Efficient OLEDs Fabricated by Solution Process Based on Carbazole and Thienopyrrolediones Derivatives
Abstract
1. Introduction
2. Results and Discussions
2.1. Synthesis
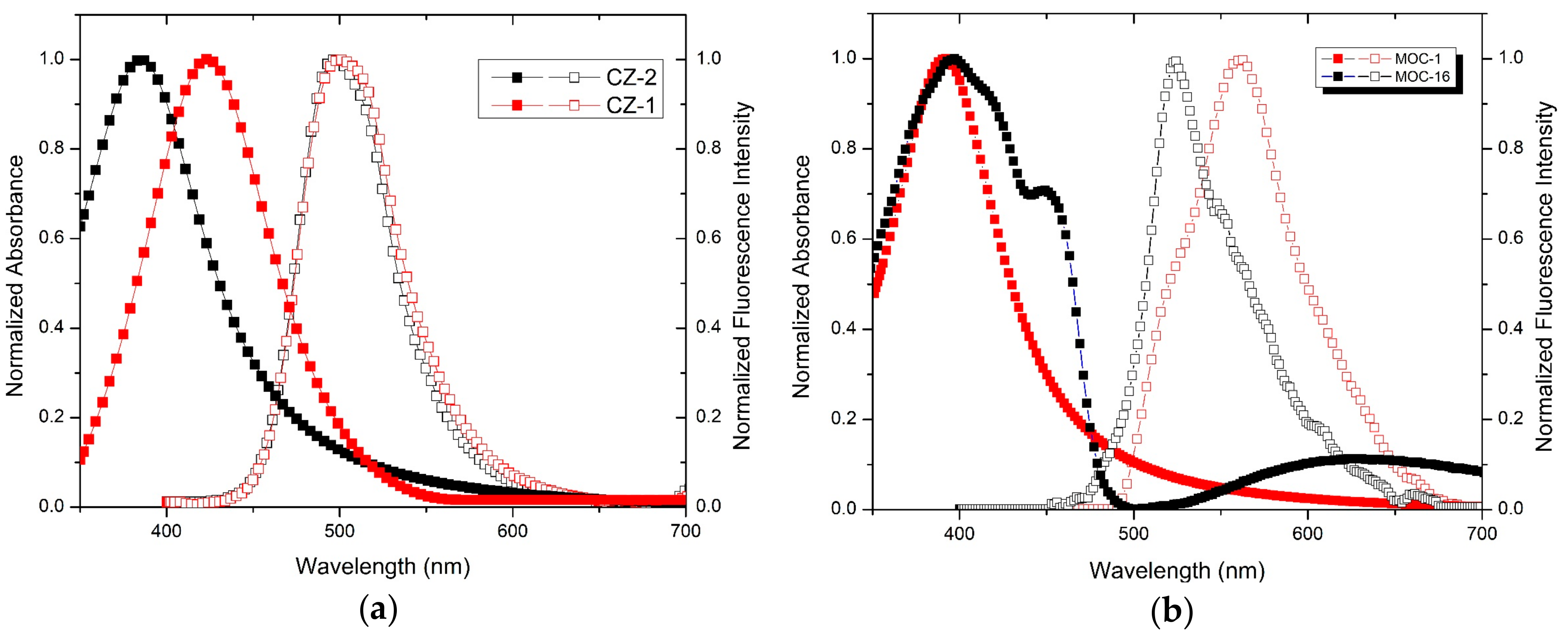
2.2. Theoretical Calculation
2.3. Electrochemical Analysis
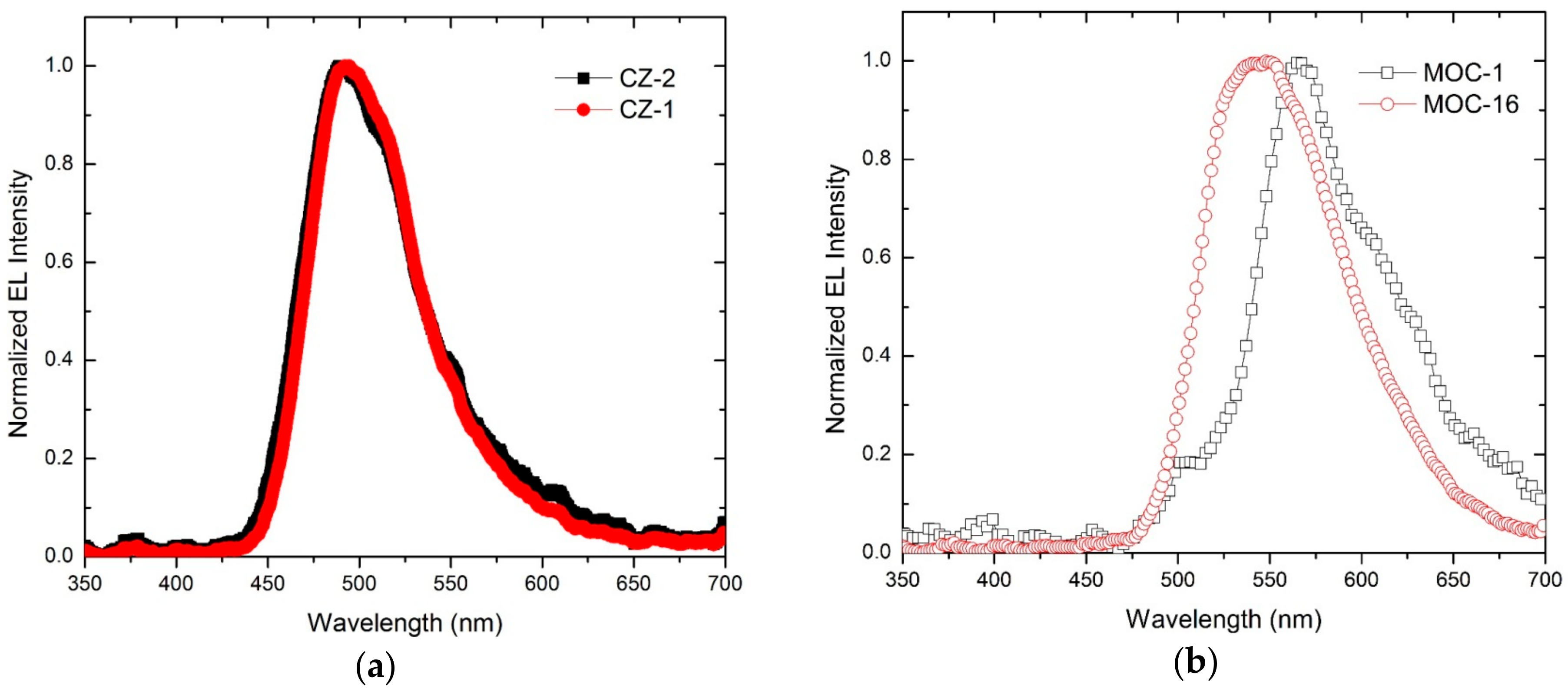
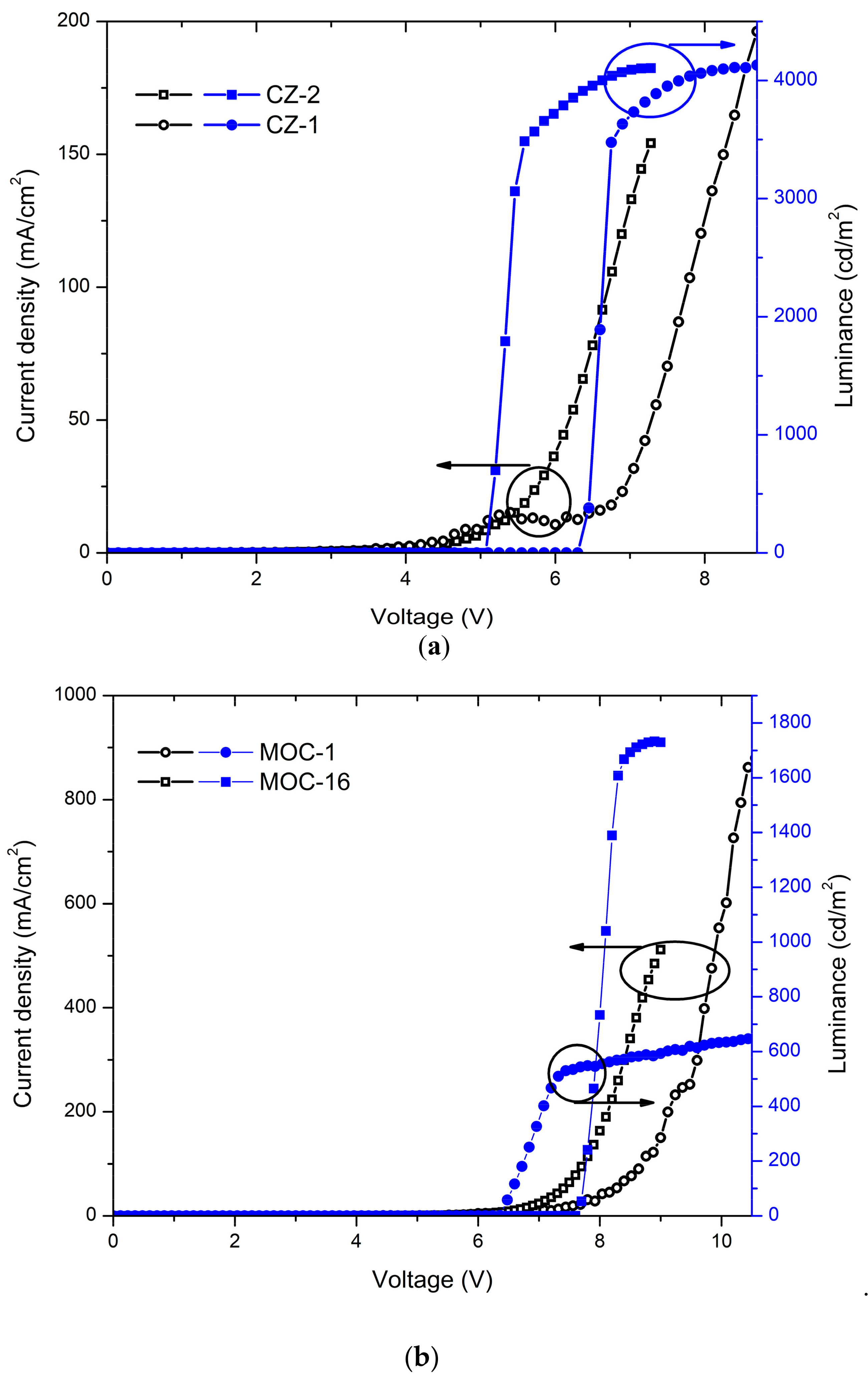
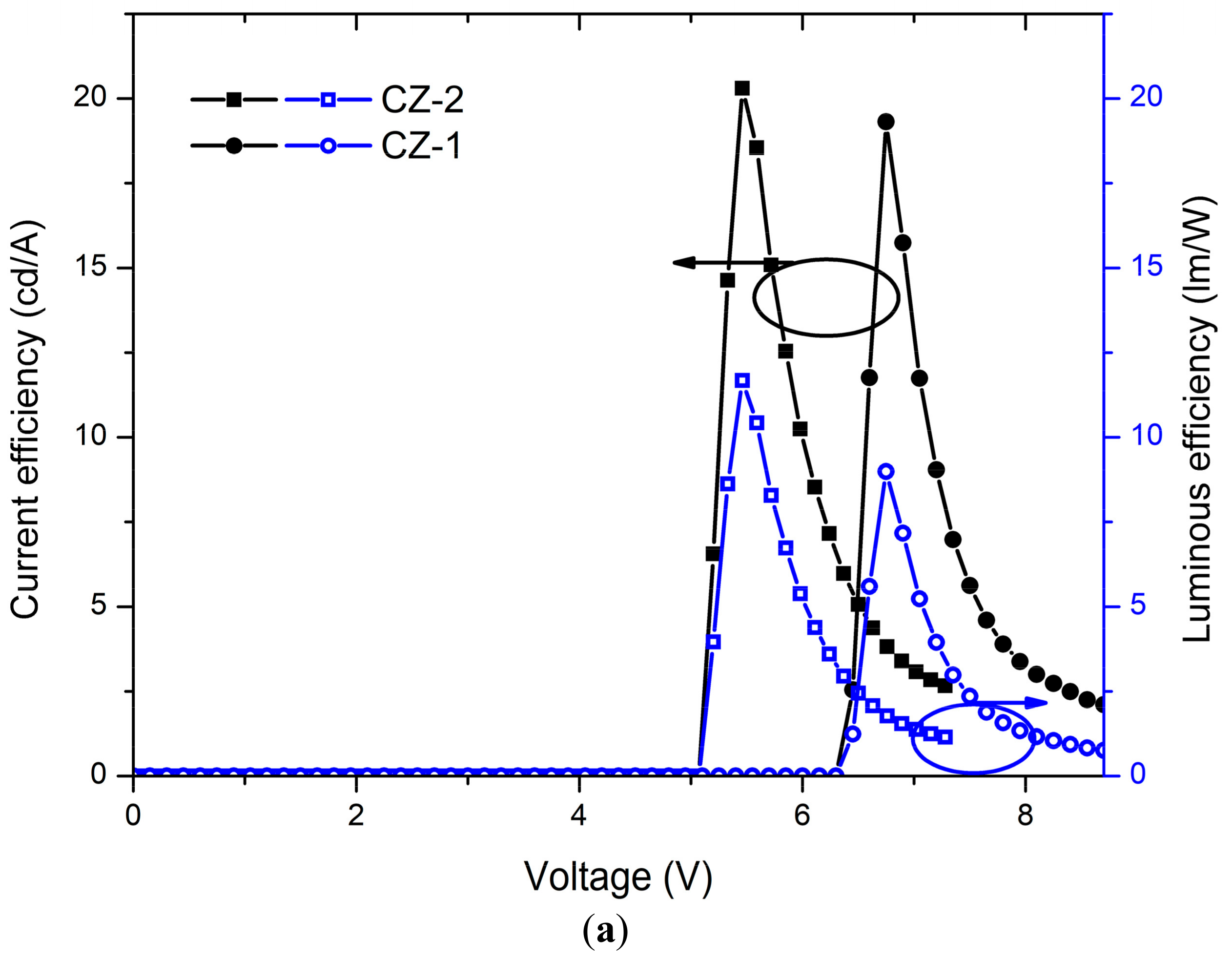
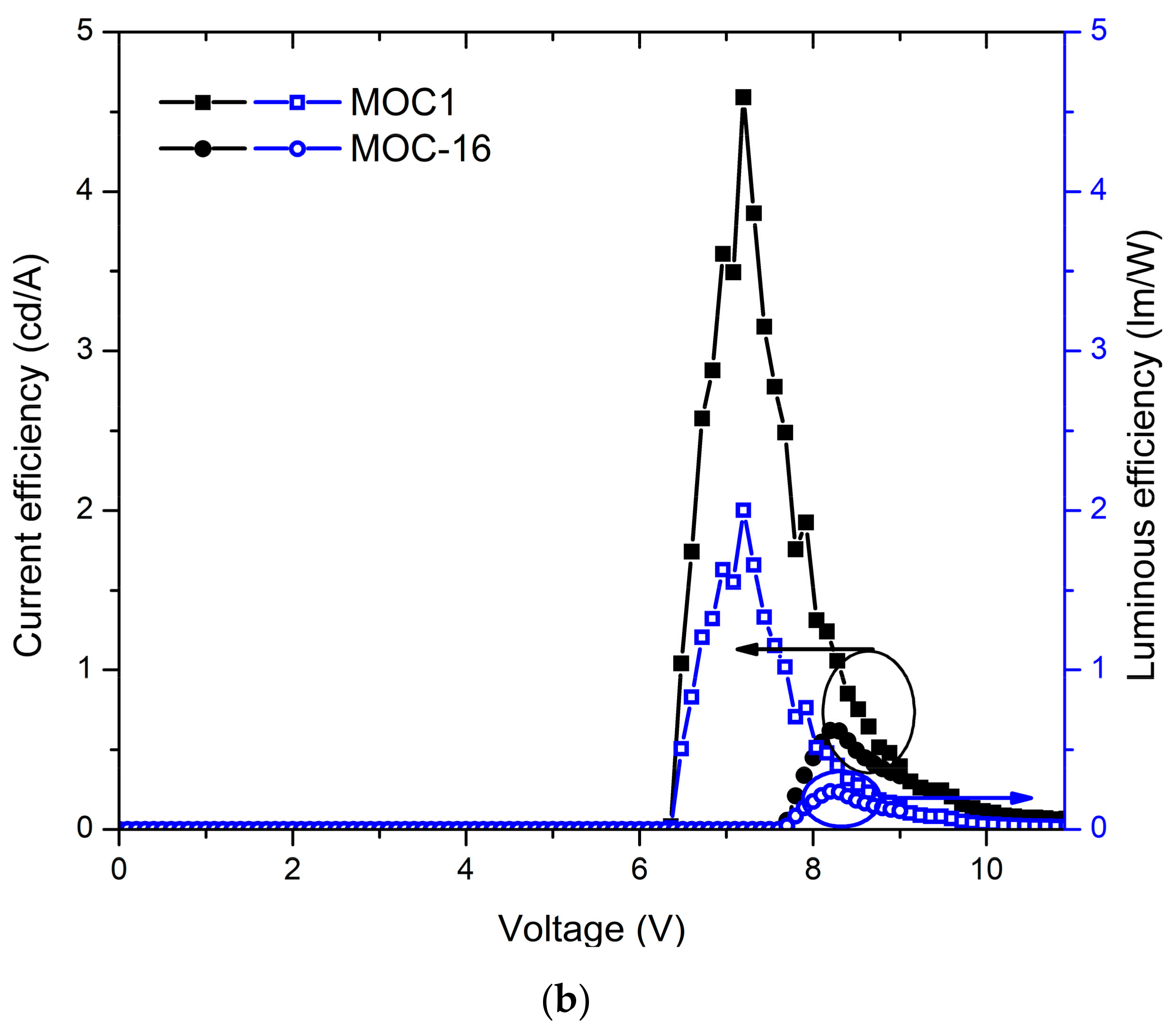
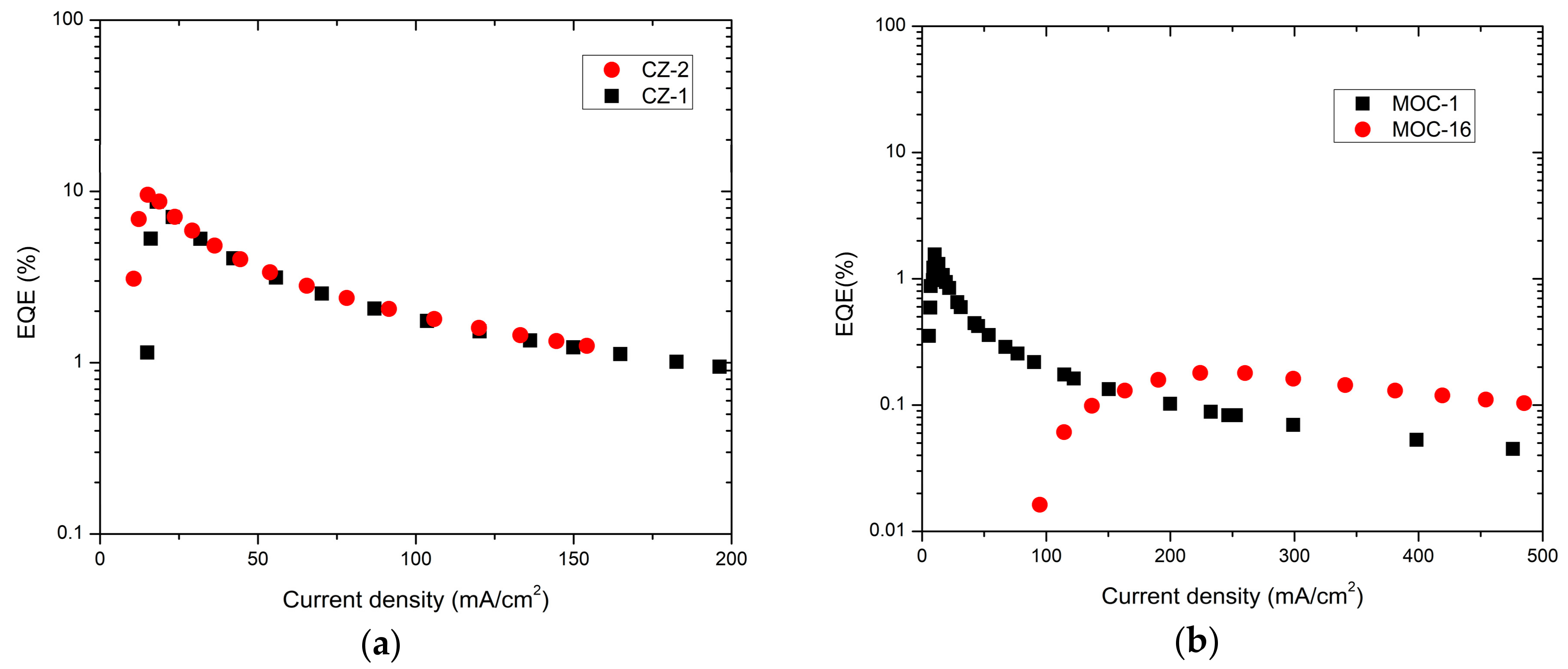
2.4. OLED Devices
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Materials
3.2.1. General Method for Knoevenagel Reaction
3.2.2. Suzuki Reaction
3.3. Electrochemical Analysis
3.4. Fabrication of the OLED Devices
3.5. OLEDs Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, C.W.; Van Slyke, S.A. Organic electroluminiscent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, H.; Li, X.; Liu, M.; Ye, H.; Su, S.-J.; Cao, Y. Solution-processed cathode-interlayer-free deep blue organic light-emitting diodes. Org. Electron. 2014, 15, 1197–1204. [Google Scholar] [CrossRef]
- Thejo Kalyani, N.; Dhoble, S. Novel materials for fabrication and encapsulation of OLEDs. Renew. Sustain. Energy Rev. 2015, 44, 319–347. [Google Scholar] [CrossRef]
- Niu, F.; Niu, H.; Liu, Y.; Lian, J.; Zeng, P. Synthesis, characterization and application of starburst 9-alkyl-1,3,6,8-tetraaryl-carbazole derivatives for blue-violet to UV OLEDs. RSC Adv. 2011, 1, 415–423. [Google Scholar] [CrossRef]
- Duan, L.; Hou, L.; Lee, T.W.; Qiao, J.; Zhang, D.; Dong, G.; Wang, L.; Qiu, Y. Solution processable small molecules for organic light-emitting diodes. J. Mater. Chem. 2010, 20, 6392–6407. [Google Scholar] [CrossRef]
- Jun, C.H.; Ohisa, S.; Pu, Y.J.; Chiba, T.; Kido, J. Comparison of spin and blade coating methods in solution-process for organic light-emitting devices. J. Photopolym. Sci. Technol. 2015, 28, 343–347. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Z.; Jin, Y.; Niu, Y.; Cao, H.; Liang, X.; Liwei, C.; Jianpu, W.; Peng, X. Solution-processed, high performance light-emitting diodes based on quantum dots. Nature 2014, 515, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Ye Ram, C.; Cho, Y.R.; Kim, H.S.; Yu, Y.J.; Suh, M.C. Highly efficient organic light emitting diodes formed by solution processed red emitters with evaporated blue common layer structure. Sci. Rep. 2015, 5, 1–8. [Google Scholar]
