Hierarchical Structure, Gelatinization, and Digestion Characteristics of Starch from Longan (Dimocarpus longan Lour.) Seeds
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Longan Seeds
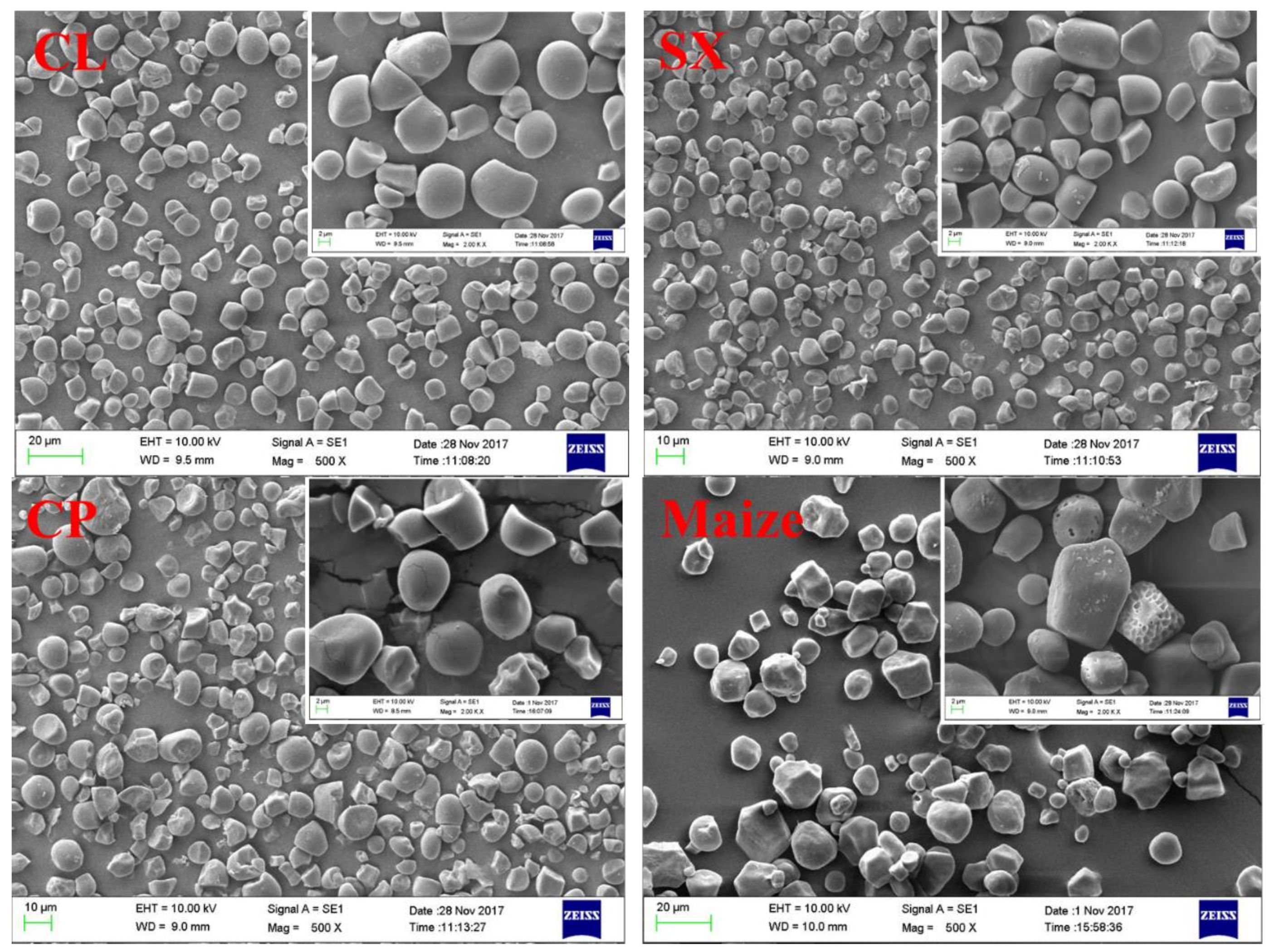
2.2. Granular Structure
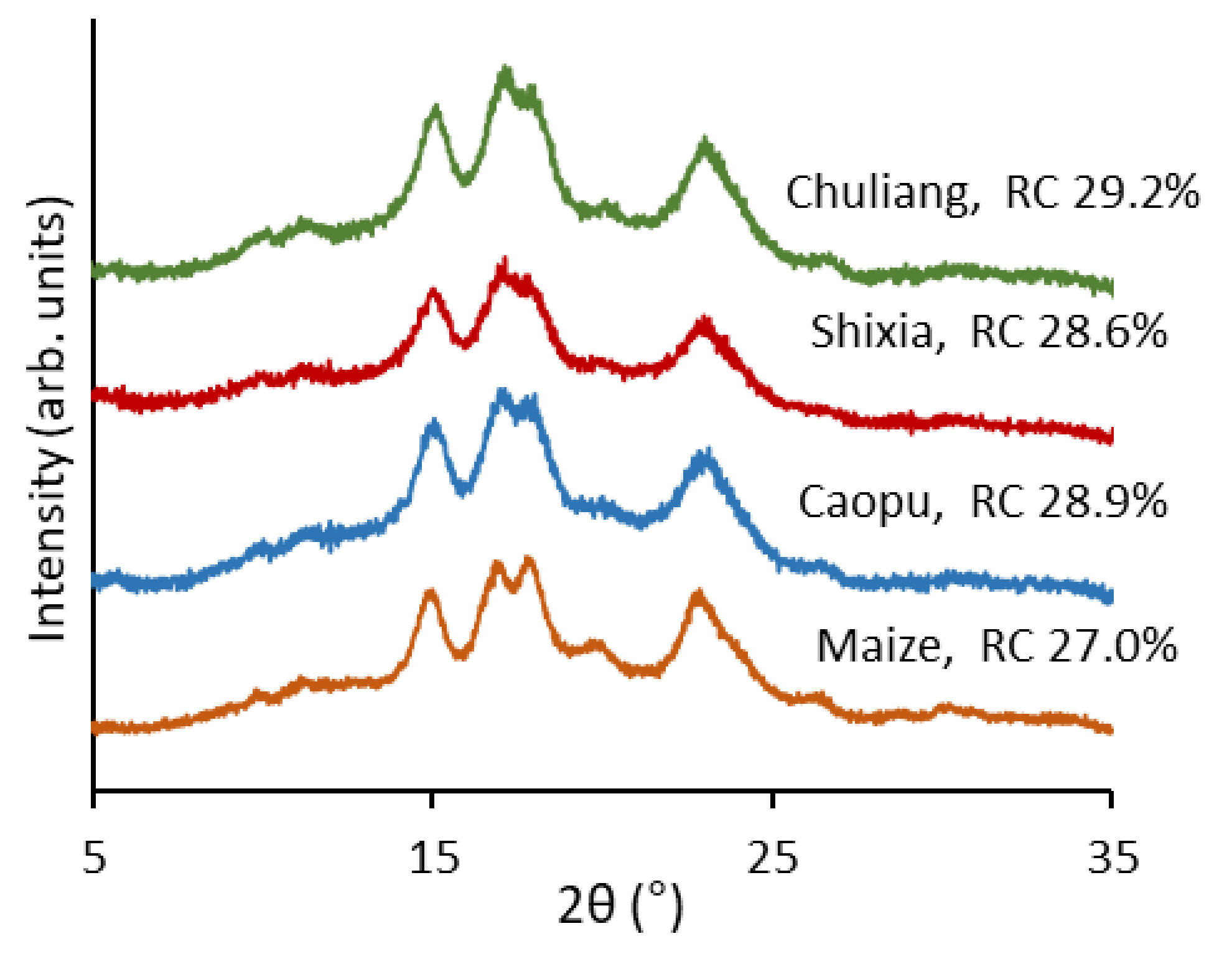
2.3. Crystalline Structure
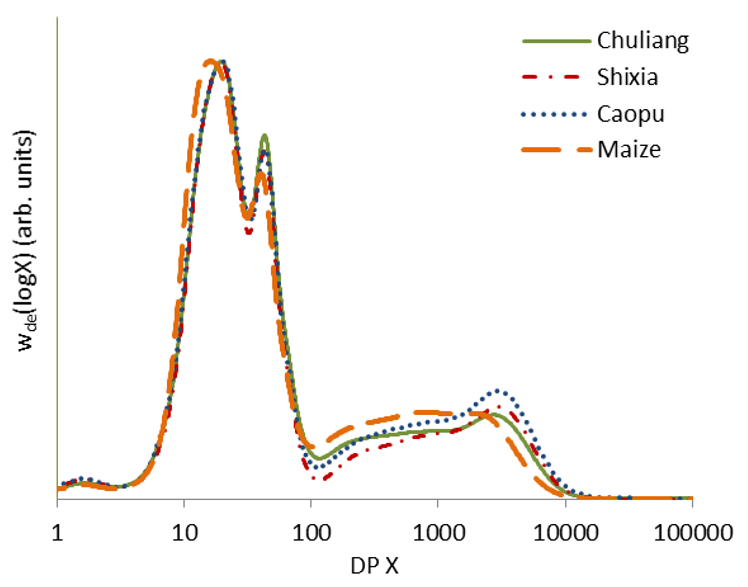
2.4. Molecular Structure
2.5. Thermal Properties
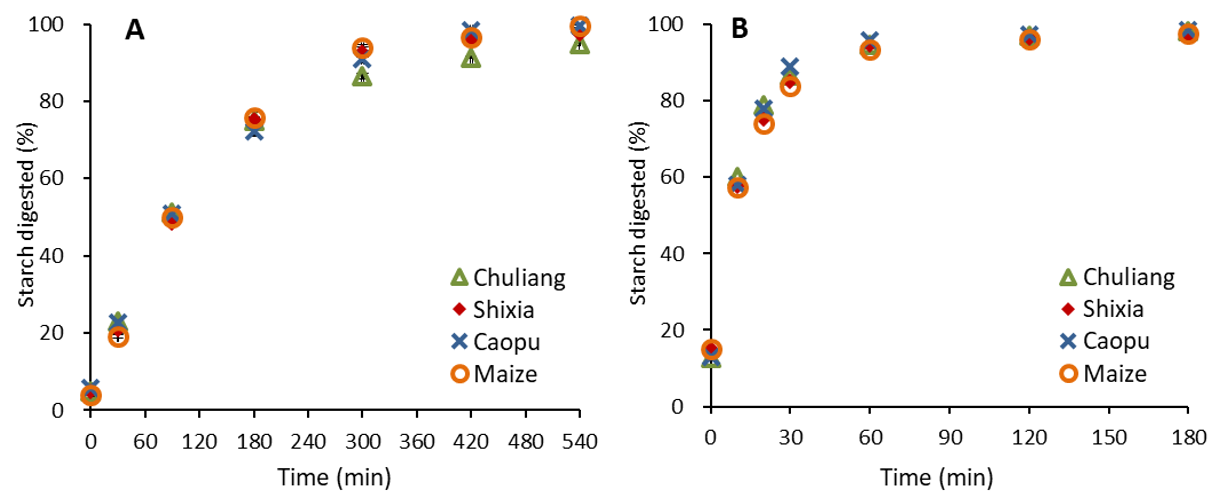
2.6. In Vitro Starch Digestibility
3. Materials and Methods
3.1. Materials
3.2. Composition of Longan Seeds
3.3. Isolation of Starch from Longan Seeds
3.4. Starch Morphology
3.5. X-ray Diffraction
3.6. Molecular Structure
3.7. Thermal Properties
3.8. In Vitro Starch Digestibility
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rangkadilok, N.; Sitthimonchai, S.; Worasuttayangkurn, L.; Mahidol, C.; Ruchirawat, M.; Satayavivad, J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem. Toxicol. 2007, 45, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Wen, L.; Chen, F.; Wu, F.; Lin, S.; Yang, B.; Jiang, Y. Structural characteristics and antioxidant activities of polysaccharides from longan seed. Carbohydr. Polym. 2013, 92, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Xu, L.; Wu, P.; Xie, H.; Jiang, Y.; Chen, F.; Wei, X. Polyphenols from longan seeds and their radical-scavenging activity. Food Chem. 2009, 116, 433–436. [Google Scholar] [CrossRef]
- Aparecida de Assis, S.; Vellosa, J.C.R.; Brunetti, I.L.; Khalil, N.M.; Leite, K.M.d.S.C.; Martins, A.B.G.; Oliveira, O.M.M.d.F. Antioxidant activity, ascorbic acid and total phenol of exotic fruits occurring in Brazil. Int. J. Food Sci. Nutr. 2009, 60, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Rangkadilok, N.; Worasuttayangkurn, L.; Bennett, R.N.; Satayavivad, J. Identification and quantification of polyphenolic compounds in longan (Euphoria longana Lam.) fruit. J. Agric. Food Chem. 2005, 53, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Huang, R.; Zeng, Q.; Chen, W.; Liu, X.; Rui, H. Nutrition composition of Longan seeds. Food Sci. Technol. 2004, 01, 93–94. [Google Scholar]
