Abstract
The chemical composition of a hexanic extract of Eryngium campestre, obtained from its aerial parts, was investigated by GC-FID, GC/MS, HRMS, NMR and VCD analyses. The main compounds were germacrene D (23.6%), eudesma-4(15)-7-dien-1-β-ol (8.2%) and falcarindiol (9.4%), which are associated with a new uncommon and naturally found 17-membered ring lactone. This 17-membered ring features conjugated acetylenic bonds, named campestrolide (23.0%). The crude extract showed moderate antitrypanosomal (Trypanosoma brucei brucei), antileishmanial (Leishmania mexicana mexicana) and anticancer (cancerous macrophage-like murine cells) activities, and also displayed cytotoxicity, (human normal fibroblasts) in similar concentration ranges (IC50 = 3.0, 3.9, 4.0 and 4.4 µg/mL respectively). Likewise, campestrolide displayed low activity on all tested cells (IC50: 12.5–19.5 µM) except on Trypanosoma, on which it was very active and moderately selective (IC50 = 2.2 µM. SI= 8.9). In conclusion, the new compound that has been described, displaying a singular structure, possesses interesting antitrypanosomal activity that should be further investigated and improved.
1. Introduction
The genus Eryngium, the most common of the Apiaceae family, comprises more than 250 species with cosmopolitan distribution in temperate regions of all continents, mainly in Eurasia, North Africa and South America [1]. This genus has been the subject of several phytochemical investigations. A remarkable richness in natural chemicals with interesting bioactivities was reported in the literature: terpenoids, polyacetylenes, saponins, steroids and phenolics (such as, flavonoids and coumarins) [2]. This phytochemical diversity can explain the large traditional uses of many Eryngium species in the treatment of emetic and gastrointestinal infections [3], several types of inflammatory disorders [4] and various parasitic infections [5]. More traditional uses of many Eryngium species include use in antidotes for poisons, hypoglycemic agents [6], diarrhea remedies, stimulants, aphrodisiacs, antitussives and diuretics [7].
Among this large variety of species is the E. campestre species (Figure 1), which is a perennial plant that measures from 30 to 60 cm in length. This species is widespread in Western and Central Europe, North Africa, the Middle East and the Caucasus [8]. The plant has been used in European herbal medicine as an infusion for the treatment of whooping cough, as well as in the treatment of kidney and urinary tract inflammations [9].

Figure 1.
Aerial parts of Erygium campestre.
Many compounds belonging to different phytochemical classes were already identified in this plant. Some of these were flavonoids and flavonoacyl derivatives, extracted from the aerial parts and the roots [10,11,12]; monoterpene glycosides with cyclohexanone moiety [13]; and a coumarin derivative [14] and triterpene saponins were found in the roots [15]. E. campestre essential oils displayed a complex chemical composition with hydrocarbon and oxygenated sesquiterpenes. The main components were germacrene D, β-curcumene, (E)-β-farnesene, spathulenol, α-bisabolol and α-cadinen-15-al [16,17].
E. campestre has been the subject of many biological investigations. The methanol extracts from the aerial parts showed very strong antitumoral activity on potatoes’ tumor cells induced by Agrobacterium tumefaciens (ATCC 23341), but no significant antimicrobial activity [18]. The flavonol-rich methanol extracts of E. campestre aerial parts exhibited moderate to strong antioxidant activity in DPPH radical scavenging and reducing power assays; in contrast, no effect on Alzheimer’s disease was reported [19]. Ethanol extracts, obtained from the aerial parts and the roots, revealed noticeable anti-inflammatory and antinociceptive activities [20].
Protozoan neglected diseases, such as African trypanosomiasis and cutaneaous leishmaniasis, are tropical infections affecting more than one billion people worldwide and lacking financial investments. The current available treatments suffer from some toxicity, administration difficulty and even resistance development. In this area, the plants and their bioactive secondary metabolites constitute a potential source of crucially needed new and effective drugs [21,22].
As part of our research work on bioactive metabolites from Algerian plants, phytochemical investigation of the hexane extracts obtained from the E. campestre aerial parts was performed. This study describes the isolation and structural elucidation of a new 17-membered ring lactone using chromatography techniques (GC-FID, GC-MS), HRMS, 1D and 2D NMR experiments and circular dichroism, along with two known polyacetylenes: falcarinol and falcarindiol. The cytotoxicity and some antiprotozoal activities of the bulk extract and the newly isolated macrocyclic lactone were then investigated in vitro, in order to evaluate their potential pharmacological properties.
2. Results and Discussion
2.1. Chemical Composition
The analysis of the filtered hexane extract of the E. campestre (HEEC) aerial parts using hyphenated methods allowed the identification of 34 components, which accounted for 84.0% of the total composition (Table 1). The chemical composition was dominated by the oxygenated compounds (52.8%). The principal classes were sesquiterpenes (48.6%) and polyacetylenes (35.2%). Hydrocarbon compounds were exclusively represented by sesquiterpenes (31.2%). The main components were germacrene D 9 (23.6%), unknown compounds 33 (23.0%), 34 (9.4%), eudesma-4(15)-7-dien-1-β-ol 27 (8.2%) and falcarinol 32 (2.8%). Thirty-two components were identified by comparison of their RI and MS data with those from our home library, “Arômes”. The spectrometric data of 33 and 34 were not found in our MS-library. Their identifications were achieved after purification by column chromatography, using a combination of analytical techniques: GC-FID, GC/MS-EI, HRMS, exhaustive NMR characterization and VCD. The structures of the main components that were identified are presented in Figure 2. The structure elucidation of 33 will be presented and discussed separately.

Table 1.
The chemical composition of the hexane extract of Eryngium campestre (HEEC).
