Interpretation of Euphorbia Kansui Stir-Fried with Vinegar Treating Malignant Ascites by a UPLC-Q-TOF/MS Based Rat Serum and Urine Metabolomics Strategy Coupled with Network Pharmacology
Abstract
1. Introduction
2. Results and Discussion
2.1. Metabolic Profiling of UPLC-Q-TOF/MS
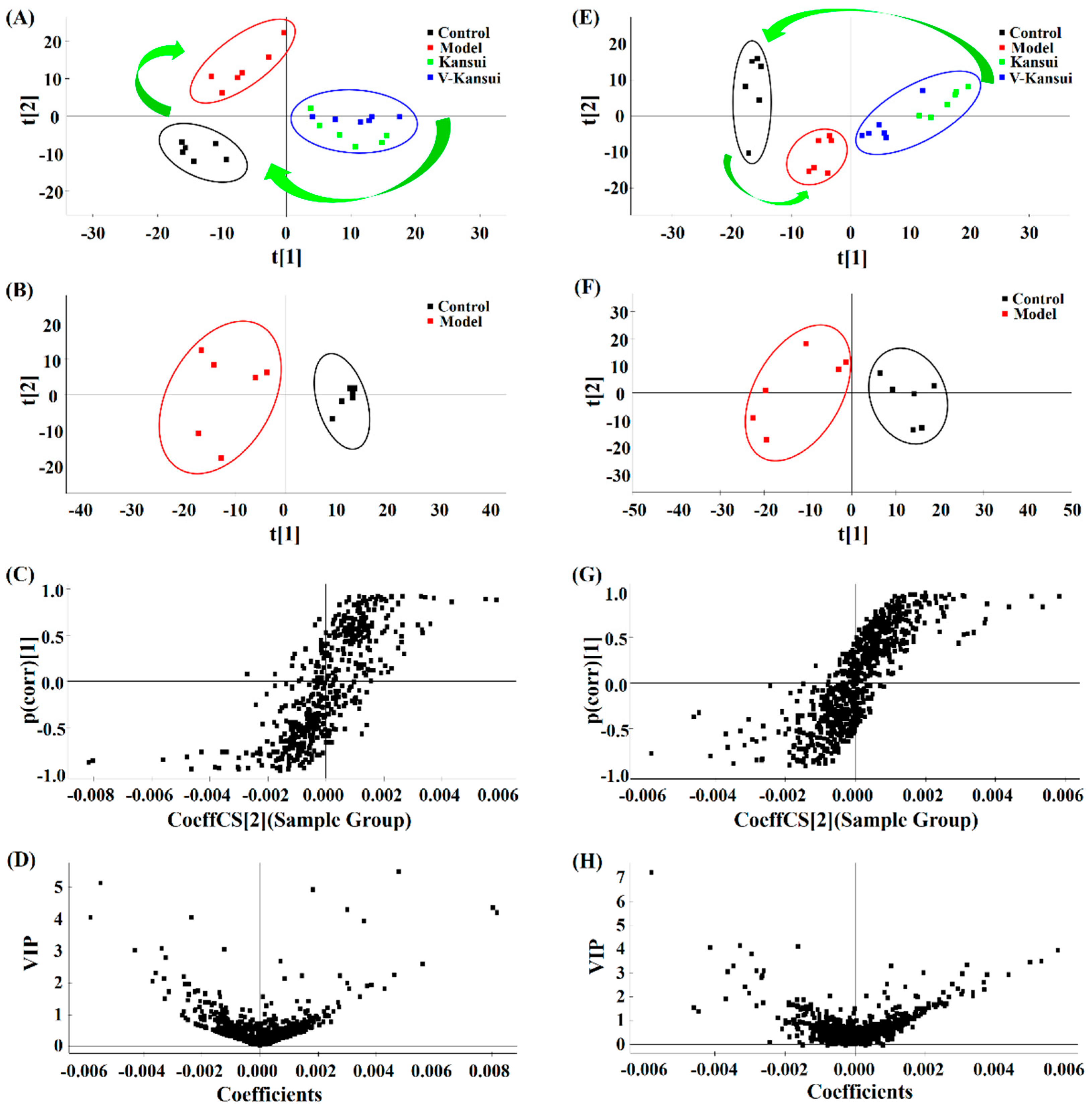
2.2. Multivariate Data Analysis
2.3. Potential Metabolites Identification
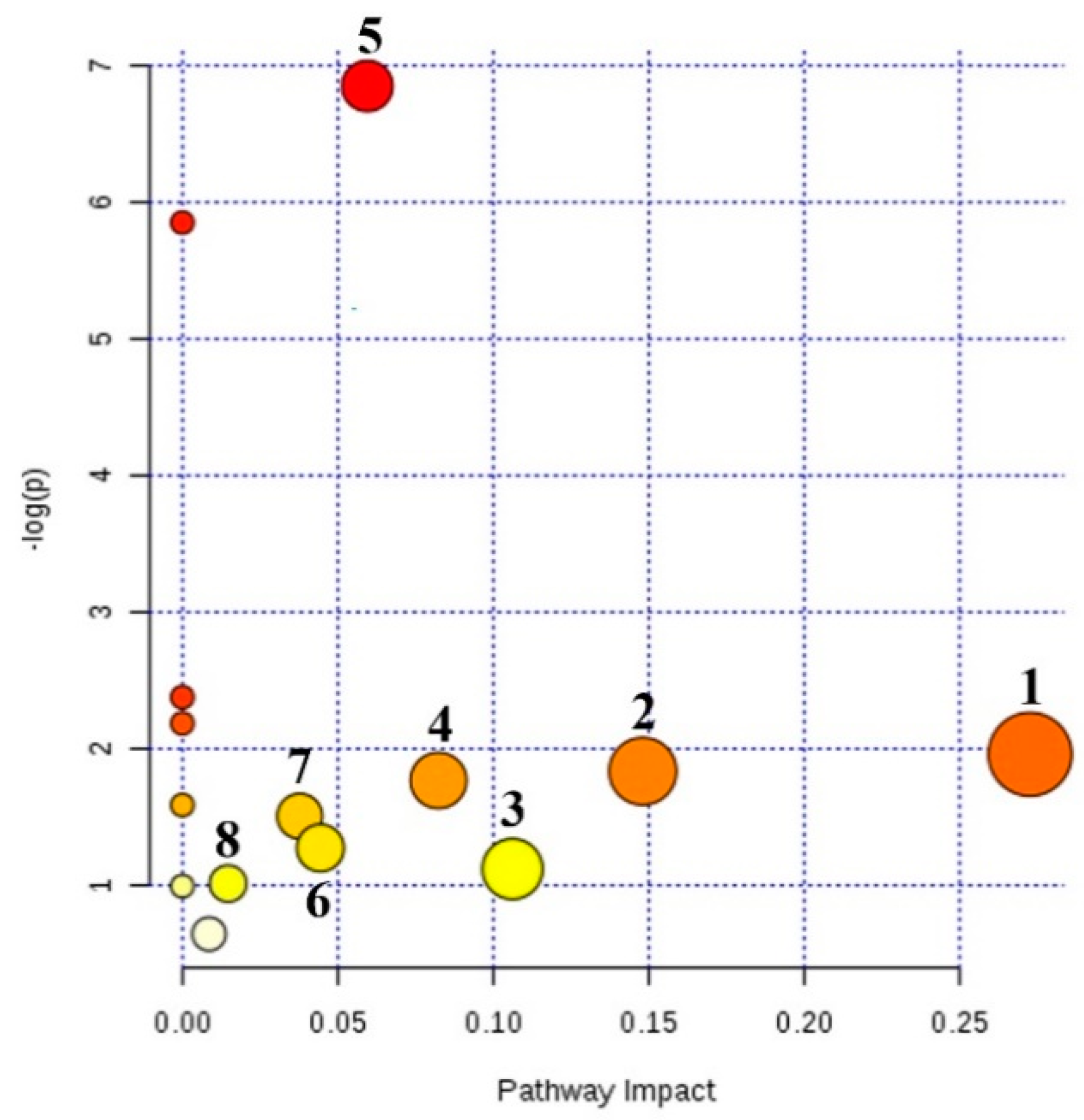
2.4. Metabolic Pathway Analysis
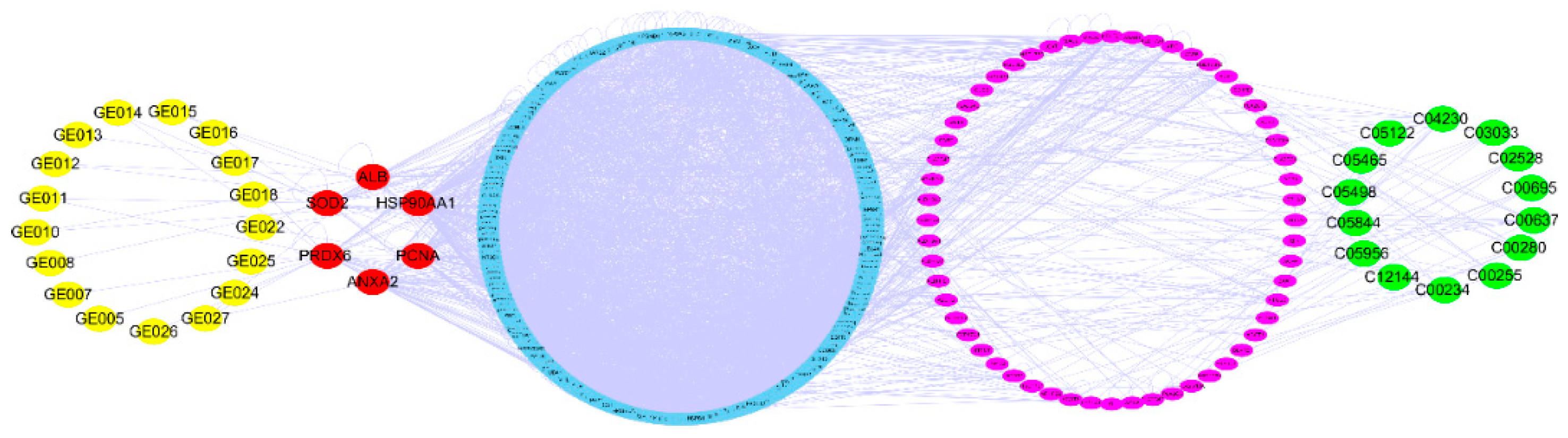
2.5. Network Pharmacology
2.6. Discussion
3. Materials and Methods
3.1. Materials, Chemicals and Reagents
3.2. Preparation of Samples of CHM Pieces
3.3. Animals and Treatment
3.4. Collection and Preparation of Serum and Urine Samples
3.5. Chromatography Conditions
3.6. Mass Spectrometry
3.7. Data processing and Pathway Analysis
3.8. Network Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zheng, L.; Xu, M.; Xu, J.; Wu, K.; Fang, Q.; Liang, Y.; Zhou, S.; Cen, D.; Ji, L.; Han, W.; et al. ELF3 promotes epithelial–mesenchymal transition by protecting ZEB1 from miR-141-3p-mediated silencing in hepatocellular carcinoma. Cell Death Dis. 2018, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Cavazzoni, E.; Bugiantella, W.; Graziosi, L.; Franceschini, M.S.; Donini, A. Malignant ascites: Pathophysiology and treatment. Int. J. Clin. Oncol. 2013, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Haberl, J.; Zollner, G.; Fickert, P.; Stadlbauer, V. To salt or not to salt?—That is the question in cirrhosis. Liver Int. 2018, 38, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Kai, J.; Tang, Y.; Zhang, L.; Su, S.; Duan, J.A. The chemical and biological properties of Euphorbia kansui. Am. J. Chin. Med. 2016, 44, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Oh, H.W.; Lee, H.R.; Yoon, S.Y.; Oh, S.R.; Ko, Y.E.; Yoo, N.; Jeong, J.; Kim, J.W. Ingenane-type diterpene compounds from Euphorbia kansui modulate IFN-γ production through NF-κB activation. J. Sci. Food Agric. 2016, 96, 2635–2640. [Google Scholar] [CrossRef]
- Lu, X.S.; Zhang, Y.; Ai, Y.H. The clinical study of kansui root therapy for severe acute pancreatitis. J. Chin. Phys. 2004, 6, 1444–1447. [Google Scholar]
- Kim, S.J.; Jang, Y.W.; Hyung, K.E.; Lee, D.K.; Hyun, K.H.; Park, S.Y.; Park, E.S.; Hwang, K.W. Therapeutic Effects of Methanol Extract from Euphorbia kansui Radix on Imiquimod-Induced Psoriasis. J. Immunol. Res. 2017, 2017. [Google Scholar] [CrossRef]
- Fan, Y. Treatment of intestinal obstruction with a large dose of kansui. J. Emerg. Tradit. Chin. Med. 2005, 14, 278–279. [Google Scholar]
- Guo, J.; Zhou, L.Y.; He, H.P.; Leng, Y.; Yang, Z.; Hao, X.J. Inhibition of 11b-HSD1 by tetracyclic triterpenoids from Euphorbia kansui. Molecules 2012, 17, 11826–11838. [Google Scholar] [CrossRef]