- Aizawa, N.; Pu, Y.-J.; Watanabe, M.; Chiba, T.; Ideta, K.; Toyota, N.; Igarashi, M.; Suzuri, Y.; Sasabe, H.; Kido, J. Solution-processed multilayer small-molecule light-emitting devices with high-efficiency white-light emission. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, X.; Wu, K.; Ding, J.; Zhang, B.; Xie, Z.; Wang, L. Efficient solution-processed blue phosphorescent organic light-emitting diodes with halogen-free solvent to optimize the emissive layer morphology. Org. Electron. 2014, 15, 1401–1406. [Google Scholar] [CrossRef]
- Lee, C.W.; Kim, O.Y.; Lee, J.Y. Organic materials for organic electronic devices. J. Ind. Eng. Chem. 2014, 20, 1198–1208. [Google Scholar] [CrossRef]
- Höfle, S.; Pfaff, M.; Do, H.; Bernhard, C.; Gerthsen, D.; Lemmer, U.; Colsmann, A. Suppressing molecular aggregation in solution processed small molecule organic light emitting diodes. Org. Electron. 2014, 15, 337–341. [Google Scholar] [CrossRef]
- Albrecht, K.; Matsuoka, K.; Fujita, K.; Yamamoto, K. Carbazole dendrimers as solution-processable thermally activated delayed-fluorescence materials. Angew. Chem. Int. Ed. 2015, 54, 5677–5682. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.-W.; Lee, K.-M.; Kim, O.Y.; Lee, J.Y.; Hwang, S.-H. Synthesis of a dibenzothiophene/carboline/carbazole hybrid bipolar host material for green phosphorescent OLEDs. Synth. Met. 2016, 213, 7–11. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, K.; Wu, H.; Chen, K.; Xie, G.; Hu, J.; Yan, G.; Ma, S.; Su, S.-J.; Cao, Y. Novel molecular host materials based on carbazole/PO hybrids with wide bandgap via unique linkages for solution-processed blue phosphorescent OLEDs. Opt. Mater. 2016, 60, 244–251. [Google Scholar] [CrossRef]
- Hong, M.; Ravva, M.K.; Winget, P.; Brédas, J.L. Effect of substituents on the electronic structure and degradation process in carbazole derivatives for blue OLED host materials. Chem. Mater. 2016, 28, 5791–5798. [Google Scholar] [CrossRef]
- Aizawa, N.; Pu, Y.J.; Sasabe, H.; Kido, J. Solution-processable carbazole-based host materials for phosphorescent organic light-emitting devices. Org. Electron. 2012, 13, 2235–2242. [Google Scholar] [CrossRef]
