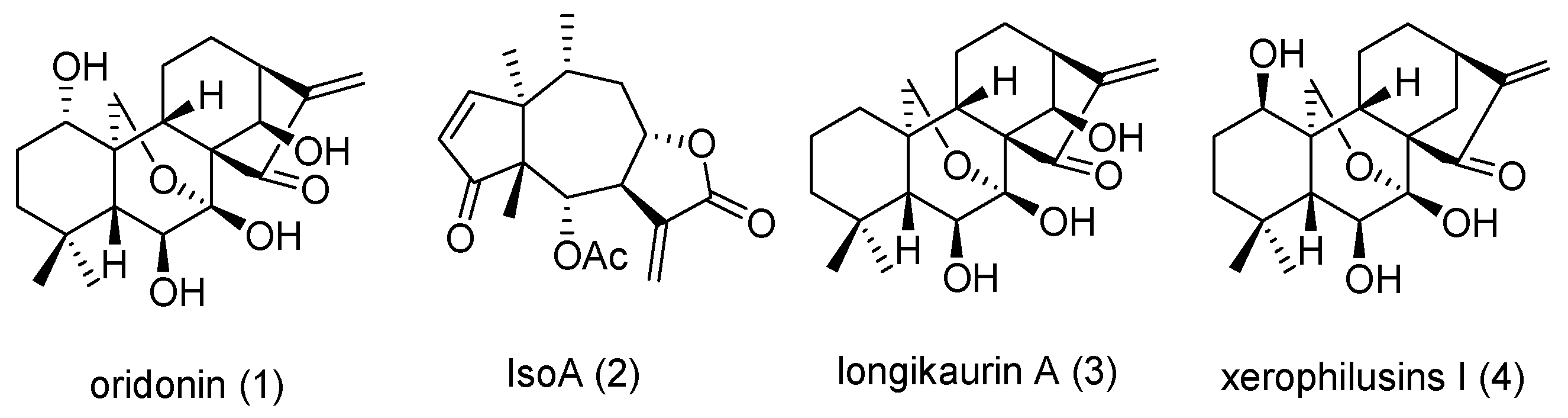
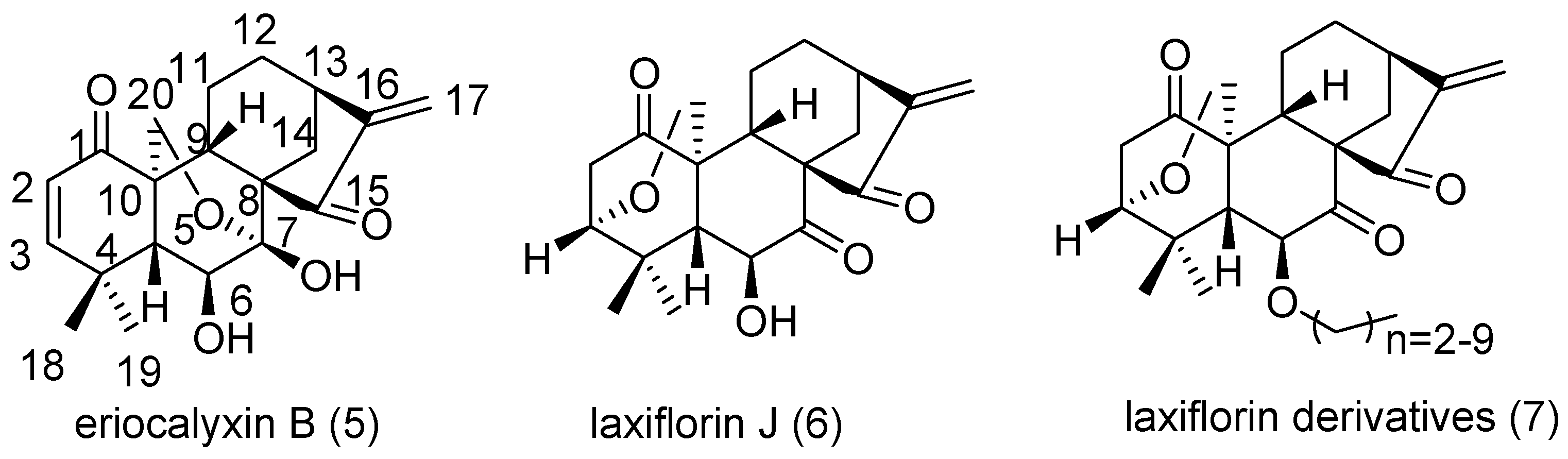
3.1. Chemistry
All regents were used as purchased from Energy Chemical (Shanghai, China), Tansoole (Shanghai, China), Acros (Beijing, China) and Aldrich (Shanghai, China) without further purification. Anhydrous CH2Cl2 was freshly distilled from CaH2, and THF was dried by Na. For reactions carried out under Ar, at least three times. The course of reaction was monitored by TLC. 1H NMR and 13C NMR spectra were recorded at ambient temperature with a Bruker Avance 300 (300 MHz for 1H and 75.5 MHz for 13C; Bruker, Germany) instrument in which TMS was used as internal standard for all measurements. MS data were recorded by using a VG Auto spec-3000 spectrometer or a Finnigan MAT 90 instrument (Bremen, Germany). IR spectra were measured as KBr pellets by using a Bio-Rad FTS-135 spectrometer (California, CA, USA). All melting points are uncorrected. CC was performed by using silica gel (100–200 mesh; Qingdao Marine Chemical, Shandong, China).