- Yan, X.J.; Zhang, L.; Guo, J.M.; Cao, Y.D.; Shang, E.X.; Tang, Y.P.; Ding, A.W.; Duan, J.A. Processing of kansui roots stir-fried with vinegar reduces kansui-induced hepatocyte cytotoxicity by decreasing the contents of toxic terpenoids and regulating the cell apoptosis pathway. Molecules 2014, 19, 7237–7254. [Google Scholar] [CrossRef]
- Tang, B.; Ding, J.; Yang, Y.; Wu, F.; Song, F. Systems biochemical responses of rats to Kansui and vinegar-processed Kansui exposure by integrated metabonomics. J. Ethnopharmacol. 2014, 153, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.J.; Lin, L.I.; Zheng-Jun, L.I.; Yuan, L.I.; Lan, G.; Cao, Y.D.; Tang, Y.P.; Zhang, L. The comparision of dose-effect relationships of crude and vinegar processed Euphorbia kansui with splenic lymphocyte activity and peritoneal macrophage NO release. Chin. Pharmacol. Bull 2011, 5, 629–632. [Google Scholar]
- Zhang, Q.; Cao, L.L.; Lou, J.W.; Li, Z.; Ding, A.W. Comparative study on expelling water retention with drastic purgative of crude and vinegar stir-fried kansui radix in cancerous ascites rats. Chin. Tradit. Herb. Drugs 2016, 47, 2492–2496. [Google Scholar]
- Fan, X.; Liu, J.L. The review of kansui. Chin. Tradit. Pat. Med. 2008, 30, 1358–1361. [Google Scholar]
- Lou, J.W.; Cao, L.L.; Zhang, Q.; Jiang, D.J.; Yao, W.F.; Bao, B.H.; Cao, Y.D.; Tang, Y.P.; Zhang, L.; Wang, K.; et al. The toxicity and efficacy evaluation of different fractions of Kansui fry-baked with vinegar on Walker-256 tumor-bearing malignant ascites effusion rats and normal rats. J. Ethnopharmacol. 2018, 219, 257–268. [Google Scholar] [CrossRef]
- Chang, J.S.; Lee, S.W.; Park, M.H.; Kim, M.S.; Hudson, B.I.; Park, S.J.; Lee, W.S.; Rho, M.C. Kansuinine A and Kansuinine B from Euphorbia kansui L. inhibit IL-6-induced Stat3 activation. Planta Med. 2010, 76, 1544–1549. [Google Scholar] [CrossRef]
- Lin, M.W.; Lin, A.S.; Wu, D.C.; Wang, S.S.; Chang, F.R.; Wu, Y.C.; Huang, Y.B. Euphol from Euphorbia tirucalli selectively inhibits human gastric cancer cell growth through the induction of ERK1/2-mediated apoptosis. Food Chem. Toxicol. 2012, 50, 4333–4339. [Google Scholar] [CrossRef]
- Xu, J.; Chen, H.B.; Li, S.L. Understanding the molecular mechanisms of the interplay between herbal medicines and gut microbiota. Med. Res. Rev. 2017, 37, 1140–1185. [Google Scholar] [CrossRef]
- Sui, Z.; Li, Q.; Zhu, L.; Wang, Z.; Lv, C.; Liu, R.; Xu, H.; He, B.; Li, Z.; Bi, K. An integrative investigation of the toxicity of Aconiti kusnezoffii radix and the attenuation effect of its processed drug using a UHPLC-Q-TOF based rat serum and urine metabolomics strategy. J. Pharm. Biomed. Anal. 2017, 145, 240–247. [Google Scholar] [CrossRef]
- Strack, L.; Stahl, U. “Omics” technologies and their input for the comprehension of metabolic systems particularly pertaining to yeast organisms. In Progress in Botany; Lüttge, U.E., Beyschlag, W., Büdel, B., Francis, D., Eds.; Springer: Heidelberg, Berlin, 2011; Volume 72, pp. 105–122. [Google Scholar]