- Guo, K.; Lin, L.; Fan, X.; Zhang, L.; Wei, C. Comparison of structural and functional properties of starches from five fruit kernels. Food Chem. 2018, 257, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.G.; Witt, T.; Hasjim, J. What is being learned about starch properties from multiple-level characterization. Cereal Chem. 2013, 90, 312–325. [Google Scholar] [CrossRef]
- Wang, K.; Henry, R.J.; Gilbert, R.G. Causal relations between starch biosynthesis, structure and properties. Springer Sci. Rev. 2014, 2, 15–33. [Google Scholar] [CrossRef]
- Oates, C.G.; Powell, A.D. Bioavailability of carbohydrate material stored in tropical fruit seeds. Food Chem. 1996, 56, 405–414. [Google Scholar] [CrossRef]
- Jane, J.L.; Kasemsuwan, T.; Leas, S.; Zobel, H.; Robyt, J.F. Anthology of starch granule morphology by scanning electron-microscopy. Starch 1994, 46, 121–129. [Google Scholar] [CrossRef]
- Shrestha, A.K.; Blazek, J.; Flanagan, B.M.; Dhital, S.; Larroque, O.; Morell, M.K.; Gilbert, E.P.; Gidley, M.J. Molecular, mesoscopic and microscopic structure evolution during amylase digestion of maize starch granules. Carbohydr. Polym. 2012, 90, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, N.W.H.; Tao, L. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Wang, K.; Hasjim, J.; Wu, A.C.; Henry, R.J.; Gilbert, R.G. Variation in amylose fine structure of starches from different botanical sources. J. Agric. Food Chem. 2014, 62, 4443–4453. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana, F.; Meng, D.; Hasjim, J.; Gilbert, R.G. Two-dimensional macromolecular distributions reveal detailed architectural features in high-amylose starches. Carbohydr. Polym. 