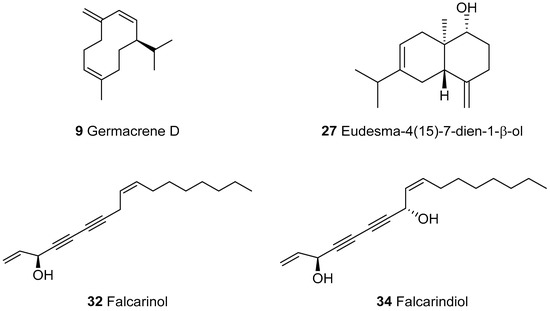
Figure 2.
The main components identified in the hexane extract of E. campestre, from Algeria.
2.2. Identification of Compounds Not Present in MS-Libraries
Column chromatography was carried out using a gradient of polarity with hexane and diisopropyl ether and then followed by UV detection, producing 75 fractions from the hexane extract of E. campestre. Among them, 34 (8 mg of an almost pure sample) was isolated in the fraction F34 obtained with diisopropyl ether/hexane (10/100). EI mass spectra of 34 exhibited a base peak at m/z 129 and a signal at m/z 260, which could be attributed to the molecular ion. ESI (+)-HRMS measurement confirms the molecular formula of C17H24O2 (detected ion C17H24O2Na+ (m/z)experimental 283.1668 and (m/z)theoretic 283.1667; error +0.4 ppm). Additionally, the acquired 13C-NMR spectra showed the presence of both oxygenated sp3 methyne groups detected at 63.50 ppm and 58.61 ppm, respectively, and four sp carbon atoms detected between 79.86 ppm and 68.69 ppm. These observations lead us to an oxygenated polyacetylene skeleton, a derivative of falcarinol 32. A comparison of mass spectra and NMR data in the literature [26] allowed the identification of falcarindiol (Figure 2—34). This polyacetylenic compound, accounting for 9.4% of the E. campestre hexane extract, is a main component of the roots of Angelica japonica [26], Daucus carota [27], and Petroselinum crispum [Mill.] Nym. ssp. tuberosum [25]. Its identification in the Eryngium species was first reported by Bohlmann and Zdero [28] in ether extracts of E. alpinum, E. coeruleum and E. giganteum. However, this is the first time it has been identified in E. campestre. Falcarindiol has been shown to have anti-inflammatory, antibacterial and anticancer activities, as well as protective effects against hepatotoxicity [29,30].
Column chromatography using the gradient of solvent diisopropyl ether/hexane (5/95) produces a yellow, odorous, oily compound (F21: 13 mg, 33 at 96%). GC/MS-EI spectra of 33 exhibited an isotopic cluster at m/z 270 and 271, and a base peak at m/z 115. Other fragment ions were observed at m/z 55, 91, 128 and 145 (Figure 3). The ESI (+)-HRMS measurement performed on 33 reveals the elemental composition of C18H22O2 (detected ion: C18H22O2Na+: (m/z)experimental 293.1513 and (m/z)theoretic 293.1512. error +0.3 ppm), which would correspond to a compound possessing an additional carbon atom and one supplementary unsaturation (DBE: double bounds equivalent = 8) as compared to the falcarindiol 34 (C17H24O2, DBE = 7). The IR spectrum exhibits absorption peaks at 1739 cm−1, 1098 cm−1 and 1225 cm−1, corresponding to C=O and C-O vibrations. This indicates the presence of a cyclic lactone function. Additionally, absorption bands at 2255 cm−1 and 2856–2928 cm−1 indicated the presence of C≡C and CH2 bonds, respectively.
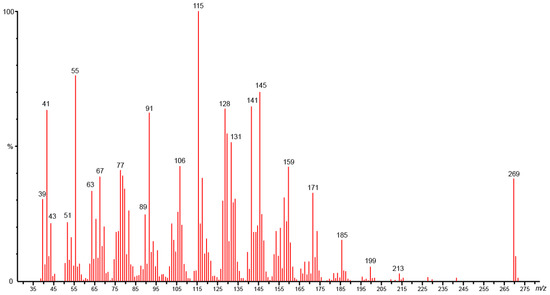
Figure 3.
Electronic impact mass spectrum of campestrolide 33 (70 eV).
A rapid analysis of the 1H- and 13C-NMR spectra (Figure 4) showed the absence of -CH3 groups and the presence of a ketone group (172.98 ppm). Furthermore, the characteristic resonances of a polyacetylene skeleton (constituted by a sequence of two consecutive triple bonds) are identified using the 13C-NMR spectrum in combination with DEPT data (Table 2).
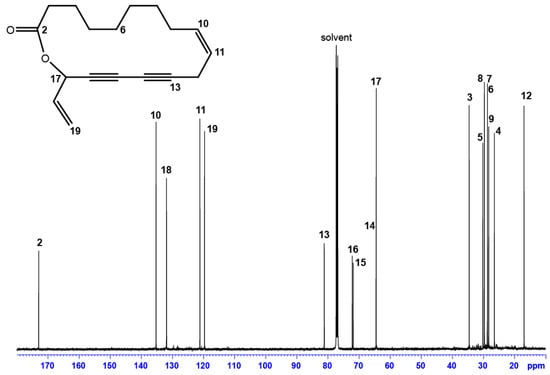
Figure 4.
13C-NMR spectrum (125.76 MHz in CDCl3, 300 K) and structure (inset) of Campestrolide 33 (N.B. NMR signal assignments refer to the numbering of the molecule illustrated on the inset; the stereochemistry of 17-CH would be revealed subsequent to the VCD experiments).

Table 2.
NMR spectroscopic data (500 MHz, CDCl3) for Campestrolide (33).
The complete assignment was achieved using the 2D NMR spectra (Figures S1–S3 in the supplementary information with this article). In addition, NOE correlations showed spatial vicinity among the aliphatic chain and the unsaturated part of the molecule, in particular between 3-CH2 → 17-CH and 7-CH2 → 10-CH (Figure S4). The combination of all spectroscopic data allowed the recognition of a seventeen-atom macrocyclic lactone and its structure is represented below (Figure 4—inset). This unprecedented 17-membered ring lactone, featuring conjugated acetylenic bonds, has been named Campestrolide. Nevertheless, the analytical data do not establish the determination of the absolute configuration of 17-CH, which has since been established by vibrational circular dichroism (VCD).