- Jiang, M.; Lu, C.; Zhang, C.; Yang, J.; Tan, Y.; Lu, A.; Chan, K. Syndrome differentiation in modern research of traditional Chinese medicine. J. Ethnopharmacol. 2012, 140, 634–642. [Google Scholar] [CrossRef]
- Zira, A.N.; Theocharis, S.E.; Mitropoulos, D.; Migdalis, V.; Mikros, E. 1H NMR metabonomic analysis in renal cell carcinoma: A possible diagnostic tool. J. Proteome Res. 2010, 9, 4038–4044. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Qiu, Y.; Ni, Y.; Su, M.; Jia, W.; Du, X. An Automated Data Analysis Pipeline for GC-TOF-MS Metabonomics Studies. J. Proteome Res. 2010, 9, 5974–5981. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, X.; Bai, C.; Zhao, C.; Lu, G.; Xu, G. LC–MS-based metabonomics analysis. J. Chromatogr. B 2008, 866, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Hao, H.; Wang, G. LC/MS based tools and strategies on qualitative and quantitative analysis of herbal components in complex matrixes. Curr. Drug Metab. 2012, 13, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C. Potential urine and serum biomarkers for patients with bladder pain syndrome/interstitial cystitis. Int. J. Urol. 2014, 21, 34–41. [Google Scholar] [CrossRef]
- Xia, H.; Tang, H.; Wang, F.; Yang, X.; Wang, Z.; Liu, H.; Pan, D.; Wang, S.; Sun, G. Metabolic effects of dietary supplementation of Lycium barbarum polysaccharides on serum and urine metabolomics in a young healthy male population. J. Funct. Foods 2018, 46, 440–448. [Google Scholar] [CrossRef]
- Yang, Q.J.; Zhao, J.R.; Hao, J.; Li, B.; Huo, Y.; Han, Y.L.; Wan, L.L.; Li, J.; Huang, J.; Lu, J.; et al. Serum and urine metabolomics study reveals a distinct diagnostic model for cancer cachexia. J. Cachexia Sarcopenia Muscle 2018, 9, 71–85. [Google Scholar] [CrossRef]
- Fadhal, E.; Gamieldien, J.; Mwambene, E.C. Protein interaction networks as metric spaces: A novel perspective on distribution of hubs. BMC Syst. Biol. 2014, 8, 6. [Google Scholar] [CrossRef]
- Li, X.; Wu, L.; Liu, W.; Jin, Y.; Chen, Q.; Wang, L.; Fan, X.; Li, Z.; Cheng, Y. A network pharmacology study of Chinese medicine QiShenYiQi to reveal its underlying multicompound, multi-target, multi-pathway mode of action. PLoS ONE 2014, 9, e95004. [Google Scholar] [CrossRef]
- Chandran, U.; Patwardhan, B. Network ethnopharmacological evaluation of the immunomodulatory activity of Withania somnifera. J. Ethnopharmacol. 2017, 197, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Li, S.; Wang, N.; Tan, H.Y.; Cheung, F.; Feng, Y. A Biomedical Investigation of the Hepatoprotective Effect of Radix salviae miltiorrhizae and Network Pharmacology-Based Prediction of the Active Compounds and Molecular Targets. Int. J. Mol. Sci. 2017, 18, 620. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zheng, Y.; Gu, J.; Cai, Y.; Wang, S.; Zhang, F.; Chen, J.; Situ, H.; Lin, Y.; Wang, Z. Network-pharmacology-based validation of TAMS/CXCL-1 as key mediator of XIAOPI formula preventing breast cancer development and metastasis. Sci. Rep. 2017, 7, 14513. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Yang, Y.; Zhang, L.; Cao, Y.; Yao, W.; Tang, Y.; Ding, A. A Natural Trieripene Derivative from Euphorbia Kansui Inhibits Cell Proliferation and Induces Apoptosis against Rat Intestinal Epithelioid Cell Line in vitro. Int. J. Mol. Sci. 2015, 16, 18956–18975. [Google Scholar] [CrossRef] [PubMed]