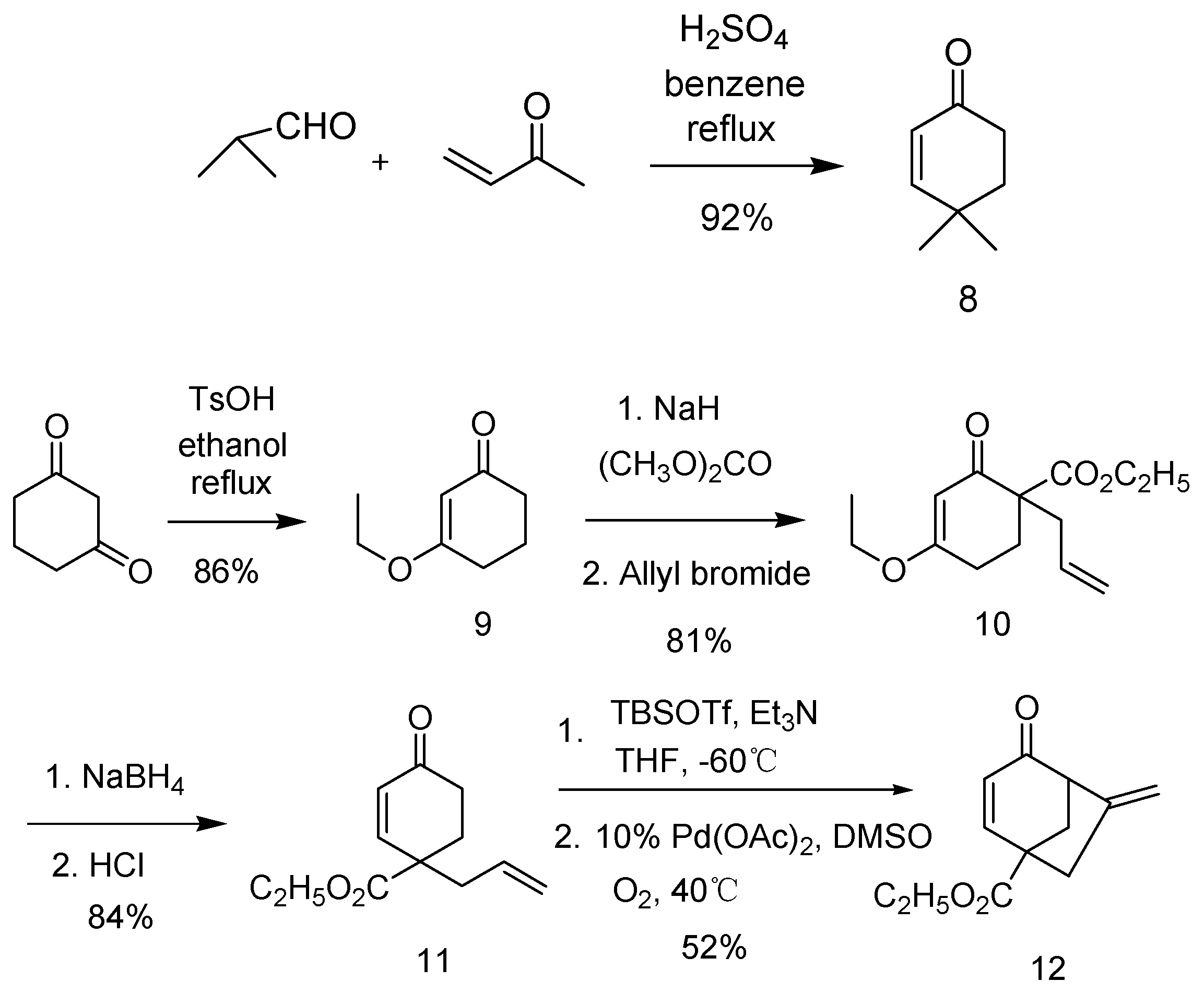
4,4-Dimethylcyclohex-2-enone (8): To a solution of isobutyraldehyde (3 g, 41.6 mmol) and methyl vinyl ketone (2.91 g, 41.6 mmol) was added 1% mmol conc. H2SO4. The resulting mixture was stirred with a Dean-Stark apparatus in refluxing benzene (20 mL) for 5 h. The reaction mixture was neutralized by sat. NaHCO3 to pH = 7. Organic layer was separated and water was extracted with EtoAc (3 × 10 mL). The organic phases were combined, dried over MgSO4, and concentrated on a rotary evaporator. The residue was purified by flash chromatography (silica, ethyl acetate:petroleum = 1:10) to give compound 8 (92%) as pale yellow liquid: 1H NMR (400 MHz, CDCl3) δ = 6.67(d, J = 6 Hz, 1H), 5.82 (d, J = 6 Hz, 1H), 2.45 (t, J = 6 Hz, 2H), 1.87 (t, J = 6 Hz, 2H), 1.17 (s, 6H) ppm; 13C NMR (100 M Hz, CDCl3) δ = 199.48, 159.79, 126.77, 36.03, 34.34, 32.77, 27.64 ppm; IR (KBr):υmax = 3441.89, 2966.23, 2926.56, 1638.51 cm−1; MS (ESI): m/z: 125; HRMS (ESI) Calcd. for [(C8H12O) + H]+: 125.0966; found: 125.0961 [M + H]+.
3-Ethoxycyclohex-2-enone (9): To a solution of cyclohexane-1,3-dione (1.12 g, 10 mmol) in dry ethanol was added 1% mmol TsOH. The resulting mixture was refluxed for overnight. Then the solvent was removed in vacuo and the residue was purified by flash column chromatography (eluting with EtOAc:petroleum = 1:20–1:10) to give compound 9 (86%) as colorless liquid: 1H NMR (300 MHz, CDCl3) δ = 4.74 (s, 1H), 3.36 (q, J = 6.9 Hz, 2H), 1.85 (t, J = 6.0 Hz, 2H), 1.74 (t, J = 6.0 Hz, 2H), 1.11 (p, J = 6.0 Hz, 2H), 0.80 (t, J = 6.9 Hz, 3H) ppm; 13C NMR (75.5 MHz, CDCl3) δ = 197.496, 176.357, 101.367, 62.996, 35.635, 27.897, 20.201, 12.970 ppm; IR (KBr):υmax = 2944.51, 1591.52 cm−1; MS (ESI): m/z: 141; HRMS (ESI) Calcd. for [(C8H12O2) + H]+: 141.0916; found: 141.0906 [M + H]+.