- He, J.; Liu, H.; Dai, Y.; Ou, X.; Wang, J.; Tao, S.; Zhang, X.; Wang, P.; Ma, D. Nonconjugated carbazoles: A series of novel host materials for highly efficient blue electrophosphorescent OLEDs. J. Phys. Chem. C 2009, 113, 6761–6767. [Google Scholar] [CrossRef]
- Jiang, W.; Ge, Z.; Cai, P.; Huang, B.; Dai, Y.; Sun, Y.; Qiao, J.; Wang, L.; Duan, L.; Qiu, Y. Star-shaped dendritic hosts based on carbazole moieties for highly efficient blue phosphorescent OLEDs. J. Mater. Chem. 2012, 22, 12016–12022. [Google Scholar] [CrossRef]
- Rehmann, N.; Hertel, D.; Meerholz, K.; Becker, H.; Susanne, H. Highly efficient solution-processed phosphorescent multilayer organic light emitting diodes based on small-molecules hosts. Appl. Phys. Lett. 2007, 91, 103507. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Yao, B.; Zhang, B.; Ding, J.; Xie, Z.; Wang, L. Solution-processible 2,2′-dimethyl-biphenyl cored carbazole dendrimers as universal host for efficient blue, green, and red phosphorescent OLEDs. Adv. Funct. Mater. 2014, 24, 3413–3421. [Google Scholar] [CrossRef]
- Yang, W.; Chen, Y.; Jiang, W.; Ban, X.; Huang, B.; Dai, Y.; Sun, Y. A carbazole-based denditric host material for efficient solution-processed blue phosphorescent OLEDs. Dyes Pigment 2013, 97, 286–290. [Google Scholar] [CrossRef]
- Yook, K.S.; Lee, J.Y. Small molecule host materials for solution processed phosphorescent organic light-emitting diodes. Adv. Mater. 2014, 26, 4218–4233. [Google Scholar] [CrossRef] [PubMed]
- Konidena, R.; Justin, K.; Sahoo, S.; Dubey, D.; Jou, J.H. Multi-substituted deep-blue emitting carbazoles: A comparative study on photophysical and electroluminescence characteristics. J. Mater. Chem. C 2017, 5, 709–726. [Google Scholar] [CrossRef]
- Agarwal, N.; Nayak, P.K.; Ali, F.; Patankar, M.P.; Narasimhan, K.L.; Periasamy, N. Tuning of HOMO levels of carbazole derivatives: New molecules for blue OLED. Synth. Met. 2011, 161, 466–473. [Google Scholar] [CrossRef]
- Justin Thomas, K.; Lin, J.T.; Tao, Y.T.; Ko, C.W. Light-emitting carbazole derivatives: Potential electroluminescent materials. J. Am. Chem. Soc. 2001, 123, 9404–9411. [Google Scholar] [CrossRef]