In the last decade, VCD spectroscopy has become a popular technique for the elucidation of absolute configurations of chiral molecules [31,32,33]. Therefore, in order to determine the stereochemistry of the asymmetrical carbon-17 of Campestrolide, the VCD spectrum of a sample containing 80% of compound 33 was measured in CD2Cl2. Despite the difficulties encountered during the determination of the absolute configuration of a molecule via the VCD spectrum of a mixture, the majority of the measured bands were in good agreement with those obtained by calculation performed on the (Z,S)-enantiomer (region I, II and III in the VCD spectrum—Figure 5a). Moreover, for the same spectral regions, a good accordance is also observed between the experimental IR spectrum recorded for the analyzed sample and the theoretical IR spectrum calculated for the (Z,S)-enantiomer (Figure 5b—region I, II and III). This observation reinforces the assignment of these bands to our molecule. Based on the analysis of the spectral data and taking into account the opposite signs between the measured and calculated VCD spectra, we could conclude that the naturally obtained Campestrolide is detected as (R)-enantiomer (Figure 5c), namely (Z)-17(R)-vinyloxacycloheptadeca-10-en-13,15-diyn-2-one. It should be mentioned that the identified configuration is perfectly consistent with those generally reported in the literature for polyacetylenes derivatives [33,34,35].
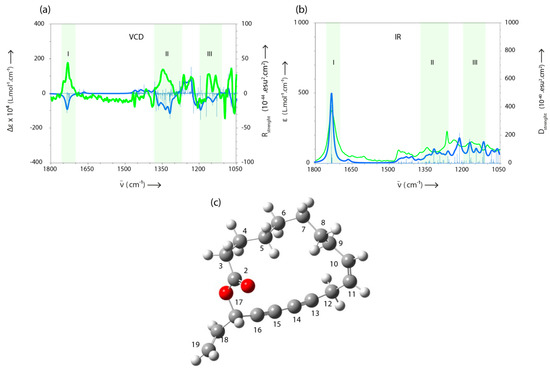
Figure 5.
A comparison between the measured (blue) and calculated (green) (a) VCD and (b) IR spectra, respectively; (c) one of the most stable simulated structures of Campestrolide (generated for (Z,S)-enantiomer). N.B. Atoms colors: oxygen in red; carbon in gray; hydrogen in white.
To our knowledge, this uncommon natural macrocyclic lactone that includes one cyclo-1,3-diynes motif has only been reported in one other compound (ivorenolide B), which was extracted from Khaya ivorensis—a dicotyledone tree from the Meliaceae family [33,36]. Furthermore, this compound, as well as its 18-memberded ring lactone analogous ivorenolide A, showed significant immunosuppressive activity [33,37,38,39]. Other macrocyclic lactones (20-membered ring) have been extracted from the marine dinoflagellate Amphidinium species. Researchers have reported cytotoxic properties for all the described amphidinolides mentioned above [40].
2.3. Biological Activities
Both the crude hexanic extract of E. campestre and compound 33 were evaluated for their in vitro cytotoxicity and antiprotozoal activities (Table 3). The plant extract showed similar moderate activities (IC50 ≈ 4 µg/mL) on all tested cells and hence, without any selectivity for the concerned parasites. The newly identified macrocyclic lactone was strongly active on Trypanosoma (IC50 = 0.6 µg/mL or 2.2 µM ≈ 2 µM), as defined by Beaufay et al. (IC50 ≤ 2 µM) [41], with a reasonable selectivity (SI = 8.9). As such, it fits the stated criteria for antiparasitic hits [42], i.e., activity in vitro with an IC50 < 1 µg/mL and at least ten-fold higher than on a mammalian cell line. As the calculated campestrolide log P value (log P = 5.56) [43] was close to the one reported for falcarinol (log P = 5.50) [44], we can assume good membrane permeability of the macrocyclic lactone. Its newly described structure could also be used as a prototype in structure–activity relation studies to improve activity/selectivity.

Table 3.
The cytotoxicity (WI38 and J774), antitrypanosomal (Tbb) and antileishmanial (Lmm) activities expressed in the IC50 (Mean ± SD in µg/mL and μM for pure compounds from at least six values).
The antileishmanial or anticancer activities were notable (IC50 = 3.4 and 4.8 µM, respectively) but not as selective as those observed for the extract. Another well-known complex polyene macrolide is amphotericin B (IC50 = 0.09 ± 0.06 µM), a reference drug widely used to treat visceral leishmaniasis but still little studied on cutaneaous one and with dose-limiting toxicity [45]. Some patents are also based on different kinds of macrolides as antiparasitics [46].
Campestrolide can partially explain all the activities observed for the crude extract but it is certainly not the only active component of the mixture, as observed with the other samples containing different percentages of campestrolide (data not shown). Therefore, other components, e.g., the identified polyacetylenes, could influence activities by addition or synergism action. This has already been observed, for example, with the polyphenolic compounds [47]. Indeed, natural polyacetylenes are highly bioactive and reactive phytochemicals and were largely isolated from the Apiaceae family, mostly due to the aliphatic C17 chains. Unstable compounds of this variety are known to exhibit some cytotoxic behaviors and an extract, rich in these unsaturated derivatives, has already been shown to possess synergetic cytotoxic activity in combination with taxol [48]. Previous researchers have also shown such compounds to possess encouraging potential against protozoan diseases. However, this requires further investigation [21]. Falcarindiol, another major compound of the crude extract, has yet to be tested for antitrypanosomal and antileishmanial activity. However, Falcarinol, also called panaxynol, was found to be strongly active and selective (IC50 = 0.01 µg/mL, corresponding to 0.04 µM, and SI = 858) on Trypanosoma brucei brucei compared to HeLa cytotoxicity. The inhibition of the trypanothione reductase was proposed as one target of this very reactive alkylating agent [49]. Another two natural polyacetylenes, 8-hydroxyheptadeca-1-ene-4,6-diyn-3-yl ethanoate and 16-acetoxy-11-hydroxyoctadeca-17-ene-12,14-diynyl ethanoate, were highly active and selective on Trypanosoma brucei rhodesiense, T. cruzi, Plasmodium falciparum and Leishmania donovani axenic and the infected macrophages amastigotes compared on L6 rat skeletal myoblasts (IC50 = 0.1–2.5 µM. SI > 10). The terminal methylene double bond seems to improve activity [50].