Ethyl 1-allyl-4-ethoxy-2-oxocyclohex-3-enecarboxylate (10): NaH (0.6 g, 15 mmol, 60% in mineral oil) was suspended in diethyl carbonate (10 mL) at 100 °C. A solution of 9 (1.4 g, 10 mmol) in diethyl carbonate (2 mL) was added to the suspension by syringe and stirring was continued for 5 h at 100 °C. Upon cooling to ambient temperature, the reaction mixture was washed with brine (2 × 10mL) and evaporated in vacuo without further purification. To a solution of crude product in dry THF was added NaH (0.4 g, 10 mmol, 60% in mineral oil) at room temperature. After 30 min, the mixture was added allyl bromide (1.2 g, 10 mmol) and refluxed for 3 h. The reaction mixture was poured into ice-water and extracted with ether. The ether layer was washed with water and dried over Na2SO4. Removal of solvent followed by CC gave compound 10 (81%) as colorless liquid: 1H NMR (300 MHz, CDCl3) δ = 5.77 (m, 1H), 5.35 (s, 1H), 5.09 (m, 2H), 4.17 (q, J = 6.9 Hz, 2H), 3.92 (q, J = 6.9 Hz, 2H), 2.74–2.32 (m, 5H), 1.97–1.91 (m, 1H), 1.19 (t, J = 6.9 Hz, 3H), 1.05 (t, J = 6.9 Hz, 3H) ppm; 13C NMR (75.5 MHz, CDCl3) δ = 194.98, 176.59, 171.13, 133.50, 118.42, 101.65, 64.24, 60.98, 55.33, 38.38, 37.95, 26.10, 13.93 ppm; IR (KBr):υmax = 2979.73, 2937.66, 1725.23, 1655.39, 1605.02, 1186.77 cm−1; MS (ESI): m/z: 275; HRMS (ESI) Calcd. for [(C14H20O4) + Na]+: 275.1259; found: 275.1254 [M + Na]+.