- Ding, J.; Gao, J.; Cheng, Z.; Xie, Y.; Wang, L.; Ma, D.; Jing, X.; Wang, F. Highly efficient green-emitting phosphorescent iridium dendrimers based on carbazole dendrons. Adv. Funct. Mater. 2006, 16, 575–581. [Google Scholar] [CrossRef]
- Adhikari, R.M.; Mondal, R.; Shah, B.K.; Neckers, D.C. Synthesis and photophysical properties of carbazole-based blue light-emitting dendrimers. J. Org. Chem. 2007, 72, 4727–4732. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Woo, H.S.; Kim, T.W.; Park, J.W. Blue organic light-emitting diodes with carbazole-based small molecules. Opt. Mater. 2002, 21, 225–229. [Google Scholar] [CrossRef]
- Sasabe, H.; Kido, J. Multifunctional materials in high-performance OLEDs: Challenges for solid-state lighting. Chem. Mater. 2011, 23, 621–630. [Google Scholar] [CrossRef]
- Shen, J.Y.; Yang, X.L.; Huang, T.H.; Lin, J.T.; Ke, T.H.; Chen, L.Y.; Wu, C.C.; Yeh, M.C. Ambipolar conductive 2,7-carbazole derivatives for electroluminescent devices. Adv. Funct. Mater. 2007, 17, 983–995. [Google Scholar] [CrossRef]
- Kotchapradist, P.; Prachumrak, N.; Tarsang, R.; Jungsuttiwong, S.; Keawin, T.; Sudyoadsuk, T.; Promarak, V. Pyrene-functionalized carbazole derivatives as non-doped blue emitters for highly efficient blue organic light-emitting diodes. J. Mater. Chem. C 2013, 1, 4916–4924. [Google Scholar] [CrossRef]
- Huang, B.; Yin, Z.; Ban, X.; Ma, Z.; Jiang, W.; Tian, W.; Yang, M.; Ye, S.; Lin, B.; Sun, Y. Nondoped deep blue OLEDs based on Bis-(-4-benzenesulfonyl-phenyl)-9-phenyl-9H-carbazoles. J. Lumin. 2016, 172, 7–13. [Google Scholar] [CrossRef]
- Wang, K.; Liu, W.; Zheng, C.J.; Shi, Y.Z.; Liang, K.; Zhang, M.; Ou, X.M.; Zhang, X.H. A comparative study of carbazole-based thermally activated delayed fluorescence emitters with different steric hindrance. J. Mater. Chem. C 2017, 5, 4797–4803. [Google Scholar] [CrossRef]
- Luo, J.; Gong, S.; Gu, Y.; Chen, T.; Li, Y.; Zhong, C.; Xie, G.; Yang, C. Multi-carbazole encapsulation as a simple strategy for the construction of solution-processed, non-doped thermally activated delayed fluorescence emitters. J. Mater. Chem. C 2016, 4, 2442–2446. [Google Scholar] [CrossRef]