In addition, germacrene D, another major identified compound, possesses some antiparasitic activity as with other non-lactonized sesquiterpenoids and eudesmanolides, with 61% growth inhibition of the T. cruzi trypomastigotes at 100 µg/mL. It also inhibited cruzain, an essential T. cruzi cysteine protease, with an IC50 of 22.1 µg/mL [21].
Concerning the reported plant extract, dichloromethane and methanol compounds from the aerial parts of the E. campestre displayed antileishmanial activity with IC50 values of 36 and 15 µg/mL, respectively, on L. donovani promastigotes. They were inactive on the P. falciparum D6 and W strains [51]. However, the petroleum ether extract exhibited more than 50% Plasmodium growth inhibition at 4.81 µg/mL [23]. Other Eryngium species have previously shown interesting antiparasitic activities (IC50 < 20 µg/mL) on T. cruzi [50] and L. donovani, but appeared less active or inactive on Plasmodium falciparum [52,53]. A daucane sesquiterpene with moderate antileishmanial activity (IC50 values of 14.33 and 7.84 µM on L. tarentolae promastigotes and L. donovani amastigotes respectively) was isolated from E. foetidum aerial parts [5].
3. Materials and Methods
3.1. Plant Material
Aerial parts of Eryngium campestre L. (Apiaceae) were collected in June 2016, from the north-western areas of Algeria. The botanical identification of the plants was performed according to the botanical determination keys summarized in the Flora of Algeria [54] and a voucher specimen has been deposited in the COSNA laboratory (Voucher Code: EC1-06-2016).
3.2. Extraction
The dried aerial parts (120 g) were powdered and weighed, then extracted with hexane (500 mL) under reflux in a Soxhlet apparatus, at 68 °C. The extract was first filtered on filter paper and then on PTFE filters (0.2 µm). After that, the extract was evaporated to dryness under reduced pressure. This resulted in a yield of 1.05%.
3.3. Compound Identification
The identification of individual compounds in the E. campestre hexanic extract was carried out using different techniques, such as GC/FID, GC/MS (EI) and 1D and 2D NMR. The identifications were mainly based on comparison of the retention indices (RI) and the MS spectra with those contained in our laboratory library “Arômes”. When spectral data were not present in the “Arômes” library, the RI and the MS data were compared with those from commercial libraries [24,55,56,57]. Compounds absent from consulted libraries were isolated by the fractionation process, using flash column chromatography and then identified mainly by NMR spectroscopy.
3.4. Fractionation
We measured 300 mg of the hexanic extract. This was then submitted to chromatography on a silica gel column (200–500 µm, 4 g, Clarisep® Bonna Agela Technologies, Willington, CT, USA), using an Automatized Combi Flash apparatus (Teledyne ISCO. Lincoln., NE, USA) equipped with a fraction collector and monitored by a UV detector. The solvents of elution were n-hexane (A) and diisopropyl ether (B), with a gradients of elution: a (A: 100%; B: 0%), b (A: 98%; B: 2%), c (A: 96.5%; B: 3.5%), d (A: 95%; B: 5%), e (A: 90%; B: 10%) and f (A: 0%; B: 100%).
3.5. GC-FID Conditions
Analyses were carried out using a Perkin-Elmer Autosystem XL GC apparatus (Walthon, MA, USA), equipped with a dual flame ionization detection (FID) system and fused-silica capillary columns, namely, Rtx-1 (polydimethylsiloxane) and Rtx-wax (polyethyleneglycol) (60 m × 0.22 mm i.d.; of a film thickness of 0.25 µm). The oven temperature was programmed from 60 to 230 °C at 2 °C/min and then held isothermally at 230 °C for 35 min and helium was employed as the carrier gas (1 mL/min). The injector and the detector temperatures were maintained at 280 °C and the samples were injected (0.2 µL of pure oil) in the split mode (1:50). The RI of each compound was determined relative to the retention times of a series of n-alkanes (C5–C30) by linear interpolation, using the Van den Dool and Kratz (1963) equation with the aid of the software from Perkin-Elmer (TotalChrom navigator, 6.3.1, Shelton, CT, USA). The relative percentages of the extract constituents were calculated from the GC peak areas by the normalization procedure, without the application of correction factors.
3.6. GC/MS-EI Conditions
Samples were analyzed with a Perkin-Elmer Turbo mass detector (quadrupole) coupled to a Perkin-Elmer Autosystem XL, equipped with fused-silica capillary columns Rtx-1 and Rtx-Wax. The oven temperature was programmed from 60 to 230 °C at 2 °C/min and then held isothermally at 230 °C (35 min) and helium was employed as the carrier gas (1 mL/min). The following chromatographic conditions were employed: the injection volume was 0.2 µL of pure oil; the injector injector temperature was 280 °C; split 1:80; the ion source temperature was 150 °C; the ionization energy was 70 eV; the MS (EI) data were acquired over the mass range 35–350 Da; and the scan rate was 1 s.