Ethyl 1-allyl-4-oxocyclohex-2-enecarboxylate (11): To a solution of 10 (1 g, 3.97 mmol) in MeOH (5 mL) was added CeCl3·7H2O (1.48 g, 3.97 mmol). After 10 min of stirring, the reaction mixture was added NaBH4 (0.3 g, 7.94 mmol) about for 30 min. Then the mixture was acidified to pH 6 by the dropwise addition of concentrated HCl, and extracted with ethyl acetate and washed with brine. The combined organic layers were dried over Na2SO4 and the solvent was removed under reduced pressure. Purification of the residue by column chromatograph afford compound 11 (84%) as pale yellow liquid: 1H NMR (300 MHz, CDCl3) δ = 6.88(d, J = 10.2 Hz, 1H), 5.97 (d, J = 10.2 Hz, 1H), 5.66 (m, 1H), 5.10 (m, 2H), 4.15 (q, J = 7.2 Hz, 2H), 2.52–2.34 (m, 5H), 1.98–1.93 (m, 1H), 1.24 (t, J = 7.2 Hz, 3H) ppm; 13C NMR (75.5 MHz, CDCl3) δ = 198.498, 172.728, 150.539, 131.920, 129.227, 119.638, 61.435, 47.502, 42.884, 34.525, 30.238, 14.184 ppm; IR (KBr):υmax = 2978.78, 2958.27, 1728.05, 1638.95, 1614.26 cm−1; MS (ESI): m/z: 209; HRMS (ESI) Calcd. for [(C12H16O3) + H]+: 209.1178; found: 209.1169 [M + H]+.
Ethyl 6-methylene-4-oxobicyclo[3.2.1]oct-2-ene-1-carboxylate (12): To a solution of 11 (6 g, 28.85 mmol, 1.0 eq) in dry THF (200 mL) under Ar at –60 °C was added Et3N (11.6 mL, 115.4 mmol, 4.0 eq). The mixture was stirred for 20 min at this temperature, and then TBSOTf (19.11 g, 72.13 mmol, 2.5 eq) was added dropwise. After full conversion was observed by TLC, the reaction mixture was quenched with saturated NaHCO3 solution and extracted with EtOAc. The combined organic layers were dried over Na2SO4 and the solvent was removed under reduced pressure. The residue was passed through a short column of silica gel to provide the corresponding silyl enol ether as a colorless oil. The obtained silyl enol ether was dissolved in DMSO (200 mL) and Pd(OAc)2 (650 mg, 2.89 mmol, 0.1 eq) was added, and the resulting solution was stirred under oxygen atmosphere (balloon with O2) at 40 °C for 6 h. The reaction was quenched with water, the mixture was extracted with Et2O, and the combined organic layers were washed with brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by flash column chromatography to afford compound 12 (40%) as pale yellow liquid: 1H NMR (300 MHz, CDCl3) δ = 7.43 (d, J = 9.9 Hz, 1H), 5.81 (d, J = 9.9 Hz, 1H), 5.26 (s, 1H), 5.05 (s, 1H), 4.19 (q, J = 7.2 Hz, 2H), 3.47 (br., 1H), 2.86 (d, J = 95.4 Hz, 1H), 2.52 (d, J = 95.4 Hz, 1H), 2.25 (m, 2H), 1.25 (t, J = 7.2 Hz, 3H) ppm; 13C NMR (75.5 MHz, CDCl3) δ = 197.515, 172.761, 152.359, 143.136, 126.641, 113.126, 61.584, 57.960, 51.617, 43.207, 41.109, 14.120 ppm; IR (KBr):υmax = 2979.98, 1729.06, 1685.40, 1280.90, 1222.04, 1193.09 cm−1; MS (ESI): m/z: 207; HRMS (ESI) Calcd. for [(C12H14O3) + H]+: 207.1021; found: 207.1015 [M + H]+.
