Structural Elucidation and Cytotoxicity of a New 17-Membered Ring Lactone from Algerian Eryngium campestre
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition
2.2. Identification of Compounds Not Present in MS-Libraries
2.3. Biological Activities
3. Materials and Methods
3.1. Plant Material
3.2. Extraction
3.3. Compound Identification
3.4. Fractionation
3.5. GC-FID Conditions
3.6. GC/MS-EI Conditions
3.7. NMR Conditions
3.8. High-Resolution Mass Spectrometry Experiments
3.9. VCD Measurements
3.10. Biological Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wörz, A. A new subgeneric classification of the genus Eryngium L. (Apiaceae, Saniculoideae). Bot. Jahrb. Syst. 2005, 126, 253–259. [Google Scholar] [CrossRef]
- Wang, P. Phytochemical constituents and pharmacological activities of eryngium l. (Apiaceae). Pharm. Crop. 2012, 3, 99–120. [Google Scholar] [CrossRef]
- Moerman, D.E. Native American Food Plants. An Ethnobotanical Dictionary; Timber Press Inc.: London, UK, 2011; p. 65. [Google Scholar]
- Suciu, S.; Pârvu, A.E. Comparative Study on the Effects of Eryngium Sp. Extracts in an Acute Inflammation Model in Rat. Annals RSCB 2012, 17, 86–91. [Google Scholar]
- Rojas-Silva, P.; Graziose, R.; Vesely, B.; Poulev, A.; Mbeunkui, F.; Grace, M.H.; Kyle, D.E.; Lila, M.A.; Raskin, I. Leishmanicidal activity of a daucane sesquiterpene isolated fromeryngium foetidum. Pharm. Biol. 2014, 52, 398–401. [Google Scholar] [CrossRef]
- Jaghabir, M. Hypoglycemic effects of Eryngium creticum. Arch. Pharm. Res. 1991, 14, 295–297. [Google Scholar] [CrossRef]
- Goleniowski, M.E.; Bongiovanni, G.; Palacio, L.; Nuñez, C.; Cantero, J. Medicinal plants from the ‘Sierra de Comechingones’, Argentina. J. Ethnopharmacol. 2006, 107, 324–341. [Google Scholar] [CrossRef] [PubMed]
- Coste, H. Flore Descriptive et Illustrée de la France, de la Corse et des Contrées Limitrophes II; Librairie Scientifique et Technique Albert Blanchart: Le Val-Saint-Germain, France, 1980. [Google Scholar]
- Pieroni, A.; Pardo-de-Santayana, M.; Firenzuoli, F.; Quave, C.L. The european heritage of folk medicines and medicinal foods: Its contribution to the cams of tomorrow. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–2. [Google Scholar] [CrossRef]
- Kartnig, T.; Wolf, J. Flavonoids from the Aboveground Parts of Eryngium campestre. Planta Med. 1993, 58, 285. [Google Scholar] [CrossRef]
- Abou El-Kassem, L.; Hawas, U.; Awad, H.; Taie, H. Flavonoids from the aerial parts of eryngium campestre l. with antioxidant and anti-alzheimer activities. Planta Medica 2013, 79. [Google Scholar] [CrossRef]
- Hohmann, J.; Páll, Z.; Günther, G.; Máthé, I. Flavonolacyl glycosides of the aerial parts of eryngium campestre. Planta Medica 1997, 63, 96. [Google Scholar] [CrossRef]
- Erdelmeier, C.A.J.; Sticher, O. A cyclohexenone and a cyclohexadienone glycoside from eryngium campestre. Phytochemistry 1986, 25, 741–743. [Google Scholar] [CrossRef]
- Erdelmeier, C.; Sticher, O. Coumarin derivatives from eryngium campestre 1. Planta Med. 1985, 51, 407–409. [Google Scholar] [CrossRef]
- Kartal, M.; Mitaine-Offer, A.-C.; Abu-Asaker, M.; Miyamoto, T.; Calis, I.; Wagner, H.; Lacaille-Dubois, M.-A. Two new triterpene saponins from eryngium campestre. Chem. Pharm. Bull. 2005, 53, 1318–1320. [Google Scholar] [CrossRef]
- Abd-Elmonem, A.R.; Shehab, N. Study of the volatile oil of Eryngium campestre l. growing in Egypt. G. Bull. Fac. Pharm. Cairo Univ. 2008, 44, 3379–3388. [Google Scholar]
- Pala-Paul, J.; Usano-Alemany, J.; Soria, A.C.; Perez-Alonso, M.J.; Brophy, J.J. Essential oil composition of Eryngium campestre L. growing in different soil types. A preliminary study. Nat. Prod. Commun. 2008, 3, 1121–1126. [Google Scholar]
- Usta, C.; Yildirim, A.B.; Turker, A.U. Antibacterial and antitumour activities of some plants grown in turkey. Biotechnol. Biotechnol. Equip. 2014, 28, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Hawas, U.W.; El-Kassem, L.A.T.; Awad, H.M.; Taie, H.A.A. Anti-Alzheimer, Antioxidant Activities and Flavonol Glycosides of Eryngium campestre L. Curr. Chem. Biol. 2013, 7, 188–195. [Google Scholar] [CrossRef]