3.7. NMR Conditions
NMR experiments were acquired in CDCl3 (EuroIsotop, Saint Aubin, France) at 300 K using a Bruker Avance DRX 500 NMR spectrometer (Karlsruhe, Germany), operating at 500.13 MHz for 1H and 125.76 MHz for 13C Larmor frequency with a double resonance broadband fluorine observe (BBFO) and 5 mm probehead. 13C-NMR experiments were recorded using the one-pulse excitation pulse sequence (90° excitation pulse), with 1H decoupling during signal acquisition (performed with WALTZ-16). The relaxation delay was set at 2 s. For each analysed sample, depending on the compound concentration, 3 k up to 5 k free induction decays (FID) 64 k complex data points were collected using a spectral width of 30,000 Hz (240 ppm). Chemical shifts (δ in ppm) were reported relative to the residual signal of CDCl3 (δC = 77.04 ppm and δH = 7.26 ppm, respectively). Complete 1H and 13C assignments of the requested compounds were obtained using 2D gradient-selected NMR experiments, 1H-1H COSY, 1H-13C HSQC, 1H-13C HMBC and 1H-1H NOESY, for which conventional acquisition parameters were used, as described in the literature [58].
3.8. High-Resolution Mass Spectrometry Experiments
High-resolution mass spectrometry (HRMS) experiments were performed with a Synapt G2 HDMS quadrupole/time-of-flight (Manchester, UK), equipped with an electrospray source operating in the positive mode, ESI(+). The samples were introduced at a 10 µL min−1 flow rate (capillary voltage +2.8 kV, sampling cone voltage +20V under a curtain gas (N2), flow of 100 L h−1 and heated at 35 °C). The samples were dissolved and further diluted in methanol (Sigma-Aldrich, St.-Louis, MO, USA) doped with sodium chloride (0.1 mM) prior to the analysis. Accurate mass experiments were performed using reference ions from an internal calibration procedure, with two reference ions formed upon electrospray of a poly (ethylene oxide) (PEO). Data analyses were conducted using MassLynx 4.1 programs, which were provided by Waters (Manchester, UK).
3.9. VCD Measurements
The infrared (IR) and vibrational circular dichroism (VCD) spectra were recorded on a Bruker PMA 50 accessory, coupled to a Vertex 70 Fourier transform infrared spectrometer (Bruker, Wissembourg, Germany). A photoelastic modulator (Hinds PEM 90, Hids Instruments, Portland, OR, USA) set at l/4 retardation was used to modulate the handedness of the circular polarized light at 50 kHz. Demodulation was performed by a lock-in amplifier (SR830 DSP, Zurich Instruments, Zurich, Switzerland). An optical low-pass filter (<1800 cm−1) was used to enhance the signal/noise ratio before use of the photoelastic modulator. A transmission cell of 200 μm optical pathlength, equipped with CaF2 windows, was used. The solutions with a concentration of 0.1 mol L−1 were prepared by dissolving the solid samples in CD2Cl2. The VCD spectrum of the enantiomer was measured at room temperature using a sample with an estimated purity of 80%. The baseline of the VCD spectrum was corrected by the subtraction of the VCD spectrum of the solvent. For each individual spectrum, about 16,000 scans were averaged at 4 cm−1 resolution (corresponding to 4 h of measurement time). For the IR absorption spectra, the cell filled with CD2Cl2 served as a reference. The spectra are presented without any smoothing and further data processing.
The calculation of the VCD and IR spectra was performed on the (S)-enantiomer of compound 33 using Gaussian 16 package [59]. The geometry optimizations, vibrational frequencies, IR absorption, and VCD intensities were calculated with the Density Functional Theory (DFT) using the B3LYP functional combined with the 6-311G (d,p) basis set. The average solvent (CH2Cl2) effects have been introduced using the implicit solvation model, SMD, which is based on the integral equation formalism of the polarizable continuum model (ief-pcm). The computed harmonic frequencies are generally larger than the fundamentals observed experimentally. They were calibrated using a scaling factor of 0.98. The IR absorption and the VCD spectra were constructed from the calculated dipole and the rotational strengths assuming the Lorentzian band shape with a half-width at a half-maximum of 8 cm−1. The computational method is detailed in the supplementary information with this article.
3.10. Biological Assays
The crude extract and the newly identified pure compound (91.9%), campestrolide, were evaluated in vitro for their cytotoxicity on two mammalian cell lines: normal human fibroblasts (WI38) and cancerous macrophage-like murine cells. Their antiprotozoal activities were also investigated on Trypanosoma brucei brucei (Tbb. strain 427) bloodstream forms and Leishmania mexicana mexicana (Lmm. MHOM/BZ/84/BEL46) promastigotes. The tests were performed using [60,61] stock solutions prepared in DMSO at 10 mg/mL. For the cytotoxicity assay, the samples were tested in eight serial 1.7-fold dilutions (150 µL transferred into 100 µL fresh medium) in the 96-well microtiter plates (concentration range: 0.7–25 µg/mL). The camptothecin (Sigma-Aldrich, St.-Louis, MO, USA) was used as a positive control with 5-fold dilutions (concentration range: 0.00032–25 µg/mL) from a 10 mg/mL DMSO stock solution. For the antiparasitic assays, the samples were tested in eight serial 2-fold dilutions (concentration range: 0.20–25 or 0.10–12 µg/mL on Leishmania and Trypanosoma, respectively). Suramine sodium and pentamidine isethionate salts (Sigma-Aldrich, St.-Louis, MO, USA) were used as a positive control with 3-fold dilutions (0.0046–10 µg/mL) from a stock solution of 2 mg/mL as well as amphotericin B. A maximum of 0.5% DMSO was previously verified to be non-toxic in all the biological assays. The IC50 values were determined using Microsoft Excel and GraphPad Prism 7.0 software (GraphPad, San Diego, CA, USA), based on a nonlinear regression. The selectivity index was calculated in comparison to the WI38 cytotoxicity to assess the therapeutic properties as antiparasitic or anticancer hits.