2014, 113, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Prakash, S.; Nicholson, T.M.; Fitzgerald, M.A.; Gilbert, R.G. The importance of amylose and amylopectin fine structure for textural properties of cooked rice grains. Food Chem. 2016, 196, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, H.; Silverio, J.; Andersson, R.; Eliasson, A.C.; Åman, P. The influence of amylose and amylopectin characteristics on gelatinization and retrogradation properties of different starches. Carbohydr. Polym. 1998, 35, 119–134. [Google Scholar] [CrossRef]
- Sasaki, T.; Yasui, T.; Matsuki, J. Effect of amylose content on gelatinization, retrogradation, and pasting properties of starches from waxy and nonwaxy wheat and their F1 seeds. Cereal Chem. 2000, 77, 58–63. [Google Scholar] [CrossRef]
- Cooke, D.; Gidley, M.J. Loss of crystalline and molecular order during starch gelatinisation: Origin of the enthalpic transition. Carbohydr. Res. 1992, 227, 103–112. [Google Scholar] [CrossRef]
- Bird, A.R.; Lopez-Rubio, A.; Shrestha, A.K.; Gidley, M.J. Resistant starch in vitro and in vivo: Factors determining yield, structure, and physiological relevance. In Modern Biopolymer Science; Kasapis, S., Norton, I.T., Ubbink, J.B., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 449–510. [Google Scholar]
- Hallström, E.; Sestili, F.; Lafiandra, D.; Björck, I.; Östman, E. A novel wheat variety with elevated content of amylose increases resistant starch formation and may beneficially influence glycaemia in healthy subjects. Food Nutr. Res. 2011, 55, 7074. [Google Scholar] [CrossRef]
- Chung, H.-J.; Liu, Q.; Lee, L.; Wei, D. Relationship between the structure, physicochemical properties and in vitro digestibility of rice starches with different amylose contents. Food Hydrocoll. 2011, 25, 968–975. [Google Scholar] [CrossRef]
- Zhu, L.J.; Liu, Q.Q.; Wilson, J.D.; Gu, M.H.; Shi, Y.C. Digestibility and physicochemical properties of rice (Oryza sativa L.) flours and starches differing in amylose content. Carbohydr. Polym. 2011, 86, 1751–1759. [Google Scholar] [CrossRef]
- Dhital, S.; Shrestha, A.K.; Gidley, M.J. Effect of cryo-milling on starches: Functionality and digestibility. Food Hydrocoll. 2010, 24, 152–163. [Google Scholar] [CrossRef]
