Pseudane-VII Regulates LPS-Induced Neuroinflammation in Brain Microglia Cells through the Inhibition of iNOS Expression
Abstract
1. Introduction
2. Results
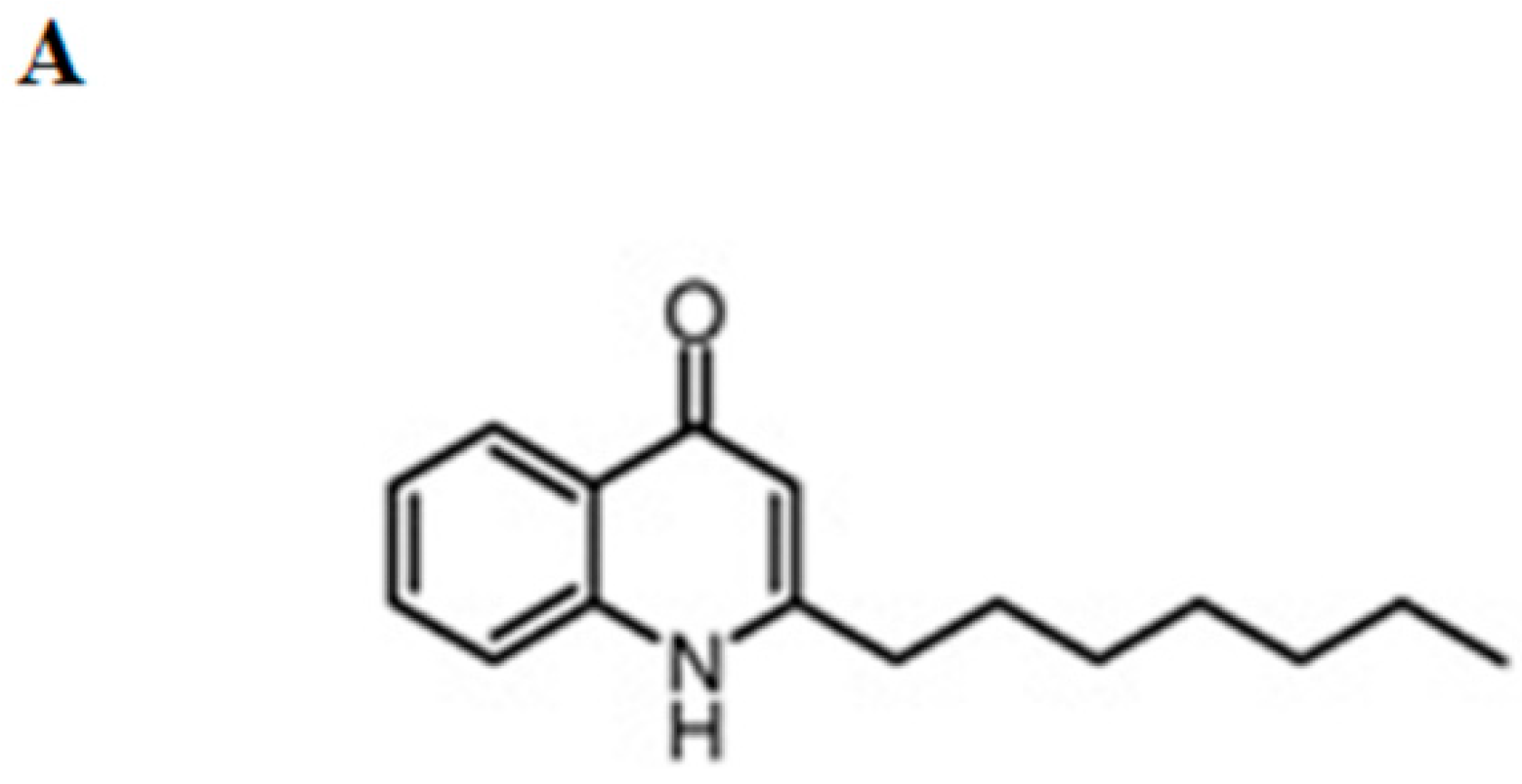