4. Conclusions
A new uncommon macrocyclic lactone named campestrolide, along with other known compounds, was isolated and identified in the Eryngium campestre hexane crude extract. This newly described compound presents a particular structure that is composed of a rigid 1,3-dynes motif and a flexible aliphatic part. Moreover, campestrolide possesses relatively strong antitrypanosomal activity but moderate selectivity. Therefore, we assume that campestrolide could be considered for further investigation to improve activity/selectivity. Furthermore, it is partially responsible for the observed antiprotozoal activities and cytotoxicity in the crude extract.
Supplementary Materials
The following are available online: 1H-NMR spectrum and 2D-NMR data of campestrolide, computational details, enthalpies and Boltzmann populations of calculated conformations, and additional comparison between calculated and experimental IR and VCD data.
Author Contributions
A.M. (Alain Muselli), A.T., J.Q.-L. and C.B. conceived and designed the experiments. A.M. (Ali Medbouhi), A.T., C.B. and J.-V.N. performed the experiments. A.M. (Ali Medbouhi), A.M. (Alain Muselli), J.Q.-L, C.B. and A.T. wrote the manuscript. N.D. and J.C. revised the manuscript. All authors read and approved the final manuscript.
Funding
This research received no external funding.
Acknowledgments
A.M. (Ali Mehboudi) is indebted to the Ministère des Affaires Etrangères et du Développement International for the research grant (Campus France, Programme d’Excellence Eiffel) and to the Ministère de l’Europe et des Affaires Etrangères for the research grant (PROFAS B+). A.T. acknowledges support from Spectropole, the Analytical Facility of Aix Marseille University, for allowing a special access to the instruments purchased with the European Funding (FEDER OBJ2142-3341).
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Wörz, A. A new subgeneric classification of the genus Eryngium L. (Apiaceae, Saniculoideae). Bot. Jahrb. Syst. 2005, 126, 253–259. [Google Scholar] [CrossRef]
- Wang, P. Phytochemical constituents and pharmacological activities of eryngium l. (Apiaceae). Pharm. Crop. 2012, 3, 99–120. [Google Scholar] [CrossRef]
- Moerman, D.E. Native American Food Plants. An Ethnobotanical Dictionary; Timber Press Inc.: London, UK, 2011; p. 65. [Google Scholar]
- Suciu, S.; Pârvu, A.E. Comparative Study on the Effects of Eryngium Sp. Extracts in an Acute Inflammation Model in Rat. Annals RSCB 2012, 17, 86–91. [Google Scholar]
- Rojas-Silva, P.; Graziose, R.; Vesely, B.; Poulev, A.; Mbeunkui, F.; Grace, M.H.; Kyle, D.E.; Lila, M.A.; Raskin, I. Leishmanicidal activity of a daucane sesquiterpene isolated fromeryngium foetidum. Pharm. Biol. 2014, 52, 398–401. [Google Scholar] [CrossRef]
- Jaghabir, M. Hypoglycemic effects of Eryngium creticum. Arch. Pharm. Res. 1991, 14, 295–297. [Google Scholar] [CrossRef]
- Goleniowski, M.E.; Bongiovanni, G.; Palacio, L.; Nuñez, C.; Cantero, J. Medicinal plants from the ‘Sierra de Comechingones’, Argentina. J. Ethnopharmacol. 2006, 107, 324–341. [Google Scholar] [CrossRef] [PubMed]
- Coste, H. Flore Descriptive et Illustrée de la France, de la Corse et des Contrées Limitrophes II; Librairie Scientifique et Technique Albert Blanchart: Le Val-Saint-Germain, France, 1980. [Google Scholar]
- Pieroni, A.; Pardo-de-Santayana, M.; Firenzuoli, F.; Quave, C.L. The european heritage of folk medicines and medicinal foods: Its contribution to the cams of tomorrow. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–2. [Google Scholar] [CrossRef]
- Kartnig, T.; Wolf, J. Flavonoids from the Aboveground Parts of Eryngium campestre. Planta Med. 1993, 58, 285. [Google Scholar] [CrossRef]
- Abou El-Kassem, L.; Hawas, U.; Awad, H.; Taie, H. Flavonoids from the aerial parts of eryngium campestre l. with antioxidant and anti-alzheimer activities. Planta Medica 2013, 79. [Google Scholar] [CrossRef]
- Hohmann, J.; Páll, Z.; Günther, G.; Máthé, I. Flavonolacyl glycosides of the aerial parts of eryngium campestre. Planta Medica 1997, 63, 96. [Google Scholar] [CrossRef]
- Erdelmeier, C.A.J.; Sticher, O. A cyclohexenone and a cyclohexadienone glycoside from eryngium campestre. Phytochemistry 1986, 25, 741–743. [Google Scholar] [CrossRef]
- Erdelmeier, C.; Sticher, O. Coumarin derivatives from eryngium campestre 1. Planta Med. 1985, 51, 407–409. [Google Scholar] [CrossRef]
- Kartal, M.; Mitaine-Offer, A.-C.; Abu-Asaker, M.; Miyamoto, T.; Calis, I.; Wagner, H.; Lacaille-Dubois, M.-A. Two new triterpene saponins from eryngium campestre. Chem. Pharm. Bull. 2005, 53, 1318–1320. [Google Scholar] [CrossRef]
- Abd-Elmonem, A.R.; Shehab, N. Study of the volatile oil of Eryngium campestre l. growing in Egypt. G. Bull. Fac. Pharm. Cairo Univ. 2008, 44, 3379–3388. [Google Scholar]
- Pala-Paul, J.; Usano-Alemany, J.; Soria, A.C.; Perez-Alonso, M.J.; Brophy, J.J. Essential oil composition of Eryngium campestre L. growing in different soil types. A preliminary study. Nat. Prod. Commun. 2008, 3, 1121–1126. [Google Scholar]
- Usta, C.; Yildirim, A.B.; Turker, A.U. Antibacterial and antitumour activities of some plants grown in turkey. Biotechnol. Biotechnol. Equip. 2014, 28, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Hawas, U.W.; El-Kassem, L.A.T.; Awad, H.M.; Taie, H.A.A. Anti-Alzheimer, Antioxidant Activities and Flavonol Glycosides of Eryngium campestre L. Curr. Chem. Biol. 2013, 7, 188–195. [Google Scholar] [CrossRef]