- Küpeli, E.; Kartal, M.; Aslan, S.; Yesilada, E. Comparative evaluation of the anti-inflammatory and antinociceptive activity of turkish eryngium species. J. Ethnopharmacol. 2006, 107, 32–37. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Khalid, S.; Romanha, A.; Alves, T.; Biavatti, M.; Brun, R.; Da Costa, R.; De Catsro, S. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases-part II. Curr. Med. Chem. 2012, 19, 2128–2175. [Google Scholar] [CrossRef]
- Franco Minguel, J.R. Report of the Second WHO Stakeholders Meeting on Rhodesiense Human African Trypanosomiasis; WHO/Department of Control of Neglected Tropical Diseases: Genève, Switzerland, 2017. [Google Scholar]
- Zimmermann, S.; Thomi, S.; Kaiser, M.; Hamburger, M.; Adams, M. Screening and HPLC-Based Activity Profiling for New Antiprotozoal Leads from European Plants. Sci. Pharm. 2012, 80, 205–213. [Google Scholar] [CrossRef]
- Joulain, D.; König, W.A. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; E.B.-Verlag: Hamburg, Germany, 1998. [Google Scholar]
- Nitz, S.; Spraul, M.-H.; Drawert, F. C17 Polyacetylenic alcohols as the major constituents in roots of Petroselinum crispum Mill. ssp. tuberosum. J. Agric. Food Chem. 1990, 38, 1445–1447. [Google Scholar] [CrossRef]
- Fujioka, T.; Furumi, K.; Fujii, H.; Okabe, H.; Mihashi, K.; Nakano, Y.; Matsunaga, H.; Katano, M.; Mori, M. Antiproliferative Constituents from Umbelliferae Plants. V. A New Furanocoumarin and Falcarindiol Furanocoumarin Ethers from the Root of Angelica japonica. Chem. Pharm. Bull. 1999, 47, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Dawid, C.; Dunemann, F.; Schwab, W.; Nothnagel, T.; Hofmann, T. Bioactive C17-Polyacetylenes in Carrots (Daucus carota L.): Current Knowledge and Future Perspectives. J. Agric. Food Chem. 2015, 63, 9211–9222. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C. Terpene derivatives from higher plants, XII. On new terpene aldehyde esters from Eryngium species. Chem. Ber. 1971, 104, 1957–1961. [Google Scholar] [CrossRef]
- Jin, H.R.; Zhao, J.; Zhang, Z.; Liao, Y.; Wang, C.-Z.; Huang, W.-H.; Li, S.-P.; He, T.-C.; Yuan, C.-S.; Du, W. The antitumor natural compound falcarindiol promotes cancer cell death by inducing endoplasmic reticulum stress. Cell Death Dis. 2012, 3, e376. [Google Scholar] [CrossRef] [PubMed]
- Meot-Duros, L.; Cérantola, S.; Talarmin, H.; Le Meur, C.; Le Floch, G.; Magné, C. New antibacterial and cytotoxic activities of falcarindiol isolated in crithmum maritimum l. leaf extract. Food Chem. Toxicol. 2010, 48, 553–557. [Google Scholar] [CrossRef]
- Batista, A.N.L.; dos Santos, F.M., Jr.; Batista, J.M., Jr.; Cass, Q.B. Enantiomeric Mixtures in Natural Product Chemistry: Separation and Absolute Configuration Assignment. Molecules 2018, 23, 492. [Google Scholar] [CrossRef]
- Said, M.E.-A.; Bombarda, I.; Naubron, J.-V.; Vanloot, P.; Jean, M.; Cheriti, A.; Dupuy, N.; Roussel, C. Isolation of the major chiral compounds from Bubonium graveolens essential oil by HPLC and absolute configuration determination by VCD. Chirality 2017, 29, 70–79. [Google Scholar] [CrossRef]
- Ungeheuer, F.; Fürstner, A. Concise Total Synthesis of Ivorenolide B1. Chem.–Eur. J. 2015, 21, 11387–11392. [Google Scholar] [CrossRef] [PubMed]
- Zidorn, C.; Jöhrer, K.; Ganzera, M.; Schubert, B.; Sigmund, E.M.; Mader, J.; Greil, R.; Ellmerer, E.P.; Stuppner, H. Polyacetylenes from the apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. J. Agric. Food Chem. 2005, 53, 2518–2523. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, A.S.; Hemscheidt, T. Olefin Cross-Metathesis as a Tool in Natural Product Degradation. The Stereochemistry of (+)-Falcarindiol. Org. Lett. 2002, 4, 4667–4668. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Q.-F.; Xue, J.-J.; Zhou, Y.; Yu, H.-C.; Yang, S.-P.; Zhang, B.; Zuo, J.-P.; Li, Y.; Yue, J.M. Ivorenolide B, an Immunosuppressive 17-Membered Macrolide from Khaya ivorensis: Structural Determination and Total Synthesis. Org. Lett. 2014, 16, 2062–2065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Y.; Yang, S.-P.; Zhou, Y.; Wu, W.-B.; Tank, W.; Zuo, J.-P.; Li, Y.; Yue, J.M. Ivorenolide A, an Unprecedented Immunosuppressive Macrolide from Khaya ivorensis: Structural Elucidation and Bioinspired Total Synthesis. J. Am. Chem. Soc. 2012, 134, 20605–20608. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Oguchi, K.; Iwamoto, R.; Okamoto, Y.; Fukushi, E.; Kawabata, J.; Ozawa, T.; Masuda, A. Iriomoteolides-1b and -1c, 20-Membered Macrolides from a Marine Dinoflagellate Amphidinium Species. J. Nat. Prod. 2007, 70, 1661–1663. [Google Scholar] [CrossRef]