- Su, W.H.; Chao, C.C.; Yeh, S.H.; Chen, D.S.; Chen, P.J.; Jou, Y.S. OncoDB.HCC: An integrated oncogenomic database of hepatocellular carcinoma revealed aberrant cancer target genes and loci. Nucleic Acids Res. 2007, 35, 727–731. [Google Scholar] [CrossRef]
- Lee, L.; Wang, K.; Li, G.; Xie, Z.; Wang, Y.; Xu, J.; Sun, S.; Pocalyko, D.; Bhak, J.; Kim, C.; et al. Liverome: A curated database of liver cancer-related gene signatures with self-contained context information. BMC Genom. 2011, 12 (Suppl. 3), S3. [Google Scholar] [CrossRef]
- Harbourt, D.E.; Fallon, J.K.; Ito, S.; Baba, T.; Ritter, J.K.; Glish, G.L.; Smith, P.C. Quantification of human uridine-diphosphate glucuronosyl transferase 1 A isoforms in liver, intestine and kidney using nanobore liquid chromatography-tandem mass spectrometry. Anal. Chem. 2012, 84, 98–105. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, L.L.; Liu, X.; Wu, D.C.; Xia, Y.L.; Ma, X.C.; Xin, Y.; Hou, J. Highly efficient preparation of bisphenol A glucuronide. Chem. J. Chin. Univ. 2014, 35, 314–318. [Google Scholar]
- Lu, F.; Cao, M.; Wu, B.; Li, X.Z.; Liu, H.Y.; Chen, D.Z.; Liu, S.M. Urinary metabonmoics study on toxicity biomarker discover in rats treated with Xanthii Fructus. J. Ethopharmacol. 2013, 149, 311–320. [Google Scholar] [CrossRef]
- Breyer, M.D.; Breyer, R.M. G Protein-Coupled Prostanoid Receptors and the Kidney. Annu. Rev. Physiol. 2001, 63, 579–605. [Google Scholar] [CrossRef]
- Hata, A.N.; Breyer, R.M. Pharmacology and signaling of prostaglandin receptors: Multiple roles in inflammation and immune modulation. Pharmacol. Ther. 2004, 103, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Briggs, J.P.; Schnermann, J. Convergence of major physiological stimuli for renin release on the Gs-alpha/cyclic adenosine monoposphate signaling pathway. Clin. Exp. Nephrol. 2012, 16, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Carl, D.E.; Ghosh, S.S.; Gehr, T.W.; Abbate, A.; Toldo, S.; Sanyal, A.J. A model of acute kidney injury in mice with cirrhosis and infection. Liver Int. 2016, 36, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; He, Q.; Tang, J.F.; Sha, M.C.; Tu, C.; Zhang, L.; Liu, Z.X.; Wang, J.B.; Xiao, X.H. Metabolomic study on immunological stress-mediated hepatotoxicity of Polygonum multiflorum and its processed products of nine times steaming and nine times sunning. Acta Pharm. Sin. 2017, 52, 1069–1076. [Google Scholar]
- Souness, G.W.; Latif, S.A.; Laurenzo, J.L.; Morris, D.J. 11 alpha- and 11 beta-hydroxyprogesterone, potent inhibitors of 11 beta-hydroxysteroid dehydrogenase (isoforms 1 and 2), confer marked mineralocorticoid activity on corticosterone in the ADX rat. Endocrinology 1995, 136, 1809–1812. [Google Scholar] [CrossRef] [PubMed]