- Küpeli, E.; Kartal, M.; Aslan, S.; Yesilada, E. Comparative evaluation of the anti-inflammatory and antinociceptive activity of turkish eryngium species. J. Ethnopharmacol. 2006, 107, 32–37. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Khalid, S.; Romanha, A.; Alves, T.; Biavatti, M.; Brun, R.; Da Costa, R.; De Catsro, S. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases-part II. Curr. Med. Chem. 2012, 19, 2128–2175. [Google Scholar] [CrossRef]
- Franco Minguel, J.R. Report of the Second WHO Stakeholders Meeting on Rhodesiense Human African Trypanosomiasis; WHO/Department of Control of Neglected Tropical Diseases: Genève, Switzerland, 2017. [Google Scholar]
- Zimmermann, S.; Thomi, S.; Kaiser, M.; Hamburger, M.; Adams, M. Screening and HPLC-Based Activity Profiling for New Antiprotozoal Leads from European Plants. Sci. Pharm. 2012, 80, 205–213. [Google Scholar] [CrossRef]
- Joulain, D.; König, W.A. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; E.B.-Verlag: Hamburg, Germany, 1998. [Google Scholar]
- Nitz, S.; Spraul, M.-H.; Drawert, F. C17 Polyacetylenic alcohols as the major constituents in roots of Petroselinum crispum Mill. ssp. tuberosum. J. Agric. Food Chem. 1990, 38, 1445–1447. [Google Scholar] [CrossRef]
- Fujioka, T.; Furumi, K.; Fujii, H.; Okabe, H.; Mihashi, K.; Nakano, Y.; Matsunaga, H.; Katano, M.; Mori, M. Antiproliferative Constituents from Umbelliferae Plants. V. A New Furanocoumarin and Falcarindiol Furanocoumarin Ethers from the Root of Angelica japonica. Chem. Pharm. Bull. 1999, 47, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Dawid, C.; Dunemann, F.; Schwab, W.; Nothnagel, T.; Hofmann, T. Bioactive C17-Polyacetylenes in Carrots (Daucus carota L.): Current Knowledge and Future Perspectives. J. Agric. Food Chem. 2015, 63, 9211–9222. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C. Terpene derivatives from higher plants, XII. On new terpene aldehyde esters from Eryngium species. Chem. Ber. 1971, 104, 1957–1961. [Google Scholar] [CrossRef]
- Jin, H.R.; Zhao, J.; Zhang, Z.; Liao, Y.; Wang, C.-Z.; Huang, W.-H.; Li, S.-P.; He, T.-C.; Yuan, C.-S.; Du, W. The antitumor natural compound falcarindiol promotes cancer cell death by inducing endoplasmic reticulum stress. Cell Death Dis. 2012, 3, e376. [Google Scholar] [CrossRef] [PubMed]
- Meot-Duros, L.; Cérantola, S.; Talarmin, H.; Le Meur, C.; Le Floch, G.; Magné, C. New antibacterial and cytotoxic activities of falcarindiol isolated in crithmum maritimum l. leaf extract. Food Chem. Toxicol. 2010, 48, 553–557. [Google Scholar] [CrossRef]
- Batista, A.N.L.; dos Santos, F.M., Jr.; Batista, J.M., Jr.; Cass, Q.B. Enantiomeric Mixtures in Natural Product Chemistry: Separation and Absolute Configuration Assignment. Molecules 2018, 23, 492. [Google Scholar] [CrossRef]
- Said, M.E.-A.; Bombarda, I.; Naubron, J.-V.; Vanloot, P.; Jean, M.; Cheriti, A.; Dupuy, N.; Roussel, C. Isolation of the major chiral compounds from Bubonium graveolens essential oil by HPLC and absolute configuration determination by VCD. Chirality 2017, 29, 70–79. [Google Scholar] [CrossRef]
- Ungeheuer, F.; Fürstner, A. Concise Total Synthesis of Ivorenolide B1. Chem.–Eur. J. 2015, 21, 11387–11392. [Google Scholar] [CrossRef] [PubMed]
- Zidorn, C.; Jöhrer, K.; Ganzera, M.; Schubert, B.; Sigmund, E.M.; Mader, J.; Greil, R.; Ellmerer, E.P.; Stuppner, H. Polyacetylenes from the apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. J. Agric. Food Chem. 2005, 53, 2518–2523. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, A.S.; Hemscheidt, T. Olefin Cross-Metathesis as a Tool in Natural Product Degradation. The Stereochemistry of (+)-Falcarindiol. Org. Lett. 2002, 4, 4667–4668. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Q.-F.; Xue, J.-J.; Zhou, Y.; Yu, H.-C.; Yang, S.-P.; Zhang, B.; Zuo, J.-P.; Li, Y.; Yue, J.M. Ivorenolide B, an Immunosuppressive 17-Membered Macrolide from Khaya ivorensis: Structural Determination and Total Synthesis. Org. Lett. 2014, 16, 2062–2065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Y.; Yang, S.-P.; Zhou, Y.; Wu, W.-B.; Tank, W.; Zuo, J.-P.; Li, Y.; Yue, J.M. Ivorenolide A, an Unprecedented Immunosuppressive Macrolide from Khaya ivorensis: Structural Elucidation and Bioinspired Total Synthesis. J. Am. Chem. Soc. 2012, 134, 20605–20608. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Oguchi, K.; Iwamoto, R.; Okamoto, Y.; Fukushi, E.; Kawabata, J.; Ozawa, T.; Masuda, A. Iriomoteolides-1b and -1c, 20-Membered Macrolides from a Marine Dinoflagellate Amphidinium Species. J. Nat. Prod. 2007, 70, 1661–1663. [Google Scholar] [CrossRef]
- Tsuda, M.; Izui, N.; Shimbo, K.; Sato, M.; Fukushi, E.; Kawabata, J.; Kobayashi, J. Amphidinolide Y, a Novel 17-Membered Macrolide from Dinoflagellate Amphidinium sp.: Plausible Biogenetic Precursor of Amphidinolide X. J. Org. Chem. 2003, 68, 9109–9112. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J. Amphidinolides and Its Related Macrolides from Marine Dinoflagellates. J. Antibiot. 2008, 61, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Beaufay, C.; Bero, J.; Quetin-Leclercq, J. Antimalarial Terpenic Compounds Isolated from Plants Used in Traditional Medicine (2010–July 2016). In Natural Antimicrobial Agents. Sustainable Development and Biodiversity; Mérillon, J.M., Riviere, C., Eds.; Springer: Cham, Switzerland, 2018; Volume 19, pp. 247–268. [Google Scholar]
- Pink, R.; Hudson, A.; Mouriès, M.-A.; Bendig, M. Opportunities and challenges in antiparasitic drug discovery. Nat. Rev. Drug Discov. 2005, 4, 727–740. [Google Scholar] [CrossRef]
- Molinspiration Cheminformatics, Nova ulica, SK-900 26 Slovensky Grob, Slovak Republic. Available online: http://www.molinspiration.com/cgi-bin/properties (accessed on 23 October 2018).