- Zhang, M.; Xue, S.; Dong, W.; Wang, Q.; Fei, T.; Gu, C.; Ma, Y. Highly-efficient solution-processed OLEDs based on new bipolar materials. Chem. Commun. 2010, 46, 3923–3925. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Lam, J.; Zhao, Z.; Chen, S.; Lu, P.; Sung, H.; Kwok, H.; Ma, Y.; Williams, I.; Tang, B.Z. Aggregation-induced emission, mechanochromism and blue electroluminescence of carbazole and triphenylamine-substituted ethenes. J. Mater. Chem. C 2014, 2, 4320–4327. [Google Scholar] [CrossRef]
- Li, J.; Han, X.; Bai, Q.; Shan, T.; Lu, P.; Ma, Y. Electropolymerized AIE-active polymer film with high quantum efficiency and its application in OLED. J. Polym. Sci. A Polym. Chem. 2016, 55, 707–715. [Google Scholar] [CrossRef]
- Garcias-Morales, C.; Romero-Borja, D.; Maldonado, J.L.; Roa, A.E.; Rodríguez, M.; García-Merinos, J.P.; Ariza-Castolo, A. Small molecules derived from Thieno[3,4-c]pyrrole-4,6-dione (TPD) and their use in solution processed organic solar cells. Molecules 2017, 22, 1607. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Wong, H.; Moh, L.; Chen, Z. Polymer solar cells based on copolymers of dithieno[3,2-b:2′,3′-d]silole and thienopyrroledione. Chem. Commun. 2011, 47, 4920–4922. [Google Scholar] [CrossRef] [PubMed]
- Kadam, V.S.; Patel, A.L.; Zade, S.S. Single precursor for the synthesis of donor and acceptor units of the low band gap polymers: Synthesis of benzodithiophene and thienopyrroledione from maleic anhydride. Tetrahedron Lett. 2016, 57, 2608–2611. [Google Scholar] [CrossRef]
- Ottone, C.; Berrourad, P.; Louarn, G.; Beaupré, S.; Gendron, D.; Zagorska, M.; Rannou, P.; Najari, A.; Sadki, S.; Leclerc, M.; Pron, A. Donor-acceptor alternating copolymers containing thienopyrroledione electron accepting units: Preparation, redox behaviour, and application to photovoltaic cell. Polym. Chem. 2012, 3, 2355–2365. [Google Scholar] [CrossRef]
- Chang, S.Y.; Lin, P.H.; Liu, C.Y. Pd-catalyzed direct C-H arylation of thieno[3,4-c]pyrrole-4,6-dione (TPD): A step-economical synthetic alternative to access TPD-centred symmetrical small molecules. RSC Adv. 2014, 4, 35868–35878. [Google Scholar] [CrossRef]
