Protection Against Acute Experimental Autoimmune Encephalomyelitis. Molecules 2018, 23, 304. https://doi.org/10.3390/molecules23020304
Emmanouil M, Tseveleki V, Triantafyllakou I, Nteli A, Tselios T, Probert L. A Cyclic Altered Peptide Analogue Based on Myelin Basic Protein 87–99 Provides Lasting Prophylactic and Therapeutic Protection Against Acute Experimental Autoimmune Encephalomyelitis. Molecules. 2018; 23(2):304. https://doi.org/10.3390/molecules23020304
Chicago/Turabian StyleEmmanouil, Mary, Vivian Tseveleki, Iro Triantafyllakou, Agathi Nteli, Theodore Tselios, and Lesley Probert. 2018. "A Cyclic Altered Peptide Analogue Based on Myelin Basic Protein 87–99 Provides Lasting Prophylactic and Therapeutic Protection Against Acute Experimental Autoimmune Encephalomyelitis" Molecules 23, no. 2: 304. https://doi.org/10.3390/molecules23020304
APA StyleEmmanouil, M., Tseveleki, V., Triantafyllakou, I., Nteli, A., Tselios, T., & Probert, L. (2018). A Cyclic Altered Peptide Analogue Based on Myelin Basic Protein 87–99 Provides Lasting Prophylactic and Therapeutic Protection Against Acute Experimental Autoimmune Encephalomyelitis. Molecules, 23(2), 304. https://doi.org/10.3390/molecules23020304