- U.S. National Library of Medicine. National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/332#section=Chemical-and-Physical-Properties (accessed on 23 October 2018).
- Balasegaram, M.; Ritmeijer, K.; Lima, M.; Burza, S.; Genovese, G.; Milani, B.; Gaspani, S.; Potet, J.; Chappuis, F. Liposomal amphotericin B as a treatment for human leishmaniasis. Expert Opin. Emerg. Drugs 2012, 17, 493–510. [Google Scholar] [CrossRef]
- Fountain, M.W.; Weiss, S.J.; Lenk, R.P.; Propescu, M.C.; Ginsberg, R.S. Enhancement of Pharmaceutical Activity. U.S. Patent 5000958, 26 July 1984. [Google Scholar]
- Brglez Mojzer, E.; Knez Hrncic, M.; Škerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Cheung, S.S.C.; Tai, J.; Hasman, D.; Ou, D.; Warnock, G.L. Inhibition of Human Pancreatic Cancer Cell Proliferation by Devil’s Club Oplopanax horridus and Its Polyacetylene Bioactive Compound. Nutr. Cancer 2015, 67, 954–964. [Google Scholar] [CrossRef]
- Herrmann, F.; Sporer, F.; Tahrani, A.; Wink, M. Antitrypanosomal Properties of Panax ginseng C. A. Meyer: New Possibilities for a Remarkable Traditional Drug. Phytother. Res. 2013, 27, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Senn, M.; Gunzenhauser, S.; Brun, R.U.; Séquin, U. Antiprotozoal polyacetylenes from the Tanzanian medicinal plant Cussonia zimmermannii. J. Nat. Prod. 2007, 70, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Fokialakis, N.; Kalpoutzakis, E.; Tekwani, B.L.; Khan, S.I.; Kobaisy, M.; Skaltsounis, A.L.; Duke, S.O. Evaluation of the antimalarial and antileishmanial activity of plants from the Greek island of Crete. J. Nat. Med. 2007, 61, 38–45. [Google Scholar] [CrossRef]
- Molina-Garza, Z.J.; Bazaldúa-Rodríguez, A.F.; Quintanilla-Licea, R.; Galaviz-Silva, L. Anti-Trypanosoma cruzi activity of 10 medicinal plants used in northeast Mexico. Acta Trop. 2014, 136, 14–18. [Google Scholar] [CrossRef]
- Roumy, V.; Garcia-Pizango, G.; Gutierrez-Choquevilca, A.L.; Ruiz, L.; Jullian, V.; Winterton, P.; Fabre, N.; Moulis, C.; Valentin, A. Amazonian plants from Peru used by Quechua and Mestizo to treat malaria with evaluation of their activity. J. Ethnopharmacol. 2007, 112, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Quézel, P.; Santa, S. Nouvelle Flore de l’Algérie et des Régions Désertiques Méridionales; Éditions du Centre national de la Recherche scientifique: Paris, France, 1962. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- National Institute of Standards and Technology. NIst webBook. 2015. Available online: http://webbook.nist.gov/chemistry/ (accessed on 24 January 2017).
- König, W.A.; Joulain, D.; Hochmuth, D.H. Terpenoids and Related Constituents of Essential Oils, Library of Mass Finder 2.1; Institute of Organic Chemistry, University of Hamburg: Hamburg, Germany, 2011. [Google Scholar]
- Braun, S.; Kalinowski, H.-O.; Berger, S. 150 and More Basic NMR Experiments. A Practical Course; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision A.03; Barone, V., Petersson, G.A., Nakatsuji, H., Eds.; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Hoet, S.; Stévigny, C.; Block, S.; Opperdoes, F.; Colson, P.; Baldeyrou, B.; Lansiaux, A.; Bailly, C.; Quetin-Leclercq, J. Alkaloids from Cassytha filiformis and related aporphines: Antitrypanosomal activity, cytotoxicity, and interaction with DNA and topoisomerases. Planta Med. 2004, 70, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Le, T.B.; Beaufay, C.; Nghiem, D.T.; Mingeot-Leclercq, M.-P.; Quetin-Leclercq, J. In Vitro Anti-Leishmanial Activity of Essential Oils Extracted from Vietnamese Plants. Molecules 2017, 22, 1071. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 32, 33 and 34 are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).