- Palakollu, V.; Kanvah, S. a-Cyanostilbene based fluorophores: Aggregation-induced enhanced emission, solvatochromism and the PH effect. New J. Chem. 2014, 38, 5736–5746. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Menucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical considerations for determining absolute frontier orbital levels of conjugated polymers for solar cell applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef] [PubMed]
- Abro, H.A.; Zhou, T.; Han, W.; Xue, T.; Wang, T. Carbazole-based compounds containing aldehyde and cyanoacetic acid: Optical properties and applications in photopolymerization. RSC Adv. 2017, 7, 55382–55388. [Google Scholar] [CrossRef]
- Jacques, E.; Romain, M.; Yassin, A.; Bebiche, S.; Harnois, M.; Mohammed-Brahim, T.; Rault-Berthelot, J.; Poriel, C. An electron deficient dicyanovinylene-ladder-type pentaphenylene derivative for n-type organic field effect transistors. J. Mater. Chem. C 2014, 17, 3292–3302. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Z.; Shan, T.; Liu, Y.; Shen, F.; Pan, Y.; Zhang, H.; He, X.; Lu, P.; Yang, B.; Ma, Y. High-efficiency deep blue fluorescent emitters based on phenanthro[9,10-d]imidazole substituted carbazole and their applications in organic light emitting diodes. Org. Electron. 2014, 15, 2667–2676. [Google Scholar] [CrossRef]
- Huang, Y.; Du, X.; Tao, S.; Yang, X.; Zheng, C.J.; Zhang, X.; Lee, C.S. High efficiency non-doped deep-blue and fluorescent/phosphorescent white organic light-emitting diodes based on an anthrance derivative. Synth. Met. 2015, 203, 49–53. [Google Scholar] [CrossRef]
- Tian, G.; Liang, W.; Chen, Y.; Xiang, N.; Dong, Q.; Huang, J.; Su, J.H. A novel spiro-annulated host based on carbazole with good thermal stability and high triplet energy for efficient blue and green phosphorescent organic light-emitting diodes. Dyes Pigments 2016, 126, 296–302. [Google Scholar] [CrossRef]
- Fisher, A.L.; Linton, K.E.; Kamtekar, K.T.; Pearson, C.; Bryce, M.R.; Petty, M.C. Efficient deep-blue electroluminescence from an ambipolar fluorescent emitter in a single-active-layer device. Chem. Mater. 2011, 23, 1640–1642. [Google Scholar] [CrossRef]
