Aquaporin 11-Dependent Inhibition of Proliferation by Deuterium Oxide in Activated Hepatic Stellate Cells
Abstract
1. Introduction
2. Results
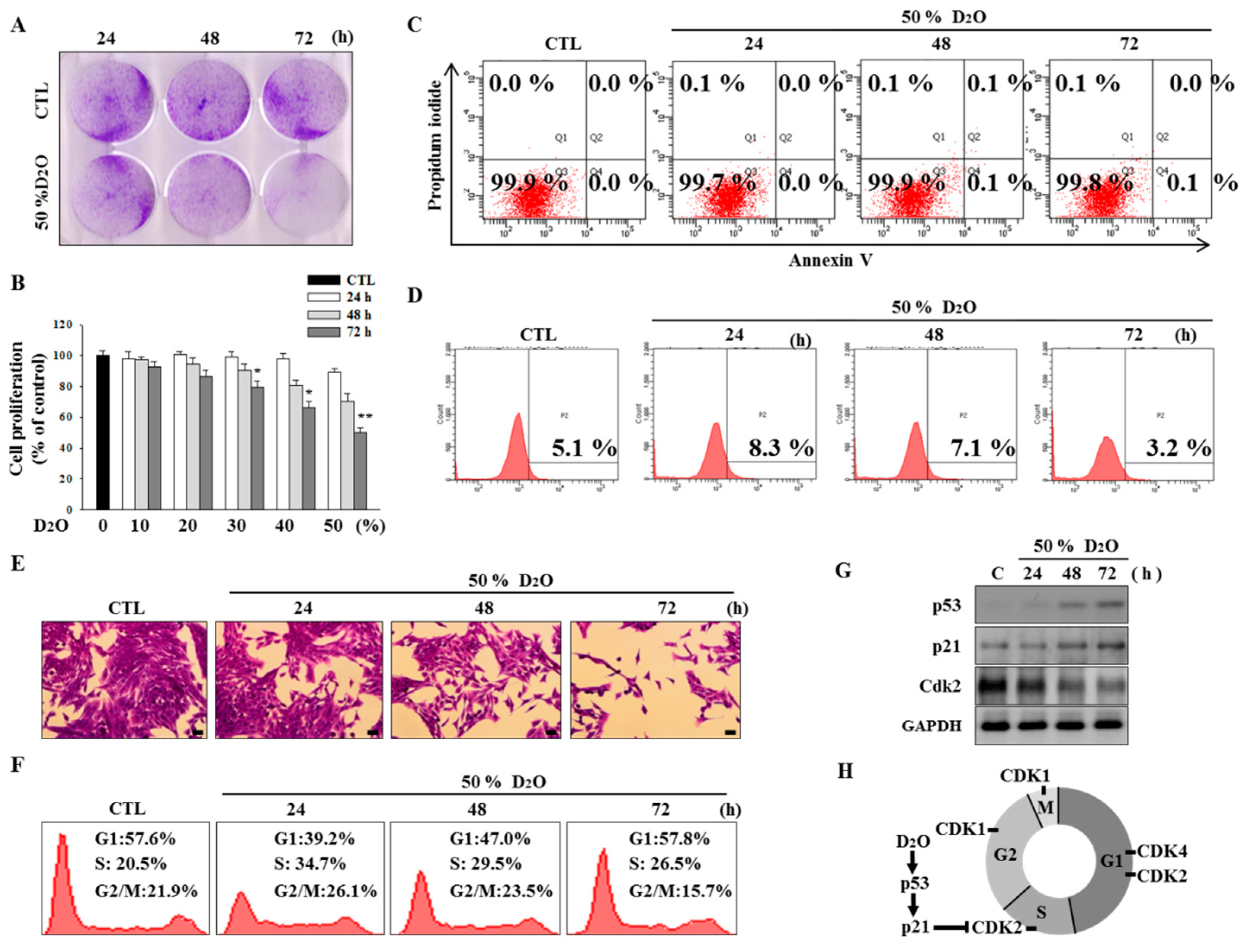
2.1. Activation of the p53-Cyclin-Dependent Pathway by D2O Leads to Cell Cycle Arrest
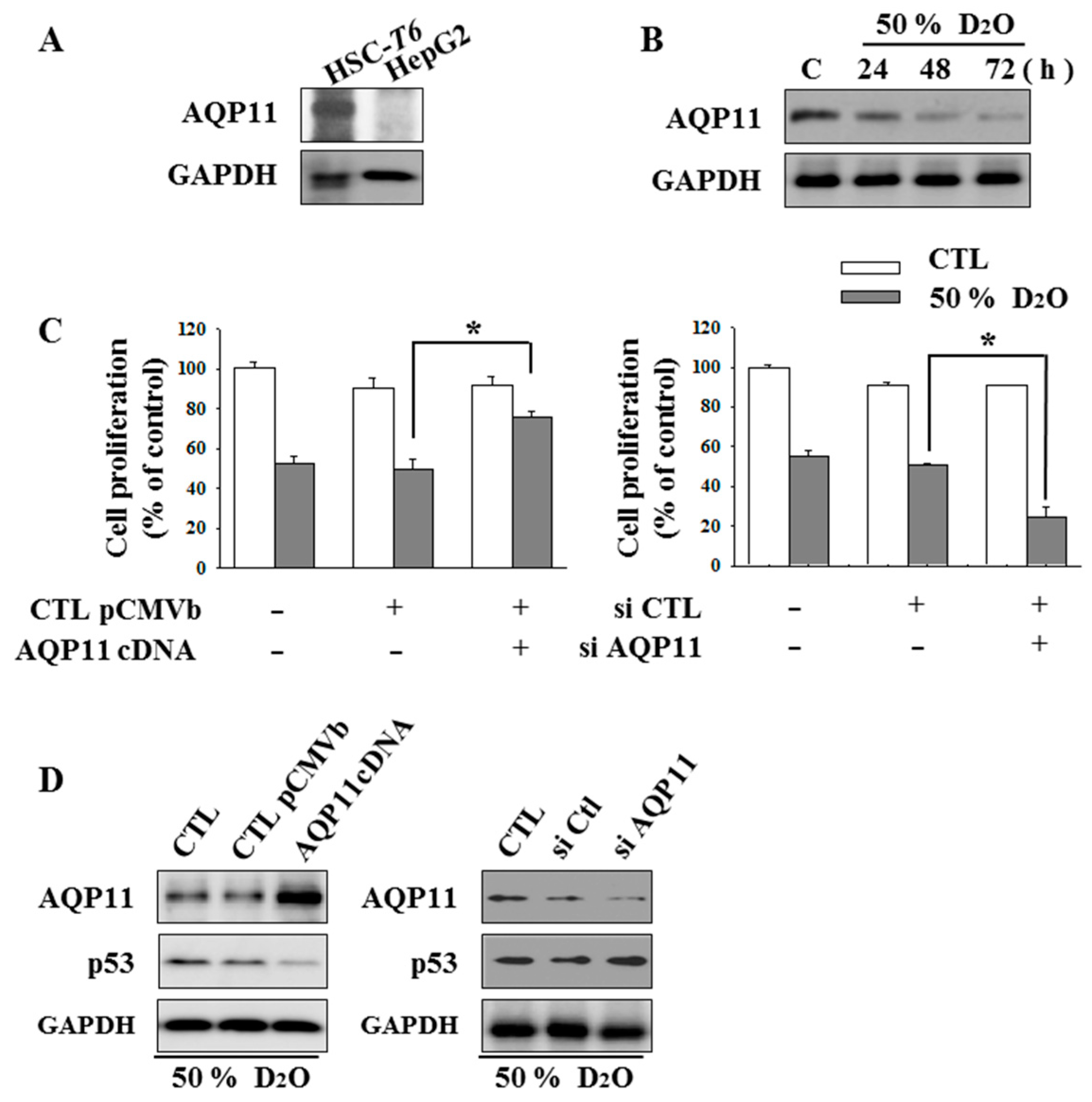
2.2. HSC Proliferation Is AQP11 Dependent
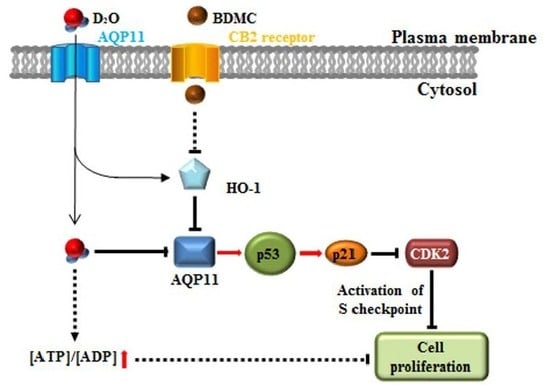
2.3. Inhibition of HO-1 Activity Increases the Antifibrotic Effect of D2O
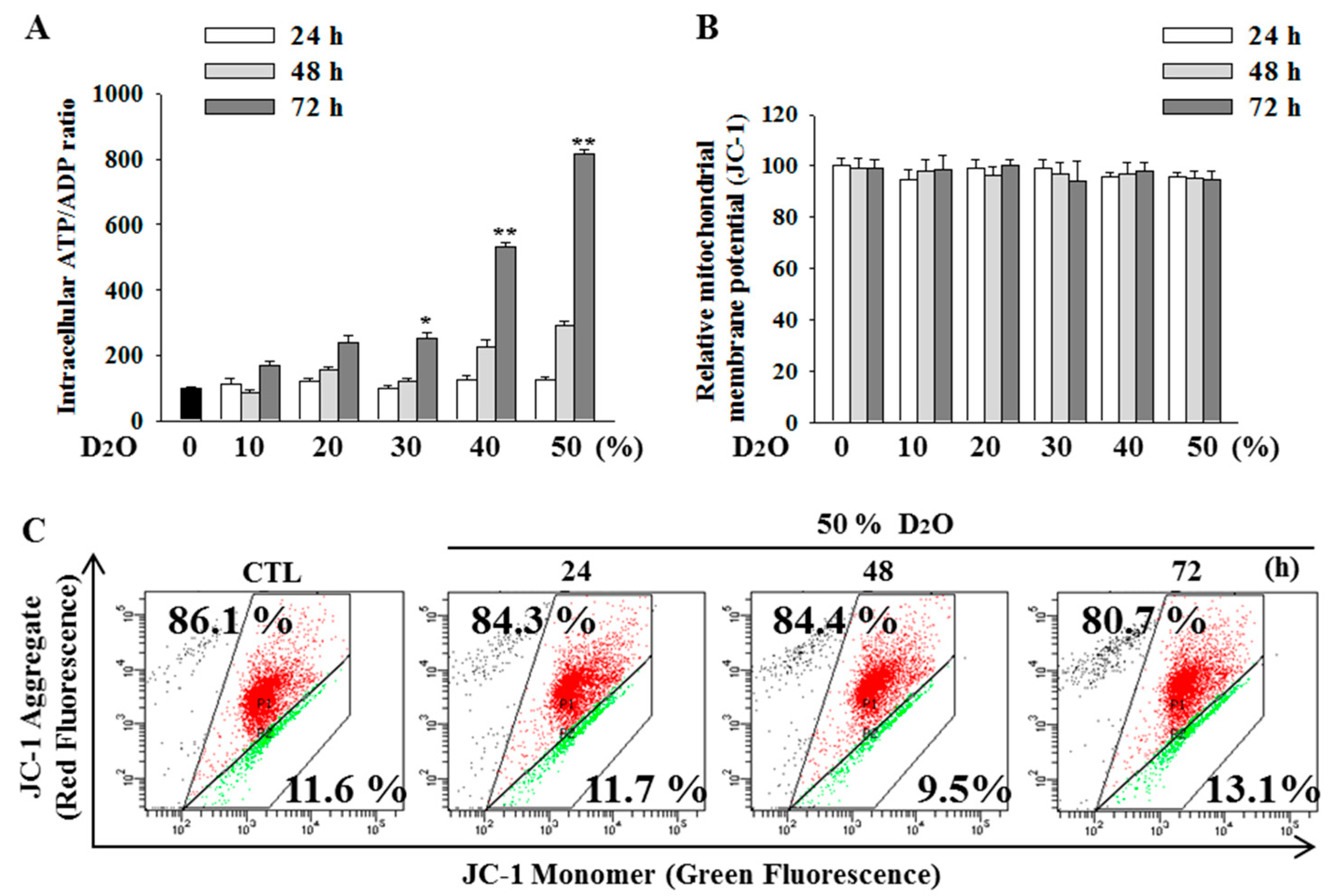
2.4. Excess Accumulation of ATP Diminishes Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Cell and Tissue Cultures
4.2. Reagents and Chemicals
4.3. Cell Proliferation
4.4. An Apoptosis Assay
4.5. Western Blotting and Antibodies
4.6. The Intracellular ATP/ADP Ratio
4.7. Analysis of the Cell Cycle
4.8. Intracellular ROS Levels
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Friedman, S.L. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N. Engl. J. Med. 2006, 328, 1828–1835. [Google Scholar]
- Kim, H.P.; Lee, E.J.; Kim, Y.C.; Kim, J.; Kim, H.K.; Park, J.H.; Kim, S.Y.; Kim, Y.C. Zeaxanthin dipalmitate from Lycium chinense fruit reduces experimentally induced hepatic fibrosis in rats. Biol. Pharm. Bull. 2002, 25, 390–392. [Google Scholar] [CrossRef]
- Baroni, G.S.; D’Ambrosio, L.; Curto, P.; Casini, A.; Mancini, R.; Jezequel, A.M.; Benedetti, A. Interferon gamma decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology 1996, 23, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Geerts, A.; Lazou, J.M.; De Bleser, P.; Wisse, E. Tissue distribution, quantitation and proliferation kinetics of fat-storing cells in carbon tetrachloride-injured rat liver. Hepatology 1991, 13, 1193–1202. [Google Scholar] [PubMed]