- Chamulitrat, W.; Zhang, W.; Xu, W.; Pathil, A.; Setchell, K.; Stremmel, W. Hepatoprotectant ursodeoxyl lysophospatidy lethanolamide increasing phosphatidylcholine levels as a potential therapy of acute liver injury. Front. Physiol. 2012, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Gumprecht, L.A.; Beasley, V.R.; Weigel, R.M.; Parker, H.M.; Tumbleson, M.E.; Bacon, C.W.; Meredith, F.I.; Haschek, W.M. Development of fumonisin-induced hepatotoxicity and pulmonary edema in orally dosed swine; morphological and biochemical alterations. Toxicol. Pathol. 1998, 26, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Wan, D.; Zhao, C.; Chen, J.; Zhao, X.; Wang, W.; Lu, X.; Yang, S.; Gu, J.; Xu, G. A metabonomic study of hepatitis B-induced liver cirrhosis and hepatocellular carcinoma by using RP-LC and HILIC coupled with mass spectrometry. Mol. Biosyst. 2009, 5, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Tsugane, K.; Tamiya-Koizumi, K.; Nagino, M.; Nimura, Y.; Yoshida, S. A possible role of nuclear ceramide and sphingosine in hepatocyte apoptosis in rat liver. J. Hepatol. 1999, 31, 8–17. [Google Scholar] [CrossRef]
- Yamashita, A.; Tanaka, K.; Kamata, R.; Kumazawa, T.; Suzuki, N.; Koga, H.; Waku, K.; Sugiura, T. Subcellular localization and lysophospolipase transacylation activities of human group IVC phospholipase A2. Biochim. Biophys. Acta 2009, 1791, 1011–1022. [Google Scholar] [CrossRef]
- Olofsson, K.E.; Andersson, L.; Nilsson, J.; Björkbacka, H. Nano molar concentrations of lysophospatidylcholine recruit monocyes and induce pro-inflammatory cytokine production in macrophages. Biochem. Biophys. Res. Commun. 2008, 370, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xiao, Y.; Elson, P.; Tan, H.; Plummer, S.J.; Berk, M.; Aung, P.P.; Lavery, I.C.; Achkar, J.P.; Li, L.; et al. Plasma lysophosphatidycholine Levels: Potential Biomarkers for Colorectal Cancer. J. Clin. Oncol. 2007, 25, 2696–2701. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.Y.; Han, T.; Zhang, L.; Li, Y.; Liu, H.; Xiao, S.X.; Li, Y.; Kang, H.; Liu, S.Y. Cross-sectional and dynamic change of serum metabolite profiling for Hepatitis B-related acute-on-chronic liver failure by UHPLC/MS. J. Viral Hepat. 2014, 21, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut microbiome–mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018, 360, eaan5931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, A.G. Clinical application of determination of tryptophan and its metabolites. Pract. Prev. Med. 2012, 19, 633–634. [Google Scholar]
- Fan, J.; Ye, J.B.; Jurre, J. Quantitaive flux analysis reveals folate-dependent NADPH production. Nature 2014, 510, 298–302. [Google Scholar] [CrossRef]