- Chen, W.C.; Yuan, Y.; Ni, S.F.; Zhu, Z.L.; Zhang, J.; Jiang, Z.Q.; Liao, L.S.; Wong, F.L.; Lee, C.S. Highly efficient deep-blue electroluminescence from a charge-transfer emitter with stable donor skeleton. ACS Appl. Mater. Interfaces 2017, 9, 7331–7338. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H.; Suzuki, H.; Fukushima, T.; Shizu, K.; Suzuki, K.; Kubo, S.; Komino, T.; Oiwa, H.; Suzuki, F.; Wakamiya, A.; Murata, Y.; Adachi, C. Purely organic electroluminescent material realizing 100% conversion from electricity to light. Nat. Commun. 2015, 6, 8476. [Google Scholar] [CrossRef] [PubMed]
- Romero-Servin, S.; Lozano-Hernández, L.A.; Maldonado, J.L.; Carriles, R.; Ramos-Ortíz, G.; Peréz-Gutiérrez, E.; Scherf, U.; Zolotukin, M.G. Light emission properties of a cross-conjugated fluorene polymer: Demonstration of its use in electro-luminiscence and lasing devices. Polymers 2016, 8, 43. [Google Scholar] [CrossRef]
- Shukla, M.; Brahme, N.; Kher, R.S.; Khokhar, M.S.K. Elementary approach to calculate quantum efficiency of polymer light emitting diodes. Indian J. Pure Appl. Phys. 2011, 49, 142–145. [Google Scholar]
- Chen, S. Design of a Test Box for OLED Devices. Ph.D. Thesis, Queen’s University, Kingston, ON, Canada, 2014. [Google Scholar]
Sample Availability: Samples of the compounds CZ-2, CZ-1, MOC-1 and MOC-16 are available from the authors. |


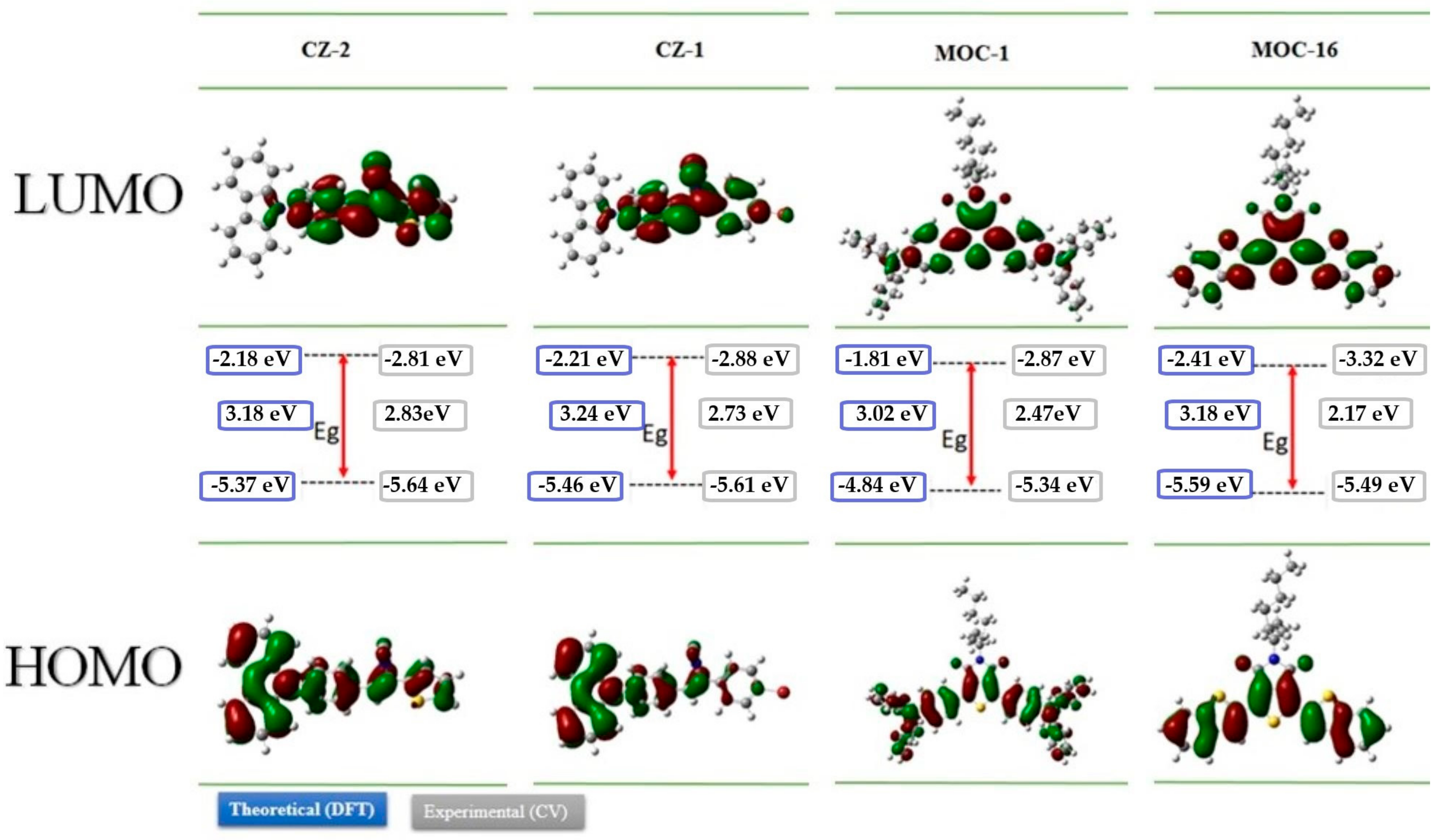


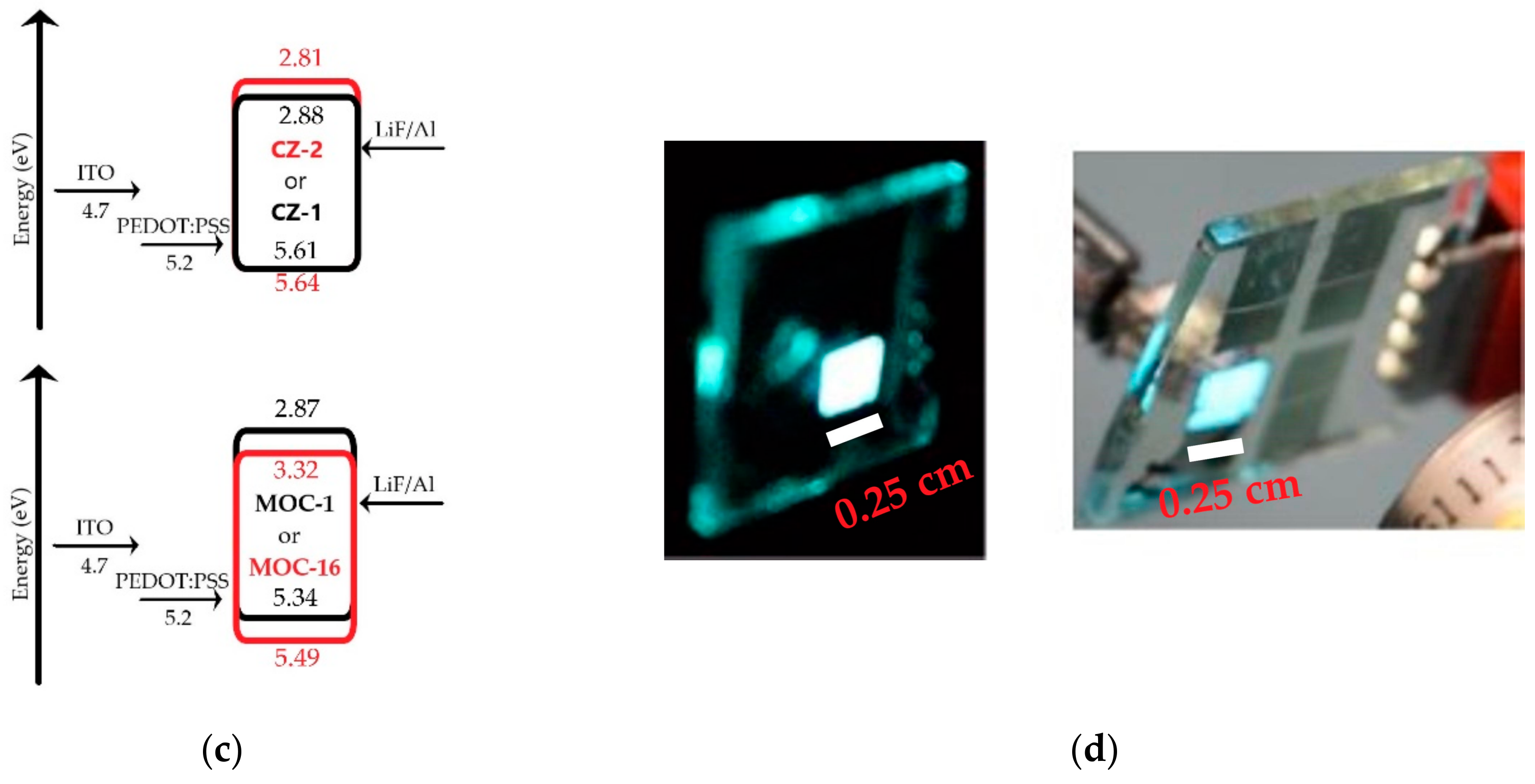

| Calculated | Experimental | |||||
|---|---|---|---|---|---|---|
| HOMO (eV) | LUMO (eV) | Egcal (eV) | HOMO (eV) | LUMO (eV) | Egexp (eV) | |
| CZ-2 | −5.37 | −2.18 | 3.18 | −5.64 | −2.81 | 2.83 |
| CZ-1 | −5.46 | −2.21 | 3.24 | −5.61 | −2.88 | 2.73 |
| MOC-1 | −4.84 | −1.81 | 3.02 | −5.34 | −2.87 | 2.47 |
| MOC-16 | −5.59 | −2.41 | 3.18 | −5.49 | −3.32 | 2.17 |
| EML | EQEmax (%) | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CZ-2 d | 5.2 | 4104 | 7.2 | 2.6 | 20.2 | 3062 | 9.5 | 488 | This work |
| CZ-1 d | 6.5 | 4130 | 8.7 | 2.1 | 19.3 | 3476 | 8.6 | 492 | ” |
| MOC-1 d | 6.2 | 651 | 10.6 | 0.07 | 4.5 | 467 | 1.5 | 564 | ” |
| MOC-16 d | 7.7 | 1729 | 7.9 | 0.34 | 0.61 | 1388 | 0.1 | 547 | ” |
| CP3 (a),(c),m | 3 | 8235 | 8.8 | --- | 2.53 | --- | --- | 474 | [32] |
| CP3 *,(a),(c),m | 2.7 | 24,442 | 9 | --- | 6.9 | --- | --- | 501 | [32] |
| TCBzC (a),m | 2.5 | 9226 | --- | --- | 31.6 | --- | --- | 534 | [36] |