- Milani, S.; Herbst, H.; Schuppan, D.; Surrenti, C.; Riecken, E.O.; Stein, H. Cellular localization of type I III and IV procollagen gene transcripts in normal and fibrotic human liver. Am. J. Pathol. 1990, 137, 59–70. [Google Scholar] [PubMed]
- Gressner, O.A.; Weiskirchen, R.; Gressner, A.M. Evolving concepts of liver fibrogenesis provide new diagnostic and therapeutic options. Comp. Hepatol. 2007, 30, 6–7. [Google Scholar] [CrossRef]
- Iwaisako, K.; Brenner, D.A.; Kisseleva, T. What’s new in liver fibrosis? The origin of myofibroblasts in liver fibrosis. J. Gastroenterol. Hepatol. 2012, 27, 65–68. [Google Scholar] [CrossRef]
- Lee, P.J.; Park, H.J.; Cho, N.; Kim, H.P. 3,5-Diethoxy-3′-hydroxyresveratrol (DEHR) ameliorates liver fibrosis via caveolin-1 activation in hepatic stellate cells and in a mouse model of bile duct ligation injury. Molecules 2018, 23, 2833. [Google Scholar] [CrossRef]
- Wang, J.; Feng, L.; Zhu, Z.; Zheng, M.; Wang, D.; Chen, Z.; Sun, H. Aquaporins as diagnostic and therapeutic targets in cancer: How far we are? J. Transl. Med. 2015, 21, 13–96. [Google Scholar] [CrossRef]
- Pellavio, G.; Rui, M.; Caliogna, L.; Martino, E.; Gastaldi, G.; Collina, S.; Laforenza, U. Regulation of aquaporin functional properties mediated by the antioxidant effects of natural compounds. Int. J. Mol. Sci. 2017, 18, 2665. [Google Scholar] [CrossRef]