- Tsuda, M.; Izui, N.; Shimbo, K.; Sato, M.; Fukushi, E.; Kawabata, J.; Kobayashi, J. Amphidinolide Y, a Novel 17-Membered Macrolide from Dinoflagellate Amphidinium sp.: Plausible Biogenetic Precursor of Amphidinolide X. J. Org. Chem. 2003, 68, 9109–9112. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J. Amphidinolides and Its Related Macrolides from Marine Dinoflagellates. J. Antibiot. 2008, 61, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Beaufay, C.; Bero, J.; Quetin-Leclercq, J. Antimalarial Terpenic Compounds Isolated from Plants Used in Traditional Medicine (2010–July 2016). In Natural Antimicrobial Agents. Sustainable Development and Biodiversity; Mérillon, J.M., Riviere, C., Eds.; Springer: Cham, Switzerland, 2018; Volume 19, pp. 247–268. [Google Scholar]
- Pink, R.; Hudson, A.; Mouriès, M.-A.; Bendig, M. Opportunities and challenges in antiparasitic drug discovery. Nat. Rev. Drug Discov. 2005, 4, 727–740. [Google Scholar] [CrossRef]
- Molinspiration Cheminformatics, Nova ulica, SK-900 26 Slovensky Grob, Slovak Republic. Available online: http://www.molinspiration.com/cgi-bin/properties (accessed on 23 October 2018).
- U.S. National Library of Medicine. National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/332#section=Chemical-and-Physical-Properties (accessed on 23 October 2018).
- Balasegaram, M.; Ritmeijer, K.; Lima, M.; Burza, S.; Genovese, G.; Milani, B.; Gaspani, S.; Potet, J.; Chappuis, F. Liposomal amphotericin B as a treatment for human leishmaniasis. Expert Opin. Emerg. Drugs 2012, 17, 493–510. [Google Scholar] [CrossRef]
- Fountain, M.W.; Weiss, S.J.; Lenk, R.P.; Propescu, M.C.; Ginsberg, R.S. Enhancement of Pharmaceutical Activity. U.S. Patent 5000958, 26 July 1984. [Google Scholar]
- Brglez Mojzer, E.; Knez Hrncic, M.; Škerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Cheung, S.S.C.; Tai, J.; Hasman, D.; Ou, D.; Warnock, G.L. Inhibition of Human Pancreatic Cancer Cell Proliferation by Devil’s Club Oplopanax horridus and Its Polyacetylene Bioactive Compound. Nutr. Cancer 2015, 67, 954–964. [Google Scholar] [CrossRef]
- Herrmann, F.; Sporer, F.; Tahrani, A.; Wink, M. Antitrypanosomal Properties of Panax ginseng C. A. Meyer: New Possibilities for a Remarkable Traditional Drug. Phytother. Res. 2013, 27, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Senn, M.; Gunzenhauser, S.; Brun, R.U.; Séquin, U. Antiprotozoal polyacetylenes from the Tanzanian medicinal plant Cussonia zimmermannii. J. Nat. Prod. 2007, 70, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Fokialakis, N.; Kalpoutzakis, E.; Tekwani, B.L.; Khan, S.I.; Kobaisy, M.; Skaltsounis, A.L.; Duke, S.O. Evaluation of the antimalarial and antileishmanial activity of plants from the Greek island of Crete. J. Nat. Med. 2007, 61, 38–45. [Google Scholar] [CrossRef]
- Molina-Garza, Z.J.; Bazaldúa-Rodríguez, A.F.; Quintanilla-Licea, R.; Galaviz-Silva, L. Anti-Trypanosoma cruzi activity of 10 medicinal plants used in northeast Mexico. Acta Trop. 2014, 136, 14–18. [Google Scholar] [CrossRef]
- Roumy, V.; Garcia-Pizango, G.; Gutierrez-Choquevilca, A.L.; Ruiz, L.; Jullian, V.; Winterton, P.; Fabre, N.; Moulis, C.; Valentin, A. Amazonian plants from Peru used by Quechua and Mestizo to treat malaria with evaluation of their activity. J. Ethnopharmacol. 2007, 112, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Quézel, P.; Santa, S. Nouvelle Flore de l’Algérie et des Régions Désertiques Méridionales; Éditions du Centre national de la Recherche scientifique: Paris, France, 1962. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- National Institute of Standards and Technology. NIst webBook. 2015. Available online: http://webbook.nist.gov/chemistry/ (accessed on 24 January 2017).