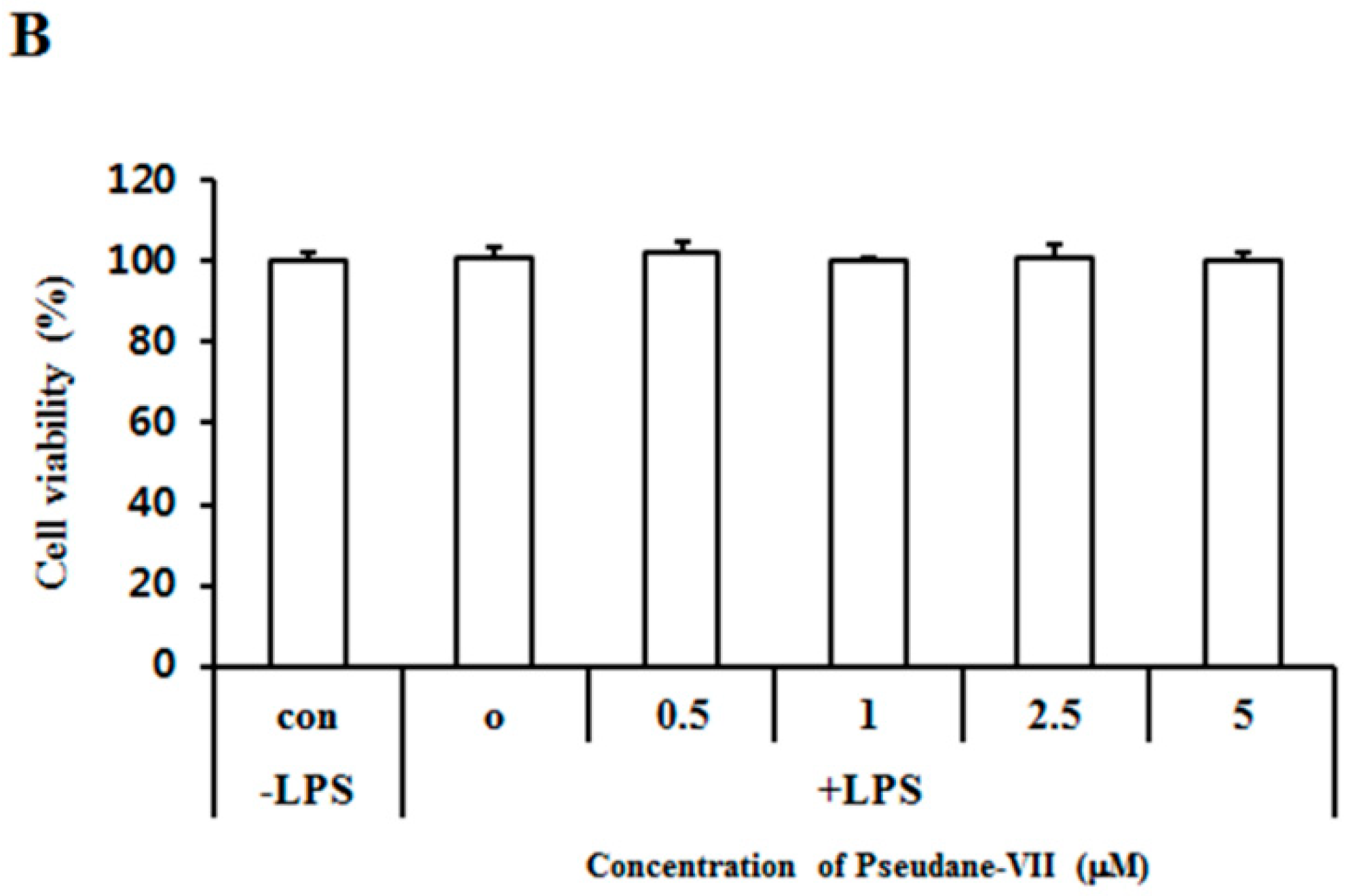
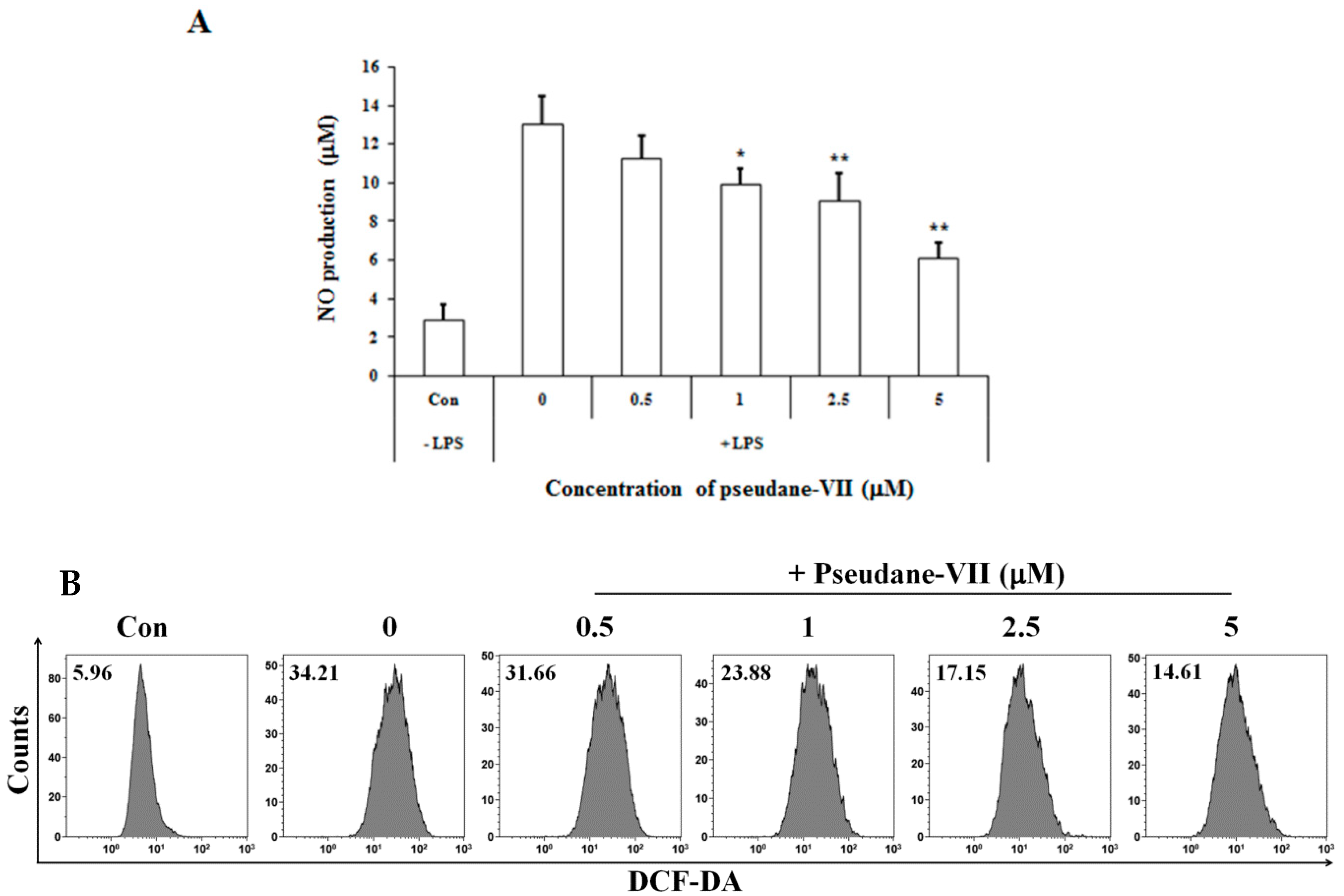