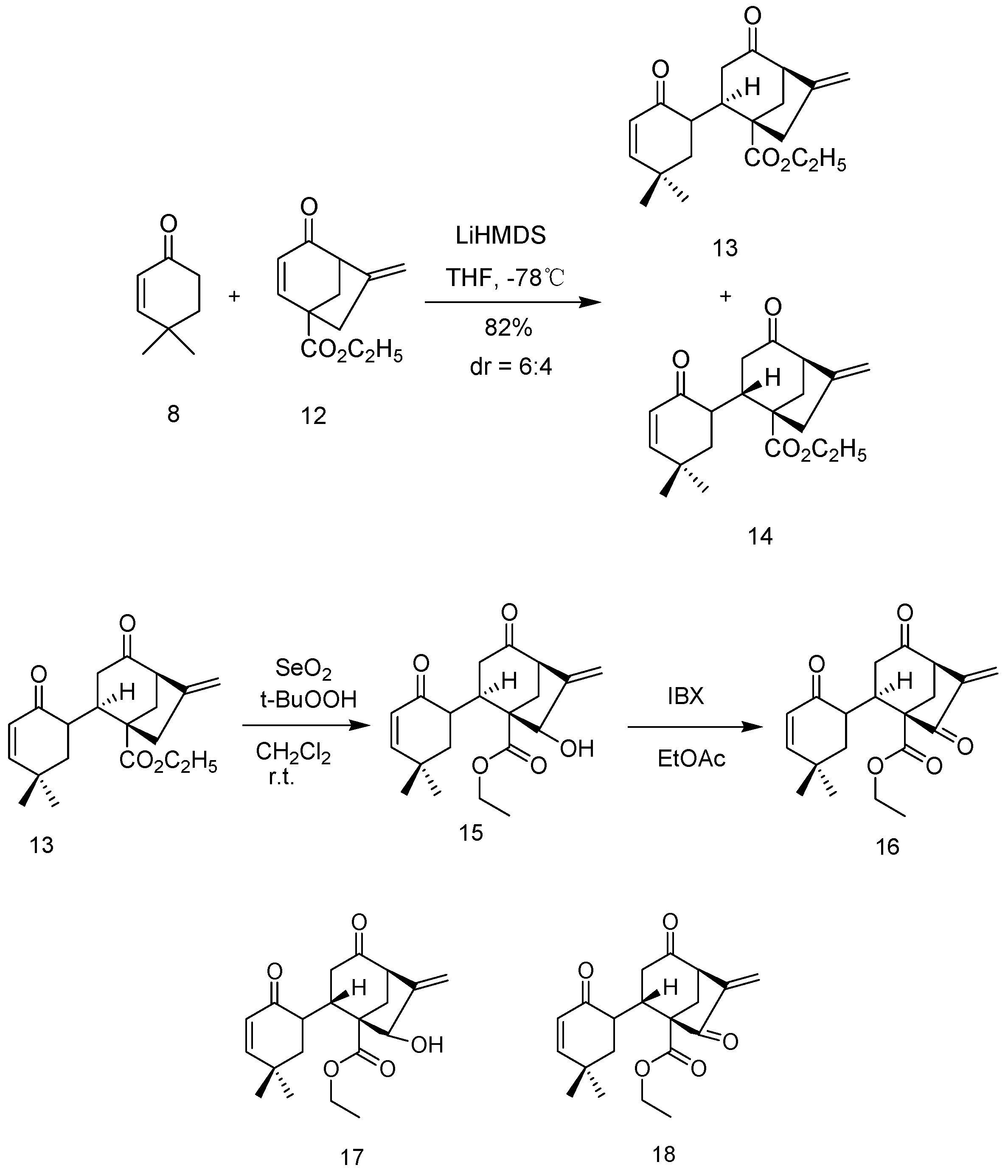
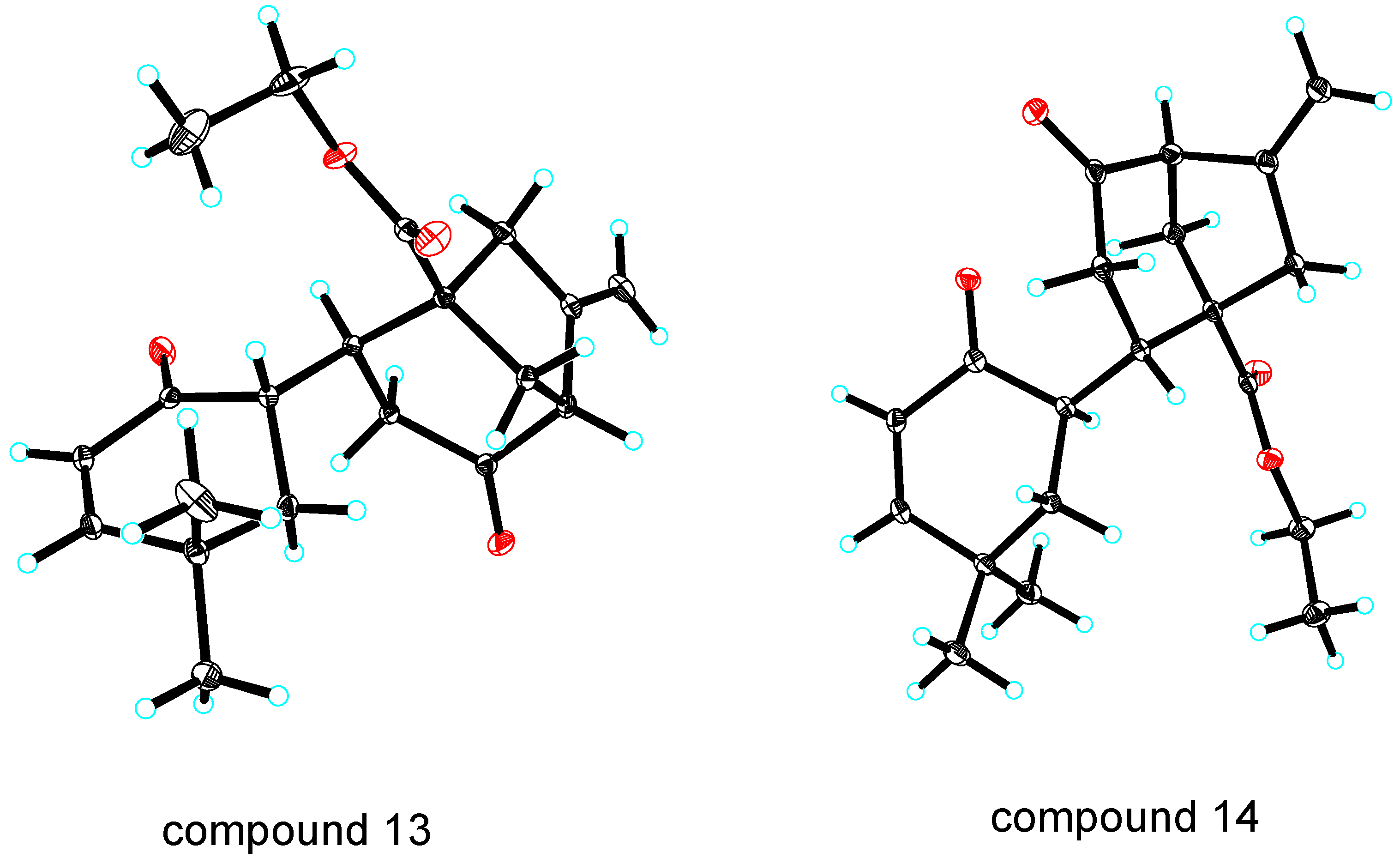
3.1.1. General Method for Synthesis of 13 and 14
To a stirred solution of 8 (1 eq) and 12 (1 eq) in dry THF under Ar at −78 °C; was added LiHMDS (2 eq, 1.3 M in THF) over a period of 5 min and stirring at −78 °C was continued for 45 min. The resulting solution was warmed to room temperature, quenched with saturated NH4Cl and extracted with Et2O. The combined organic layers were dried over Na2SO4 and the solvent was removed under reduced pressure. The residue was purified by flash column chromatography to provide 13 and 14.