- Li, E.P.; Hasjim, J.; Dhital, S.; Godwin, I.D.; Gilbert, R.G. Effect of a gibberellin-biosynthesis inhibitor treatment on the physicochemical properties of sorghum starch. J. Cereal Sci. 2011, 53, 328–334. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; AOAC International: Arlington, VA, USA, 2002. [Google Scholar]
- Wang, K.; Hasjim, J.; Wu, A.C.; Li, E.; Henry, R.J.; Gilbert, R.G. Roles of GBSSI and SSIIa in determining amylose fine structure. Carbohydr. Polym. 2015, 127, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhang, B.; Huang, Q.; Li, C.; Pletsch, E.A.; Fu, X. Variation in the rate and extent of starch digestion is not determined by the starch structural features of cooked whole pulses. Food Hydrocoll. 2018, 83, 340–347. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Fu, X.; Li, C.; He, X.; Zhang, B.; Huang, Q. Encapsulation of lutein into swelled cornstarch granules: Structure, stability and in vitro digestion. Food Chem. 2018, 268, 362–368. [Google Scholar] [CrossRef]
- Wang, K.; Wambugu, P.W.; Zhang, B.; Wu, A.C.; Henry, R.J.; Gilbert, R.G. The biosynthesis, structure and gelatinization properties of starches from wild and cultivated African rice species (Oryza barthii and Oryza glaberrima). Carbohydr. Polym. 2015, 129, 92–100. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, K.; Hasjim, J.; Li, E.; Flanagan, B.M.; Gidley, M.J.; Dhital, S. Freeze-drying changes the structure and digestibility of B-polymorphic starches. J. Agric. Food Chem. 2014, 62, 1482–1491. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, P.; Wang, K.; Li, C.; Li, E.; Gilbert, R.G. Effects of pectin on molecular structural changes in starch during digestion. Food Hydrocoll. 2017, 69, 10–18. [Google Scholar] [CrossRef]
- Butterworth, P.J.; Warren, F.J.; Grassby, T.; Patel, H.; Ellis, P.R. Analysis of starch amylolysis using plots for first-order kinetics. Carbohydr. Polym. 2012, 87, 2189–2197. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Cultivar | Starch Content (%) | Protein Content (%) | Fat Content (%) |