- König, W.A.; Joulain, D.; Hochmuth, D.H. Terpenoids and Related Constituents of Essential Oils, Library of Mass Finder 2.1; Institute of Organic Chemistry, University of Hamburg: Hamburg, Germany, 2011. [Google Scholar]
- Braun, S.; Kalinowski, H.-O.; Berger, S. 150 and More Basic NMR Experiments. A Practical Course; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision A.03; Barone, V., Petersson, G.A., Nakatsuji, H., Eds.; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Hoet, S.; Stévigny, C.; Block, S.; Opperdoes, F.; Colson, P.; Baldeyrou, B.; Lansiaux, A.; Bailly, C.; Quetin-Leclercq, J. Alkaloids from Cassytha filiformis and related aporphines: Antitrypanosomal activity, cytotoxicity, and interaction with DNA and topoisomerases. Planta Med. 2004, 70, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Le, T.B.; Beaufay, C.; Nghiem, D.T.; Mingeot-Leclercq, M.-P.; Quetin-Leclercq, J. In Vitro Anti-Leishmanial Activity of Essential Oils Extracted from Vietnamese Plants. Molecules 2017, 22, 1071. [Google Scholar] [CrossRef]
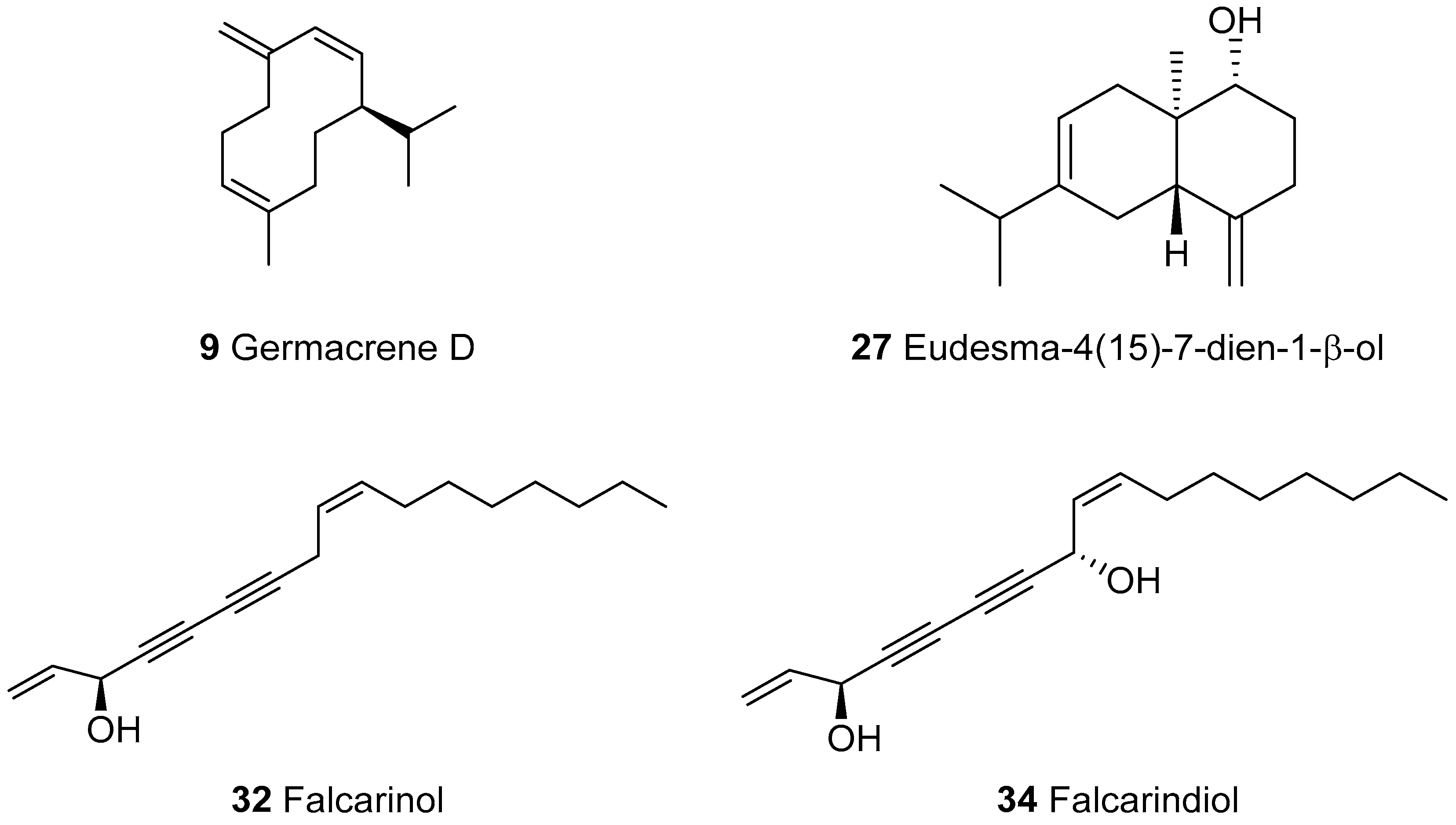
Sample Availability: Samples of the compounds 32, 33 and 34 are available from the authors. |

| No. | Components a | LRIa b | Ria c | RIp d | HEEC e | Identification f |
|---|---|---|---|---|---|---|