- Lakner, A.M.; Walling, T.L.; McKillop, I.H.; Schrum, L.W. Altered aquaporin expression and role in apoptosis during hepatic stellate cell activation. Liver Int. 2011, 31, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Knock-out models reveal new aquaporin functions. Handb. Exp. Pharmacol. 2009, 190, 359–381. [Google Scholar]
- Masyuk, A.I.; Larusso, N.F. Aquaporins in the hepatobiliary system. Hepatology 2006, 43, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, D.; Lamprecht, J.; Eckhardt, R.; Futterman, G.; Paweletz, N. Deuterium oxide (heavy water) arrests the cell cycle of PtK2 cells during interphase. Eur. J. Cell. Biol. 1992, 58, 365–370. [Google Scholar] [PubMed]
- Bader, Y.; Hartmann, J.; Horvath, Z.; Saiko, P.; Grusch, M.; Madlener, S.; Oehler, L.; Fritzer-Szekeres, M.; Heller, N.; Alken, R.G.; et al. Synergistic effects of deuterium oxide and gemcitabine in human pancreatic cancer cell lines. Cancer Lett. 2008, 259, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, J.; Schroeter, D.; Paweletz, N. Disorganization of mitosis in HeLa cells by deuterium oxide. Eur. J. Cell. Biol. 1989, 50, 360–369. [Google Scholar] [PubMed]
- Lamprecht, J.; Schroeter, D.; Paweletz, N. Mitosis arrested by deuterium oxide. Light microscopic, immunofluorescence and ultrastructural characterization. Eur. J. Cell. Biol. 1990, 51, 303–312. [Google Scholar] [PubMed]