- Zhang, Q.; Takahashi, M.; Noguchi, Y.; Sugimoto, T.; Kimura, T.; Okumura, A.; Ishikawa, T.; Kakumu, S. Plasma amino acid profiles applied for diagnosis of advanced liver fibrosis in patients with chronic hepatitis C infection. Hepatol. Res. 2006, 34, 170–177. [Google Scholar] [CrossRef]
- Xu, Q.; Tu, J.; Dou, C.; Zhang, J.; Yang, L.; Liu, X.; Lei, K.; Liu, Z.; Wang, Y.; Li, L.; et al. HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via Thr-328 phosphorylation in hepatocellular carcinoma. Mol. Cancer 2017, 16, 178. [Google Scholar] [CrossRef]
- Shi, H.; Xiao, L.; Duan, W.; He, H.; Ma, L.; Da, M.; Duan, Y.; Wang, Q.; Wu, H.; Song, X.; et al. ANXA2 enhances the progression of hepatocellular carcinoma via remodeling the cell motility associated structures. Micron 2016, 85, 26–33. [Google Scholar] [CrossRef]
- Akada, J.; Kamei, S.; Ito, A.; Ito, M.; Kitagawa, T.; Furumoto, H.; Kato, Y.; Tamesa, M.; Takashima, M.; Shirai, M.; et al. A new type of protein chip to detect hepatocellular carcinoma-related autoimmune antibodies in the sera of hepatitis C virus-positive patients. Proteome Sci. 2013, 11, 33. [Google Scholar] [CrossRef]
- Gramantieri, L.; Trerè, D.; Chieco, P.; Lacchini, M.; Giovannini, C.; Piscaglia, F.; Cavallari, A.; Bolondi, L. In human hepatocellular carcinoma in cirrhosis proliferating cell nuclear antigen (PCNA) is involved in cell proliferation and cooperates with P21 in DNA repair. J. Hepatol. 2003, 39, 997–1003. [Google Scholar] [CrossRef]
- Nojiri, S.; Joh, T. Albumin suppresses human hepatocellular carcinoma proliferation and the cell cycle. Int. J Mol. Sci. 2014, 15, 5163–5174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Weng, H.Z.; Huang, J.L.; Tang, G.H.; Yin, S. Anti-inflammatory ingenane diterpenoids from the roots of euphorbia kansui. Planta Med. 2018, 84, 1334–1339. [Google Scholar] [CrossRef]
- Jiang, D.; Kang, A.; Yao, W.; Lou, J.; Zhang, Q.; Bao, B.; Cao, Y.; Yu, S.; Guo, S.; Zhang, Y.; Tang, Y.; Zhang, L. Euphorbia kansui fry-baked with vinegar modulates gut microbiota and reduces intestinal toxicity in rats. J. Ethnopharmacol. 2018, 226, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, K.X.; Zhou, Y.Z.; Fang, J.S.; Qin, X.M.; Du, G.H. Uncovering the anticancer mechanism of Compound Kushen Injection against HCC by integrating quantitative analysis, network analysis and experimental validation. Sci. Rep. 2018, 8, 624. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the kansui and V-kansui are available from the authors. |
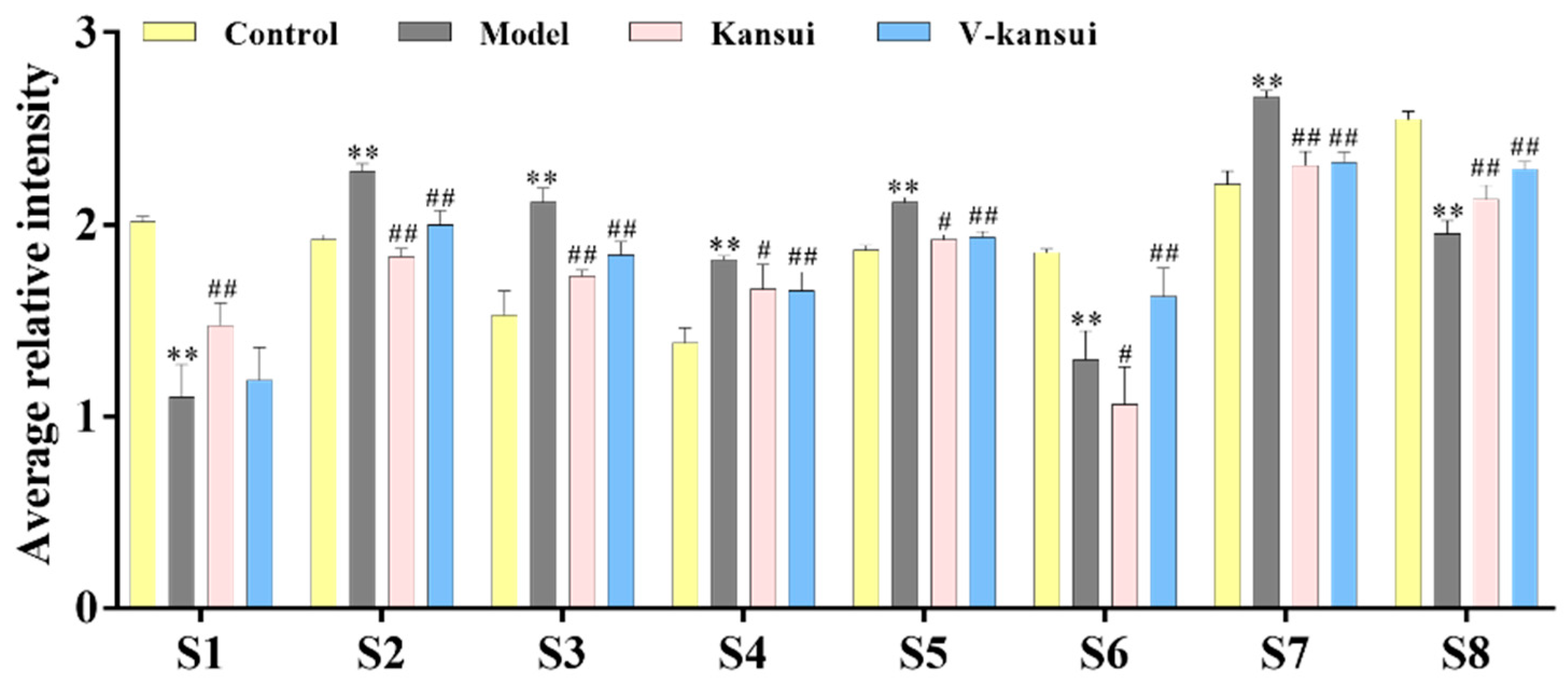
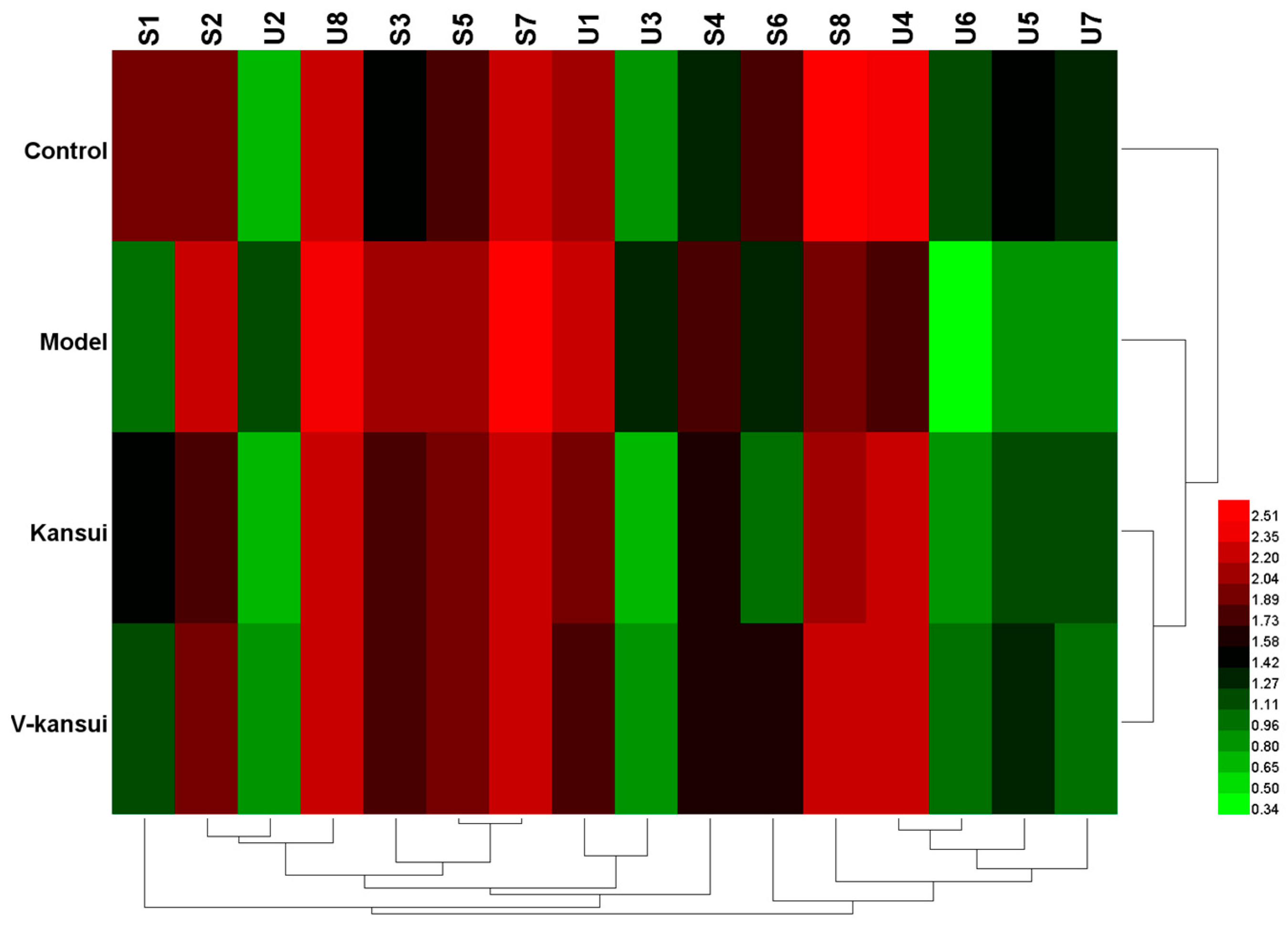
| No. | RT | m/z | Mass Error (ppm) | VIP | Metabolites | Adduct | Formula | KEGG | Trend | Pathway |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 1.18 | 160.0698 | 8.7 | 2.6 | Indoleacetaldehyde | M + H | C10H9NO | C00637 | ↓ | Tryptophan metabolism |
| S2 | 2.88 | 514.2942 | 5.0 | 1.6 | Taurocholic acid | M − H | C26H45NO7S | C05122 | ↑ | Primary bile acid biosynthesis, Taurine and hypotaurine metabolism |