| 1 | α-Copaene | 1379 | 1375 | 1438 | 0.4 | RI, MS |
| 2 | β-Bourbonene | 1385 | 1383 | 1515 | 0.1 | RI, MS |
| 3 | β-Elemene | 1388 | 1387 | 1589 | 0.5 | RI, MS |
| 4 | β-Ylangene | 1420 | 1416 | 1562 | 0.6 | RI, MS |
| 5 | β-Copaene | 1431 | 1432 | 1581 | 0.2 | RI, MS |
| 6 | Alloaromadendrene | 1451 | 1454 | 1631 | tr | RI, MS |
| 7 | diepi-4,5-Aristolochene | 1467 | 1465 | 1665 | 0.4 | RI. MS |
| 8 | α-Curcumene | 1470 | 1471 | 1742 | 1.0 | RI, MS |
| 9 | Germacrene D | 1476 | 1480 | 1704 | 23.6 | RI, MS |
| 10 | β-Selinene | 1483 | 1484 | 1712 | 0.8 | RI, MS |
| 11 | α-Muurolene | 1496 | 1503 | 1720 | 0.2 | RI, MS |
| 12 | β-Bisabolene | 1500 | 1500 | 1720 | 1.2 | RI, MS |
| 13 | Sesquicineole | 1505 | 1506 | 1737 | 1.1 | RI, MS |
| 14 | τ-Cadinene | 1507 | 1509 | 1752 | 0.2 | RI, MS |
| 15 | β-Curcumene | 1509 | 1510 | 1733 | 0.5 | RI, MS |
| 16 | δ-Cadinene | 1516 | 1514 | 1752 | 1.0 | RI, MS |
| 17 | α-Cadinene | 1535 | 1533 | 1743 | 0.1 | RI, MS |
| 18 | 1,5-Epoxysalvial4(14)-ene | 1545 | 1548 | 1941 | 0.8 | RI, MS |
| 19 | Germacrene B | 1553 | 1551 | 1827 | 0.4 | RI, MS |
| 20 | Spathulenol | 1563 | 1562 | 2103 | 1.3 | RI, MS |
| 21 | β-Copaene-4-α-ol | 1575 | 1573 | 2141 | 1.0 | RI, MS |
| 22 | Salvial-4(14)-en-1-one | 1583 | 1585 | 2005 | 0.5 | RI, MS |