Compound 13: The compound is obtained as crystal; m.p.: 112.0–114.6 °C; 1H NMR (300 MHz, CDCl3) δ = 6.56 (d, J = 6.0 Hz, 1H), 5.80 (d, J = 6.0 Hz, 1H), 5.10 (s, 1H), 5.01 (s, 1H), 4.15 (q, J = 6.0 Hz, 2H), 3.50 (m, 1H), 3.28 (s, 1H), 2.78 (s, 2H), 2.74–2.65 (m, 2H), 2.28 (m, 1H), 1.92–1.66 (m, 4H), 1.29 (t, J = 6.0 Hz, 3H), 1.26 (s, 3H), 1.25 (s, 3H) ppm; 13C NMR (75.5 MHz, CDCl3) δ = 208.93, 198.35, 174.52, 158.35, 145.59, 126.50, 109.91, 61.00, 58.73, 52.97, 46.11, 43.09, 38.80, 38.56, 36.41, 36.11, 33.70, 30.64, 24.95, 14.21 ppm; IR (KBr):υmax = 2962.26, 2901.21, 1722.91, 1710.24, 1672.31, 1229.31, 1194.50 cm−1; MS (ESI): m/z: 353; HRMS (ESI) Calcd. for [(C20H26O4) + Na]+: 353.1729; found: 353.1724 [M + Na]+.
Compound 14: The compound is obtained as crystal; m.p.:103.2–105.8 °C; 1H NMR (300 MHz, CDCl3) δ = 6.46(d, J = 6.0 Hz, 1H), 5.68 (d, J = 6.0 Hz, 1H), 5.04 (s, 1H), 4.88 (s,1H), 4.11 (q, J = 6.0 Hz, 2H), 3.30 (s, 1H), 2.65–2.39 (m, 6H),1.96 (m, 1H), 1.78–1.22 (m, 3H), 1.01 (t, J = 6.0 Hz, 3H), 0.88 (s, 6H) ppm; 13C NMR (75.5 MHz, CDCl3) δ = 206.74, 199.50, 175.14, 157.76, 146.00, 127.27, 109.41, 60.96, 59.66, 52.69, 45.89, 44.46, 43.35, 42.79, 37.86, 35.31, 34.03, 30.39, 24.74, 14.26 ppm; IR (KBr):υmax = 2958.90, 1716.70, 1673.18, 1538.19, 1234.84, 1215.98 cm−1; MS (ESI): m/z: 353; HRMS (ESI) Calcd. for [(C20H26O4) + Na]+: 353.1729; found: 353.1732 [M + Na]+.
3.1.2. General Method for Synthesis of 15 and 17
To a stirred solution of 13 (1.0 eq) in DCM, SeO2 (0.9 eq) was added in one portion, and a solution of t-BuOOH (5.5 M in decane, 3 eq) was added dropwise. The solution was vigorously stirred at room temperature overnight, and then the mixture was filtered through a short pad of silica gel washed with EtOAc. The filtrate was concentrated under vacuum and purified by flash column chromatography to provide allylic alcohol 15.
Compound 15: The compound is obtained as pale yellow liquid; 1H NMR (300 MHz, CDCl3) δ = 6.52 (d, J = 6 Hz, 1H), 5.75 (d, J = 6 Hz, 1H), 5.40 (s, 1H), 5.30 (s, 1H), 4.58 (s, 1H), 4.16 (q, J = 6 Hz, 2H), 3.32 (m, 2H), 2.57 (m, 3H), 2.17–1.69 (m, 5H), 1.21 (t, J = 6 Hz, 3H), 1.08 (s, 6H) ppm; 13C NMR (75.5 MHz, CDCl3) δ = 208.33, 198.08, 172.65, 158.66, 149.08, 126.53, 115.31, 79.98, 61.18, 59.47, 56.68, 46.35, 37.91, 36.43, 35.20, 33.54, 32.64, 30.62, 25.07, 14.28 ppm; IR (KBr):υmax = 3451.12, 2961.46, 2927.59, 2871.68, 1714.49, 1669.43, 1280.80, 1223.47, 1199.50, 1059.84, 1037.64 cm−1; MS (ESI): m/z: 369; HRMS (ESI) Calcd. for [(C20H26O5) + Na]+: 369.1678; found: 369.1677 [M + Na]+.