|---|---|---|---|
| Chuliang | 44.9 ± 0.4 a | 6.0 ± 0.0 c | 3.4 ± 0.2 a |
| Shixia | 46.2 ± 1.5 a | 8.1 ± 0.0 a | 2.0 ± 0.0 b |
| Caopu | 49.5 ± 1.6 a | 7.6 ± 0.0 b | 2.5 ± 0.0 b |
| Source | Amylose Content (%) | To | Tp | Tc | ∆H |
|---|---|---|---|---|---|
| (°C) | (°C) | (°C) | (J/g) | ||
| Chuliang | 26.7 ± 0.9 b | 72.3 ± 0.4 b | 80.5 ± 0.1 a | 88.3 ± 0.3 a | 16.4 ± 0.4 a |
| Shixia | 26.6 ± 0.3 b | 72.8 ± 0.1 b | 79.0 ± 0.1 a,b | 85.6 ± 0.0 b | 15.6 ± 0.2 a |
| Caopu | 30.1 ± 0.8 a | 69.6 ± 0.4 c | 76.7 ± 0.3 b | 84.4 ± 0.5 c | 13.5 ± 0.5 b |
| Maize | 29.1 ± 0.2 a,b | 75.4 ± 0.8 a | 79.2 ± 0.9 a,b | 88.0 ± 0.0 a | 10.3 ± 0.6 c |
| Source | k (10−3 min−1) | |
|---|---|---|
| Raw Starch | Gelatinized Starch | |
| Chuliang | 7.7 ± 0.2 b | 45.2 ± 2.8 a |
| Shixia | 8.9 ± 0.4 a | 45.1 ± 1.1 a |
| Caopu | 6.6 ± 0.0 c | 44.3 ± 0.6 a |
| Maize | 8.5 ± 0.0 a,b | 45.8 ± 0.6 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Zhao, L.; Hu, Z.; Wang, K. Hierarchical Structure, Gelatinization, and Digestion Characteristics of Starch from Longan (Dimocarpus longan Lour.) Seeds. Molecules 2018, 23, 3262. https://doi.org/10.3390/molecules23123262
Hu Z, Zhao L, Hu Z, Wang K. Hierarchical Structure, Gelatinization, and Digestion Characteristics of Starch from Longan (Dimocarpus longan Lour.) Seeds. Molecules. 2018; 23(12):3262. https://doi.org/10.3390/molecules23123262
Chicago/Turabian StyleHu, Ziman, Lei Zhao, Zhuoyan Hu, and Kai Wang. 2018. "Hierarchical Structure, Gelatinization, and Digestion Characteristics of Starch from Longan (Dimocarpus longan Lour.) Seeds" Molecules 23, no. 12: 3262. https://doi.org/10.3390/molecules23123262
APA StyleHu, Z., Zhao, L., Hu, Z., & Wang, K. (2018). Hierarchical Structure, Gelatinization, and Digestion Characteristics of Starch from Longan (Dimocarpus longan Lour.) Seeds. Molecules, 23(12), 3262. https://doi.org/10.3390/molecules23123262