2.1. Pseudane-VII Inhibits LPS-Induced NO Production in BV-2 Microglial Cells
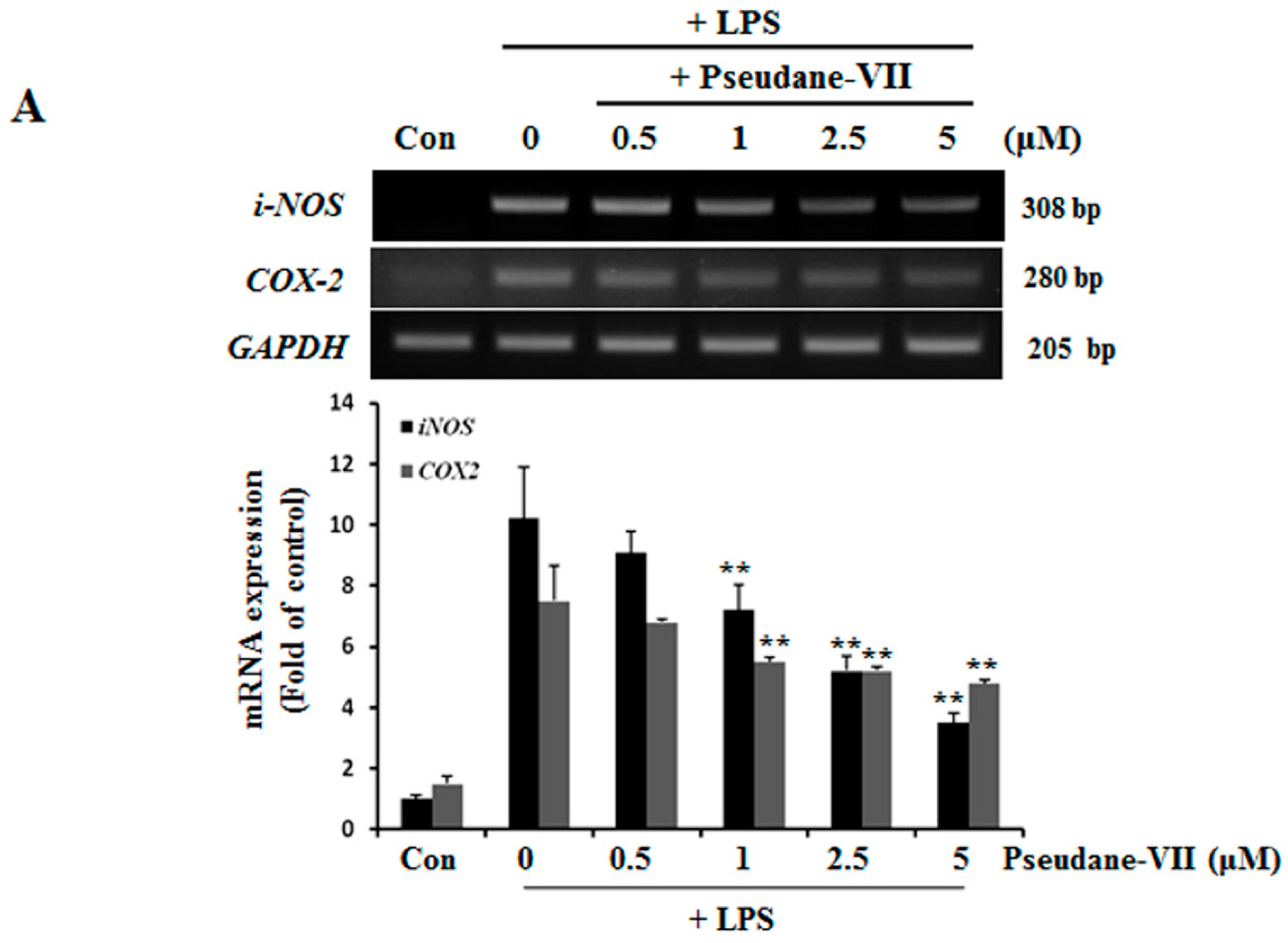
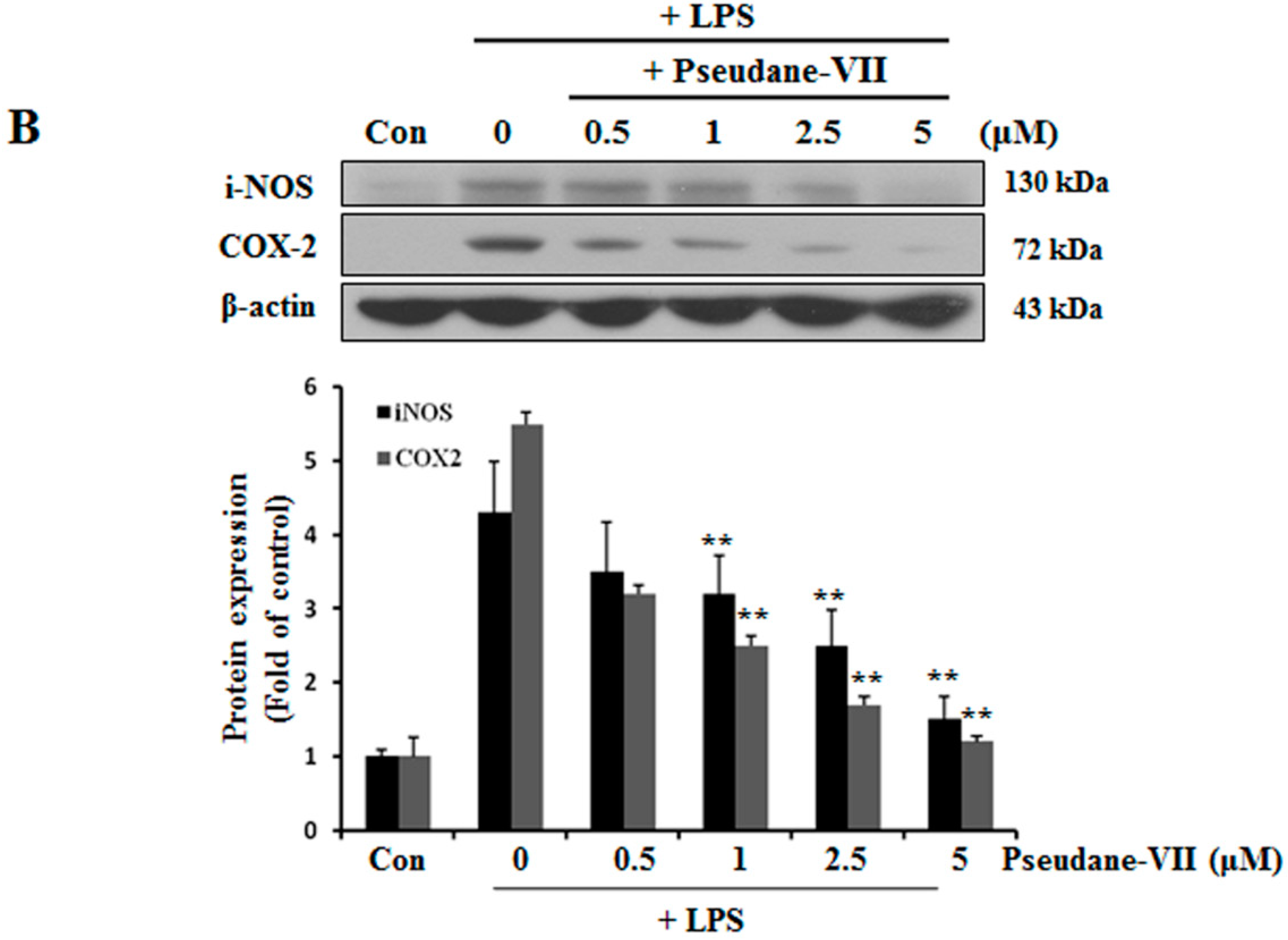
2.2. Pseudane-VII