- Bila, W.C.; Mariano, R.M.D.S.; Silva, V.R.; Dos Santos, M.E.S.M.; Lamounier, J.A.; Ferriolli, E.; Galdino, A. Applications of deuterium oxide in human health. Isotopes Environ. Health Stud. 2017, 53, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.J.; Woo, S.J.; Jee, J.G.; Sung, S.H.; Kim, H.P. Bisdemethoxycurcumin induces apoptosis in activated hepatic stellate cells via cannabinoid receptor 2. Molecules 2015, 20, 1277–1292. [Google Scholar] [CrossRef]
- Tardelli, M.; Bruschi, F.V.; Claudel, T.; Moreno-Viedma, V.; Halilbasic, E.; Marra, F.; Herac, M.; Stulnig, T.M.; Trauner, M. AQP3 is regulated by PPARγ and JNK in hepatic stellate cells carrying PNPLA3 I148M. Sci. Rep. 2017, 7, 14661. [Google Scholar] [CrossRef]
- Kushner, D.J.; Baker, A.; Dunstall, T.G. Pharmacological uses and perspective of heavy water and deuterated compounds. Can. J. Physiol. Pharmacol. 1999, 7, 79–88. [Google Scholar] [CrossRef]
- Marchetti, A.; Cecchinelli, B.; D’Angelo, M.; D’Orazi, G.; Crescenzi, M.; Sacchi, A.; Soddu, S. p53 can inhibit cell proliferation through caspase-mediated cleavage of ERK2/MAPK. Cell. Death Differ. 2004, 11, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Wilkening, S.; Stahl, F.; Bader, A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab. Dispos. 2003, 31, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R.K.; Checcarelli, N.; Naini, A.; De Vivo, D.C.; DiMauro, S.; Sue, C.M. Measurement of ATP production in mitochondrial disorders. J. Inherit. Metab. Dis. 2006, 29, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Galan-Cobo, A.; Ramirez-Lorca, R.; Echeyarria, M. Role of aquaporins in cell proliferation: What else beyond water permeability? Channels 2016, 10, 185–201. [Google Scholar] [CrossRef]