| S3 | 3.91 | 498.2991 | 4.8 | 2.1 | Taurochenodesoxycholic acid | M − H | C26H45NO6S | C05465 | ↑ | Primary bile acid biosynthesis |
| S4 | 4.46 | 407.2886 | 2.4 | 5.2 | Cholic acid | M − H | C24H40O5 | C00695 | ↑ | Primary bile acid biosynthesis |
| S5 | 4.79 | 318.2912 | −5.3 | 1.9 | Phytosphingosine | M + H | C18H39NO3 | C12144 | ↑ | Sphingolipid metabolism |
| S6 | 6.15 | 391.2934 | 2.0 | 2.1 | Chenodeoxycholic acid | M − H | C24H40O4 | C02528 | ↓ | Primary bile acid biosynthesis |
| S7 | 8.27 | 522.3438 | −8.2 | 2.3 | LysoPC(18:1) a | M + H | C26H52NO7P | C04230 | ↑ | Glycerophospholipid metabolism |
| S8 | 10.88 | 327.2400 | −0.6 | 2.8 | Docosahexaenoic acid | M − H | C22H32O2 | C06429 | ↓ | Biosynthesis of unsaturated fatty acids |
| U1 | 3.73 | 340.0978 | 7.1 | 2.8 | 5-Hydroxy-6-methoxyindole glucuronide/6-Hydroxy-5-methoxyindole glucuronide | M + H | C15H17NO8 | C03033 | ↓ | Pentose and glucuronate interconversions |
| U2 | 6.46 | 413.2165 | −9.0 | 1.5 | Prostaglandin G2 | M + FA − H | C20H32O6 | C05956 | ↑ | Arachidonic acid metabolism |
| U3 | 6.71 | 472.1692 | 7.0 | 1.8 | 10-Formyltetrahydrofolate | M − H | C20H23N7O7 | C00234 | ↑ | One carbon pool by folate, Glyoxylate and dicarboxylate metabolism, Aminoacyl-tRNA biosynthesis |
| U4 | 7.22 | 255.0563 | −3.5 | 4.0 | 5-L-Glutamyl-taurine | M + H | C7H14N2O6S | C05844 | ↓ | Taurine and hypotaurine metabolism |
| U5 | 8.96 | 377.1403 | 5.6 | 2.1 | Riboflavin | M + H | C17H20N4O6 | C00255 | ↓ | Riboflavin metabolism |
| U6 | 9.07 | 331.1939 | 2.1 | 1.9 | Androstenedione | M+FA-H | C19H26O2 | C00280 | ↓ | Steroid hormone biosynthesis |
| U7 | 9.64 | 331.1973 | −4.2 | 1.7 | 11b-Hydroxyprogesterone | M − H | C20H28O4 | C05498 | ↓ | Steroid hormone biosynthesis |
| U8 | 10.48 | 318.2924 | −1.6 | 2.6 | Phytosphingosine | M + H | C18H39NO3 | C12144 | ↑ | Sphingolipid metabolism |
| Symbol | Molecule Name | OB (%) | DL |
|---|---|---|---|
| GE01 | citric acid | 56.22 | 0.05 |
| GE02 | OXL | 29.68 | 0.01 |
| GE03 | 24-Methylenecycloartanol | 10.4 | 0.79 |
| GE04 | β-sitosterol | 5.84 | 0.71 |
| GE05 | 20-O-(2,3-dimethylbutanoyl)-13-O-dodecanoylingenol | 24.17 | 0.61 |
| GE06 | 3-O-Benzoyl-20-deoxyingenol | 12.27 | 0.8 |
| GE07 | 3-O-benzoyl-13-O-dodecanoylingenol | 28.74 | 0.57 |
| GE08 | 5-O-Benzoyl-20-deoxyingenol | 13.52 | 0.79 |
| GE09 | [(1S,2R,5S,6R)-6-methyl-2-methylol-norpinan-6-yl]methanol | 24.87 | 0.07 |
| GE10 | Euphorbetin | 35.89 | 0.54 |
| GE11 | (3S,5R,10S,13R,14R,17R)-17-[(1R)-1,5-dimethyl-4-methylenehexyl]-4,4,10,13,14-pentamethyl-2,3,5,6,7,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-3-ol | 42.37 | 0.77 |
| GE12 | Euponin | 18.64 | 0.49 |
| GE13 | Karacolidine | 60.53 | 0.71 |
| GE14 | 20-O-Benzoyl-13-O-dodeeanoyl ingenol | 28.65 | 0.56 |
| GE15 | (1S,4aS,10aR)-7-isopropyl-1,4a-dimethyl-5,8-dioxo-2,3,4,9,10,10a-hexahydrophenanthrene-1-carboxylic acid | 29.08 | 0.35 |
| GE16 | kansuinin A | 44.52 | 0.55 |
| GE17 | kansuiphorin A | 21.67 | 0.22 |