| Blue-1 (b),m | 3.6 | 19,283 | --- | --- | 10.8 | --- | --- | ~460 | [51] |
| G3MP(A3) (b),m | 5.3 | 9823 | --- | --- | 28.2 | --- | 12.8 | ~480 | [21] |
| G3MP(B3) (b),m | 4.5 | 2227 | --- | --- | 18.2 | --- | 10.3 | ~480 | [21] |
| M2 (a),m | 3.4 | 4543 | --- | --- | 1.53 | --- | 3.0 | 428 | [49] |
| B (a),m | 3.8 | 2267 | --- | --- | 1.8 | --- | 3.6 | 436 | [50] |
| Dev. III no. 2 (a),(c),d | 4.6 | 4390 | --- | --- | 1.0 | --- | 0.4 | 492 | [37] |
| TPE-DFCz (a),(c),d | 5.4 | 3200 | --- | --- | 1.16 | --- | 0.4 | 500 | [38] |
| DCZ-TTR (b),m | 3.2 | ~5000 | --- | --- | 59.6 | --- | 20.1 | 512 | [34] |
| CZ-TTR (b),(c),m | 3.1 | ~1000 | --- | --- | 32.5 | --- | 14.4 | 492 | [34] |
| Dev. 2 *,d | --- | --- | --- | --- | --- | --- | 4.71 | 431 | [52] |
| BITPI *,m | 2.6 | --- | --- | --- | 6.7 | --- | 7.1 | 452 | [53] |
| BITPI *,s | 3.1 | --- | --- | --- | 3.4 | --- | 2.98 | 452 | [53] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano-Hernández, L.-A.; Maldonado, J.-L.; Garcias-Morales, C.; Espinosa Roa, A.; Barbosa-García, O.; Rodríguez, M.; Pérez-Gutiérrez, E. Efficient OLEDs Fabricated by Solution Process Based on Carbazole and Thienopyrrolediones Derivatives. Molecules 2018, 23, 280. https://doi.org/10.3390/molecules23020280
Lozano-Hernández L-A, Maldonado J-L, Garcias-Morales C, Espinosa Roa A, Barbosa-García O, Rodríguez M, Pérez-Gutiérrez E. Efficient OLEDs Fabricated by Solution Process Based on Carbazole and Thienopyrrolediones Derivatives. Molecules. 2018; 23(2):280. https://doi.org/10.3390/molecules23020280
Chicago/Turabian StyleLozano-Hernández, Luis-Abraham, José-Luis Maldonado, Cesar Garcias-Morales, Arian Espinosa Roa, Oracio Barbosa-García, Mario Rodríguez, and Enrique Pérez-Gutiérrez. 2018. "Efficient OLEDs Fabricated by Solution Process Based on Carbazole and Thienopyrrolediones Derivatives" Molecules 23, no. 2: 280. https://doi.org/10.3390/molecules23020280
APA StyleLozano-Hernández, L.-A., Maldonado, J.-L., Garcias-Morales, C., Espinosa Roa, A., Barbosa-García, O., Rodríguez, M., & Pérez-Gutiérrez, E. (2018). Efficient OLEDs Fabricated by Solution Process Based on Carbazole and Thienopyrrolediones Derivatives. Molecules, 23(2), 280. https://doi.org/10.3390/molecules23020280