- Boury-Jamot, M.; Sougrat, R.; Tailhardat, M.; Le Varlet, B.; Bonté, F.; Dumas, M.; Verbavatz, J.M. Expression and function of aquaporins in human skin. Is aquaporin-3 just a glycerol transporter? Biochim. Biophys. Acta 2006, 1758, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xu, G.; Jiang, T.; Qin, Y. Pharmacologic induction of heme oxygenase-1 plays a protective role in diabetic retinopathy in rats. Invest. Ophthalmol. Vis. Sci. 2016, 53, 6541–6556. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Lo, C.S.; Zhao, X.P.; Liao, M.C.; Chenier, I.; Bouley, R.; Ingelfinger, J.R.; Chan, J.S.; Zhang, S.L. Overexpression of angiotensinogen downregulates aquaporin 1 expression via modulation of Nrf2–HO-1 pathway in renal proximal tubular cells of transgenic mice. J. Renin Angiotensin Aldosterone Syst. 2016, 17. [Google Scholar] [CrossRef]
- Trabanelli, S.; Ocadlíková, D.; Gulinelli, S.; Curti, A.; Salvestrini, V.; Vieira, R.P.; Idzko, M.; Di Virgilio, F.; Ferrari, D.; Lemoli, R.M. Extracellular ATP exerts opposite effects on activated and regulatory CD4+ T cells via purinergic P2 receptor activation. J. Immunol. 2012, 189, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Lobyshev, V.I.; Fogel, I.; Iakovenko, L.V.; Rezaeva, M.N.; Tverdislov, V.A. D2O as a modifier of ionic specificity of Na, K-ATPase. Biofizika 1982, 27, 595–603. [Google Scholar] [PubMed]