| 23 | Ledol | 1600 | 1602 | 2030 | 0.8 | RI, MS |
| 24 | τ-Cadinol | 1632 | 1638 | 2169 | 0.9 | RI, MS |
| 25 | α-Cadinol | 1645 | 1645 | 2231 | 1.6 | RI, MS |
| 26 | α-Bisabolol | 1663 | 1672 | 2199 | 0.7 | RI, MS |
| 27 | Eudesma-4(15)-7-dien-1-β-ol | 1681 | 1667 | 2333 | 8.2 | RI, MS |
| 28 | 14-hydroxy-α-Muurolene | 1755 | 1755 | 2599 | 0.3 | RI, MS |
| 29 | 14-hydroxy-τ-Cadinene | 1788 | 1784 | 2607 | 0.2 | RI, MS |
| 31 | Hexadecanoic acid | 1942 | 1941 | 2930 | 0.2 | RI, MS |
| 32 | Falcarinol | 2028 | 2026 | - | 2.8 | RI, MS, [23] |
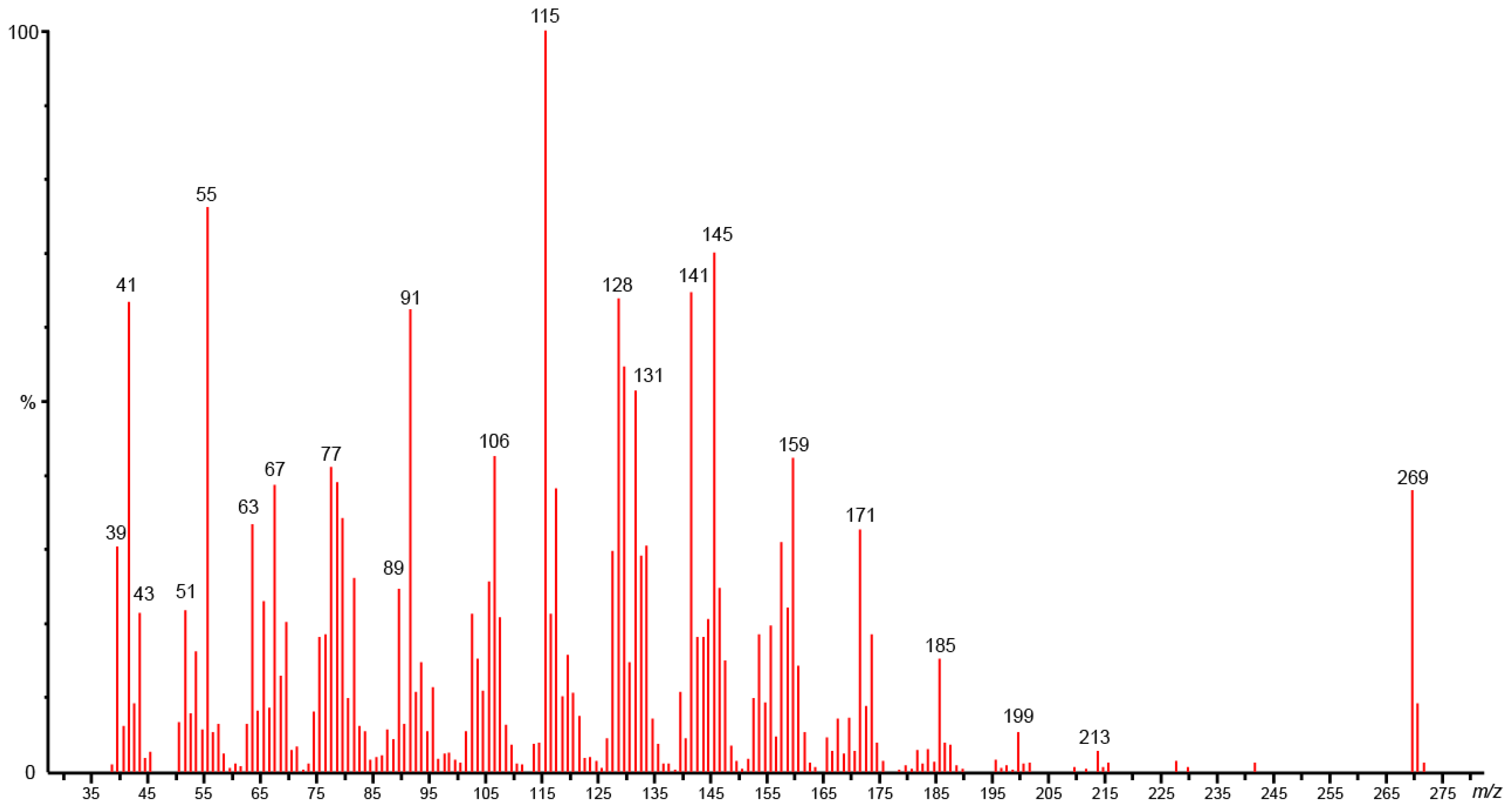
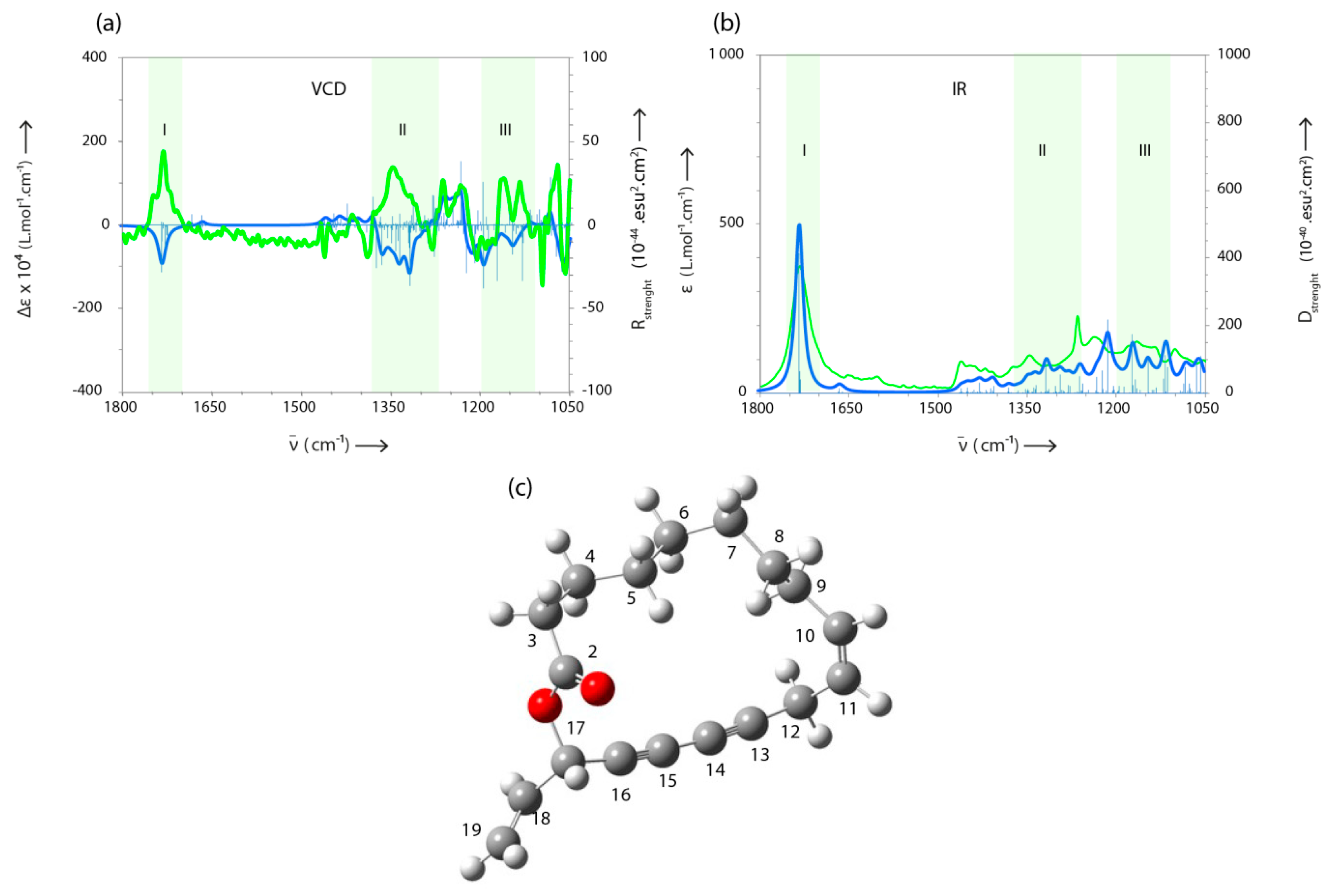
| 33 | Campestrolide | - | 2143 | 2970 | 23.0 | RI, MS, NMR |
| 34 | Falcarindiol | 2190g | 2164 | - | 9.4 | RI, MS, NMR |
| Total identification (%) | 84.0 | |||||
| Hydrocarbon compounds | 31.2 | |||||
| Oxygenated compounds | 52.8 | |||||
| Hydrocarbon sesquiterpenes | 31.2 | |||||
| Oxygenated sesquiterpenes | 17.4 | |||||
| Non terpenic compounds | 3.0 | |||||
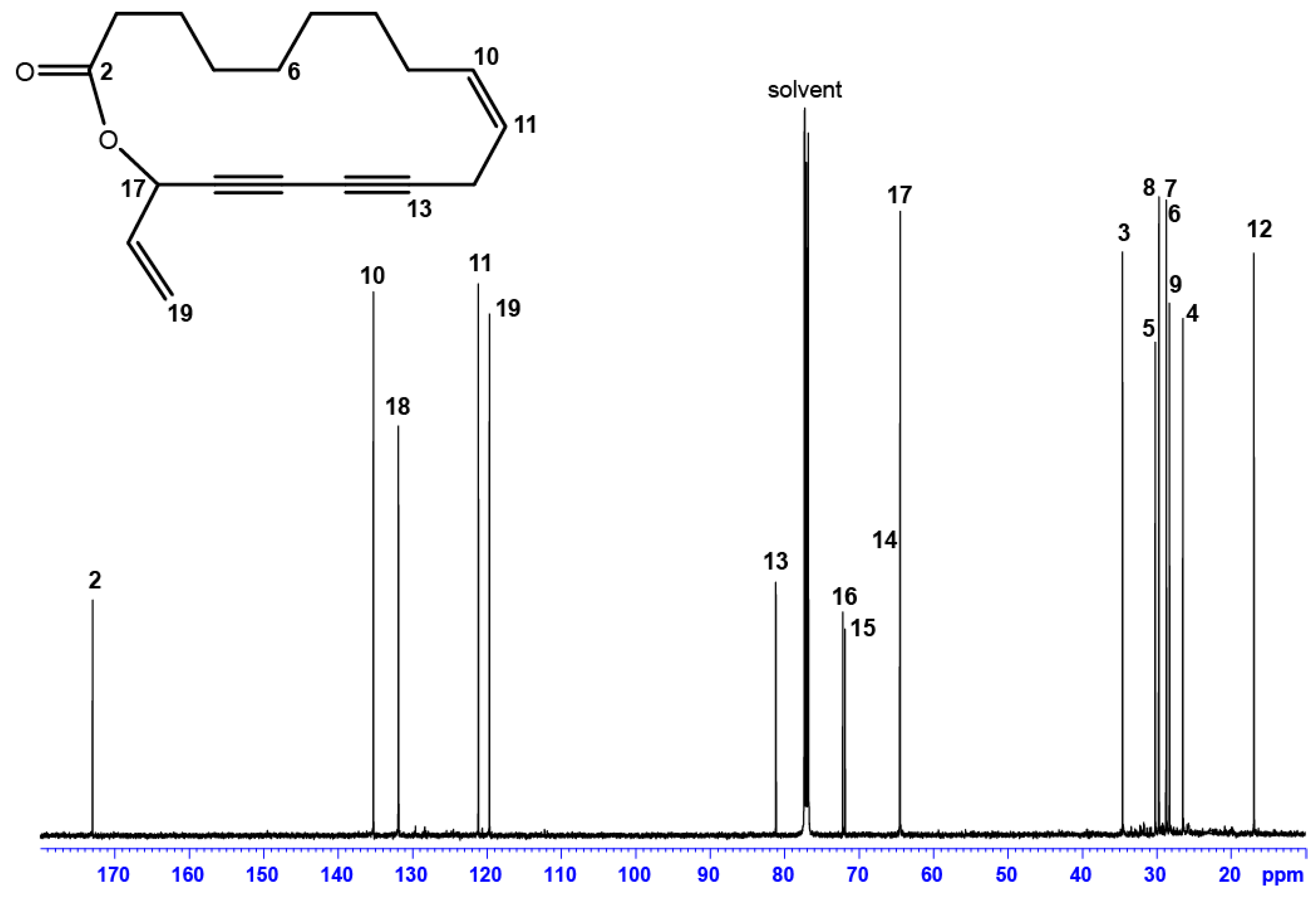
| Position | δC, Type | δH (J in Hz) | HMBC a |
|---|---|---|---|
| 2 | 172.97, C | - | 3, 4, 17, 18 |
| 3 | 34.55, CH2 | 2.41, m | 4, 5 |
| 2.38, m | |||
| 4 | 26.45, CH2 | 1.76, m | 3, 5, 6 |
| 1.67, m | |||
| 5 b | 28.68, CH2 | 1.44, m | 3, 4, 6, 7 |