| GE18 | kansuiphorin B | 19.16 | 0.2 |
| GE19 | NSC 403164 | 8.51 | 0.75 |
| GE20 | Euphol | 42.12 | 0.75 |
| GE21 | 20-OD-ingenol Z | 32.05 | 0.85 |
| GE22 | Kanziol | 41.65 | 0.75 |
| GE23 | Glycerite | 14.97 | 0.03 |
| GE24 | 3-O-(2,3-Dimethylbutanoyl)-13-O-decanoyl ingenol | 24.75 | 0.71 |
| GE25 | 3-O-(2,3-Dimethylbutanoyl)-13-O-dodecanoyl-20-O-acetylingenol | 25.44 | 0.54 |
| GE26 | 3-O-(2,3-Dimethylbutanoyl)-13-O-dodecanoyl-20-deoxyingenol | 30.82 | 0.65 |
| GE27 | 3-O-(2,3-dimethyl-butanoyl)-13-dodecanoylingenol | 24.3 | 0.63 |
| GE28 | Isoscopoletin | 23.46 | 0.08 |
| GE29 | Scopoletol | 27.77 | 0.08 |
| GE30 | palmitic acid | 19.3 | 0.1 |
| GE31 | HMF | 45.07 | 0.02 |
| Genes | Description | Average Shortest Path Length | Betweenness Centrality | R |
|---|---|---|---|---|
| HSP90AA1 | Heat shock protein HSP 90-alpha | 2.17 | 0.06665 | 0.0000 |
| PCNA | proliferating cell nuclear antigen | 2.37 | 0.00782 | 0.1193 |
| ANXA2 | annexin A2 | 2.40 | 0.01316 | 0.1352 |
| PRDX6 | peroxiredoxin 6 | 2.53 | 0.02880 | 0.1455 |
| PC | pyruvate carboxylase | 2.61 | 0.00365 | 0.1696 |
| ALB | albumin | 2.61 | 0.01804 | 0.1797 |
| SOD2 | superoxide dismutase 2 | 2.63 | 0.02969 | 0.2294 |
| APOA1 | apolipoprotein A-I | 2.68 | 0.01036 | 0.3243 |
| FGA | fibrinogen alpha chain | 2.80 | 0.00247 | 0.4764 |
| CTH | cystathionase | 3.07 | 0.00195 | 0.6409 |
| AKR1C2 | aldo-keto reductase family 1, member C2 | 3.15 | 0.00131 | 0.8362 |
| CES1 | carboxylesterase 1 | 3.64 | 0.00184 | 0.8523 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Gao, J.; Zhang, Q.; Wang, K.; Yao, W.; Bao, B.; Zhang, L.; Tang, Y. Interpretation of Euphorbia Kansui Stir-Fried with Vinegar Treating Malignant Ascites by a UPLC-Q-TOF/MS Based Rat Serum and Urine Metabolomics Strategy Coupled with Network Pharmacology. Molecules 2018, 23, 3246. https://doi.org/10.3390/molecules23123246
Zhang Y, Gao J, Zhang Q, Wang K, Yao W, Bao B, Zhang L, Tang Y. Interpretation of Euphorbia Kansui Stir-Fried with Vinegar Treating Malignant Ascites by a UPLC-Q-TOF/MS Based Rat Serum and Urine Metabolomics Strategy Coupled with Network Pharmacology. Molecules. 2018; 23(12):3246. https://doi.org/10.3390/molecules23123246
Chicago/Turabian StyleZhang, Yi, Jing Gao, Qiao Zhang, Kan Wang, Weifeng Yao, Beihua Bao, Li Zhang, and Yuping Tang. 2018. "Interpretation of Euphorbia Kansui Stir-Fried with Vinegar Treating Malignant Ascites by a UPLC-Q-TOF/MS Based Rat Serum and Urine Metabolomics Strategy Coupled with Network Pharmacology" Molecules 23, no. 12: 3246. https://doi.org/10.3390/molecules23123246
APA StyleZhang, Y., Gao, J., Zhang, Q., Wang, K., Yao, W., Bao, B., Zhang, L., & Tang, Y. (2018). Interpretation of Euphorbia Kansui Stir-Fried with Vinegar Treating Malignant Ascites by a UPLC-Q-TOF/MS Based Rat Serum and Urine Metabolomics Strategy Coupled with Network Pharmacology. Molecules, 23(12), 3246. https://doi.org/10.3390/molecules23123246