- Silver, R.B.; Breton, S.; Brown, D. Potassium depletion increases proton pump (H(+)-ATPase) activity in intercalated cells of cortical collecting duct. Am. J. Physiol. Renal Physiol. 2000, 279, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Di Noia, M.A.; Van Driesche, S.; Palmieri, F.; Yang, L.M.; Quan, S.; Goodman, A.I.; Abraham, N.G. Heme oxygenase-1 enhances renal mitochondrial transport carriers and cytochrome C oxidase activity in experimental diabetes. J. Biol. Chem. 2006, 281, 15687–15693. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, P.J.; Park, H.-J.; Cho, N.; Kim, H.P. Aquaporin 11-Dependent Inhibition of Proliferation by Deuterium Oxide in Activated Hepatic Stellate Cells. Molecules 2018, 23, 3209. https://doi.org/10.3390/molecules23123209
Lee PJ, Park H-J, Cho N, Kim HP. Aquaporin 11-Dependent Inhibition of Proliferation by Deuterium Oxide in Activated Hepatic Stellate Cells. Molecules. 2018; 23(12):3209. https://doi.org/10.3390/molecules23123209
Chicago/Turabian StyleLee, Phil Jun, Hye-Jin Park, Namki Cho, and Hong Pyo Kim. 2018. "Aquaporin 11-Dependent Inhibition of Proliferation by Deuterium Oxide in Activated Hepatic Stellate Cells" Molecules 23, no. 12: 3209. https://doi.org/10.3390/molecules23123209
APA StyleLee, P. J., Park, H.-J., Cho, N., & Kim, H. P. (2018). Aquaporin 11-Dependent Inhibition of Proliferation by Deuterium Oxide in Activated Hepatic Stellate Cells. Molecules, 23(12), 3209. https://doi.org/10.3390/molecules23123209