| 6 | 29.67, CH2 | 1.28, m | 4, 5, 7, 8 |
| 7 | 30.17, CH2 | 1.36, m | 5, 8, 9 |
| 8 b | 28.70, CH2 | 1.44, m | 6, 7, 8 |
| 9 | 28.26, CH2 | 2.10, m | 8, 10, 11 |
| 2.15, m | |||
| 10 | 135.23, CH | 5.62, m | 8, 9, 12, |
| 11 | 121.13, CH | 5.52, dt (9.96, 7.53) | 9, 12 |
| 12 | 16.91, CH2 | 2.96, dd (18.30; 7.50) | 10, 11 |
| 3.07, dd (18.30; 7.50) | |||
| 13 | 81.15, C | - | 11, 12 |
| 14 | 64.51, C | - | 12, 17 |
| 15 | 71.87, C | - | 12, 17 |
| 16 | 72.15, C | - | 12, 18, 19 |
| 17 | 64.45, CH | 5.92, d (5.85) | 18, 19 |
| 18 | 131.88, CH | 5.91, ddd (5.85; 10.17; 16.90) | 17, 19 |
| 19 | 119.65, CH2 | 5.37, dd (16.90; 1.2) | 18 |
| 5.60, dd (10.17; 1.2) |
| Cytotoxicity | Antiparasitic Activity | Selectivity Index | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IC50 ± SD in µg/mL (µM for Pure Compound) | IC50 WI38/IC50 Parasite | ||||||||
| WI38 | J774 | Tbb | Lmm | Tbb | Lmm | ||||
| Hexanic extract | 4.44 ± 0.94 | 4.00 ± 1.07 | 3.00 ± 0.88 | 3.86 ± 0.10 | 1.5 | 1.2 | |||
| 33 | 5.20 ± 0.24 | 4.84 ± 0.10 | 0.59 ± 0.08 | 3.43 ± 0.02 | 8.9 | 1.5 | |||
| (19.24 ± 0.87) | (17.89 ± 0.35) | (2.17 ± 0.28) | (12.67 ± 0.09) | ||||||
| Positive control | 0.036 ± 0.022 | 0.007 ± 0.005 | 0.031 ± 0.012 | 0.057 ± 0.008 | |||||