Compound 17: The compound is obtained as pale yellow liquid; 1H NMR (300 MHz, CDCl3) δ = 6.48 (d, J = 9 Hz, 1H), 5.68 (d, J = 9 Hz, 1H), 5.36 (s, 1H), 5.31 (s, 1H), 4.46 (s, 1H), 4.15 (q, J = 6 Hz, 2H), 3.37 (m, 1H), 2.45–2.39 (m, 5H), 1.98 (m, 2H), 1.78 (m, 1H), 1.68 (m, 1H), 1.24 (t, J = 6 Hz, 3H), 1.06 (s, 3H), 1.05 (s, 3H) ppm; 13C NMR (75.5 MHz, CDCl3) δ = 206.21, 199.22, 173.03, 157.97, 150.47, 127.26, 114.27, 79.08, 61.06, 59.93, 57.98, 45.66, 43.62, 42.09, 37.51, 34.07, 31.78, 30.36, 24.80, 14.36 ppm; IR (KBr):υmax = 3374.71, 2985.31, 2963.32, 2945.34, 2928.06, 2873.40, 1726.97, 1705.28, 1670.93, 1633.80, 1616.71, 1228.42, 1203.67, 1085.27, 1045.22 cm−1; MS (ESI): m/z: 369; HRMS (ESI) Calcd. for [(C20H26O5) + Na]+: 369.1678; found: 369.1682 [M + Na]+.
3.1.3. General Method for Synthesis of 16 and 18
To a solution of 15 (1.0 eq) in EtOAc was added IBX (3.0 eq). The mixture was heated at 70 °C for 4 h and filtered through a short pad of silica gel washed with EtOAc. The solvent was removed under reduced pressure and the residue was purified by flash column chromatography to provide 16.
Compound 16: The compound is obtained as pale yellow liquid; 1H NMR (300 MHz, CDCl3) δ = 6.57 (d, J = 9 Hz, 1H), 6.19 (s, 1H), 5.83 (d, J = 9 Hz, 1H), 5.59 (s, 1H), 4.27 (q, J = 6 Hz, 2H), 3.68 (m, 2H), 2.77–2.68 (m, 4H), 2.11 (d, 1H), 1.85 (m, 2H), 1.29 (t, J = 6 Hz, 3H), 1.16 (s, 6H) ppm; 13C NMR (75.5 MHz, CDCl3) δ = 206.69, 198.72, 197.33, 168.85, 157.95, 142.16, 126.66, 120.53, 61.80, 61.16, 54.10, 46.06, 39.07, 37.43, 35.08, 33.73, 32.93, 30.59, 25.18, 14.19ppm; IR (KBr):υmax= 3434.29, 2962.17, 2929.37, 2871.25, 1722.07, 1675.43, 1638.37, 1249.74, 1177.04, 1111.95 cm−1; MS (ESI): m/z: 367; HRMS (ESI) Calcd. for [(C20H24O5) + Na]+: 367.1521; found: 367.1528 [M + Na]+.
Compound 18: The compound is obtained as pale yellow liquid; 1H NMR (600 MHz, CDCl3) δ = 6.58 (d, J = 9.9 Hz, 1H), 6.13 (s, 1H), 5.78 (d, J = 9.9 Hz, 1H), 5.58 (s, 1H), 4.25 (q, J = 7.1 Hz, 2H), 3.78 (s, 1H), 2.93 (d, J = 12.2 Hz, 1H), 2.66 (m, 3H), 2.45 (m, 1H), 2.13 (d, J = 17.2 Hz, 1H), 1.87 (m, 2H), 1.31 (t, J = 7.1 Hz, 3H), 1.18 (s, 3H), 1.14 (s, 3H) ppm; 13C NMR (150 MHz, CDCl3) δ = 204.14, 200.19, 199.55, 169.71, 158.69, 142.90, 127.19, 119.93, 62.59, 61.80, 55.14, 45.85, 43.86, 40.88, 37.84, 34.18, 32.82, 30.26, 24.81, 14.18 ppm; IR (KBr):υmax = 3435.06, 2961.46, 2931.17, 2871.34, 1739.98, 1720.04, 1671.03, 1638.37, 1241.36, 1228.03, 1177.31, 1142.38 cm−1; MS (ESI): m/z: 367; HRMS (ESI) Calcd. for [(C20H24O5) + Na]+: 367.1521; found: 367.1515 [M + Na]+.