| (0.103 ± 0.062) a | (0.021 ± 0.013) a | (0.022 ± 0.008) b | (0.097 ± 0.014) c | ||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medbouhi, A.; Tintaru, A.; Beaufay, C.; Naubron, J.-V.; Djabou, N.; Costa, J.; Quetin-Leclercq, J.; Muselli, A. Structural Elucidation and Cytotoxicity of a New 17-Membered Ring Lactone from Algerian Eryngium campestre. Molecules 2018, 23, 3250. https://doi.org/10.3390/molecules23123250
Medbouhi A, Tintaru A, Beaufay C, Naubron J-V, Djabou N, Costa J, Quetin-Leclercq J, Muselli A. Structural Elucidation and Cytotoxicity of a New 17-Membered Ring Lactone from Algerian Eryngium campestre. Molecules. 2018; 23(12):3250. https://doi.org/10.3390/molecules23123250
Chicago/Turabian StyleMedbouhi, Ali, Aura Tintaru, Claire Beaufay, Jean-Valère Naubron, Nassim Djabou, Jean Costa, Joëlle Quetin-Leclercq, and Alain Muselli. 2018. "Structural Elucidation and Cytotoxicity of a New 17-Membered Ring Lactone from Algerian Eryngium campestre" Molecules 23, no. 12: 3250. https://doi.org/10.3390/molecules23123250
APA StyleMedbouhi, A., Tintaru, A., Beaufay, C., Naubron, J.-V., Djabou, N., Costa, J., Quetin-Leclercq, J., & Muselli, A. (2018). Structural Elucidation and Cytotoxicity of a New 17-Membered Ring Lactone from Algerian Eryngium campestre. Molecules, 23(12), 3250. https://doi.org/10.3390/molecules23123250