Decreases mRNA and Protein Levels of iNOS and COX-2
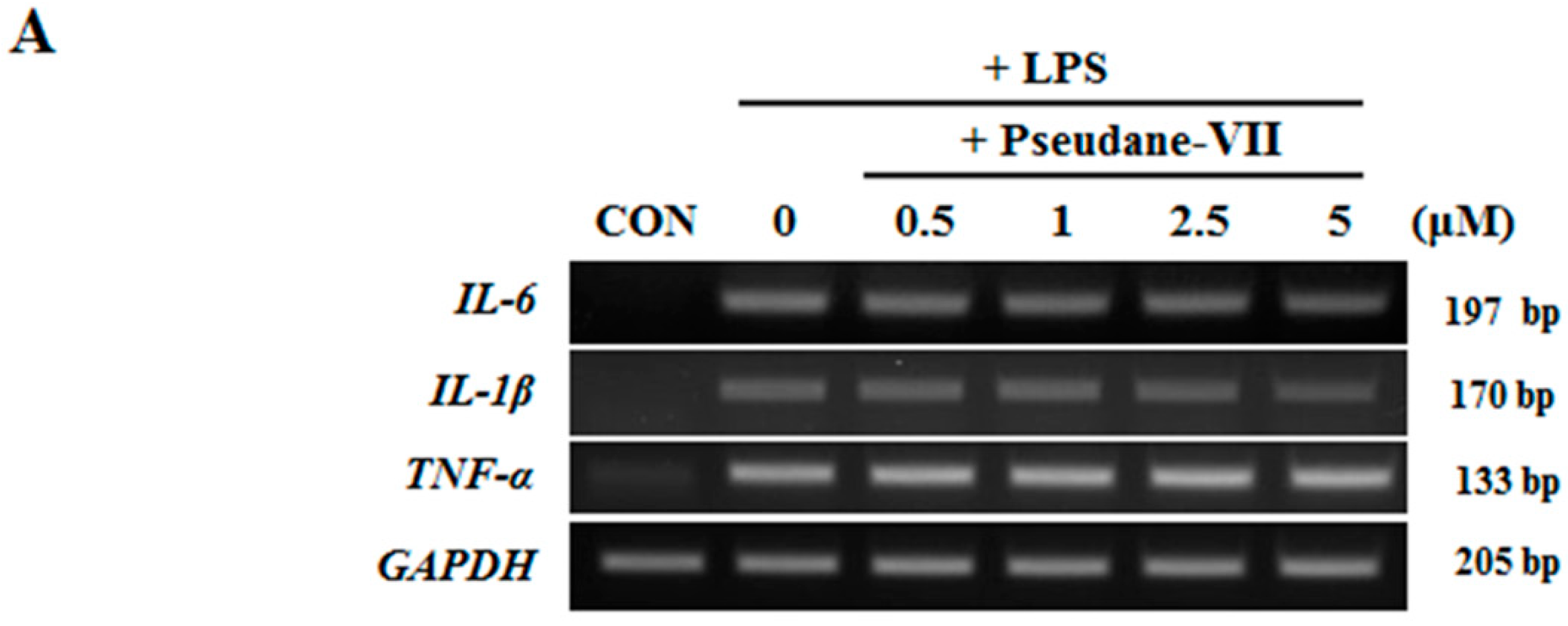
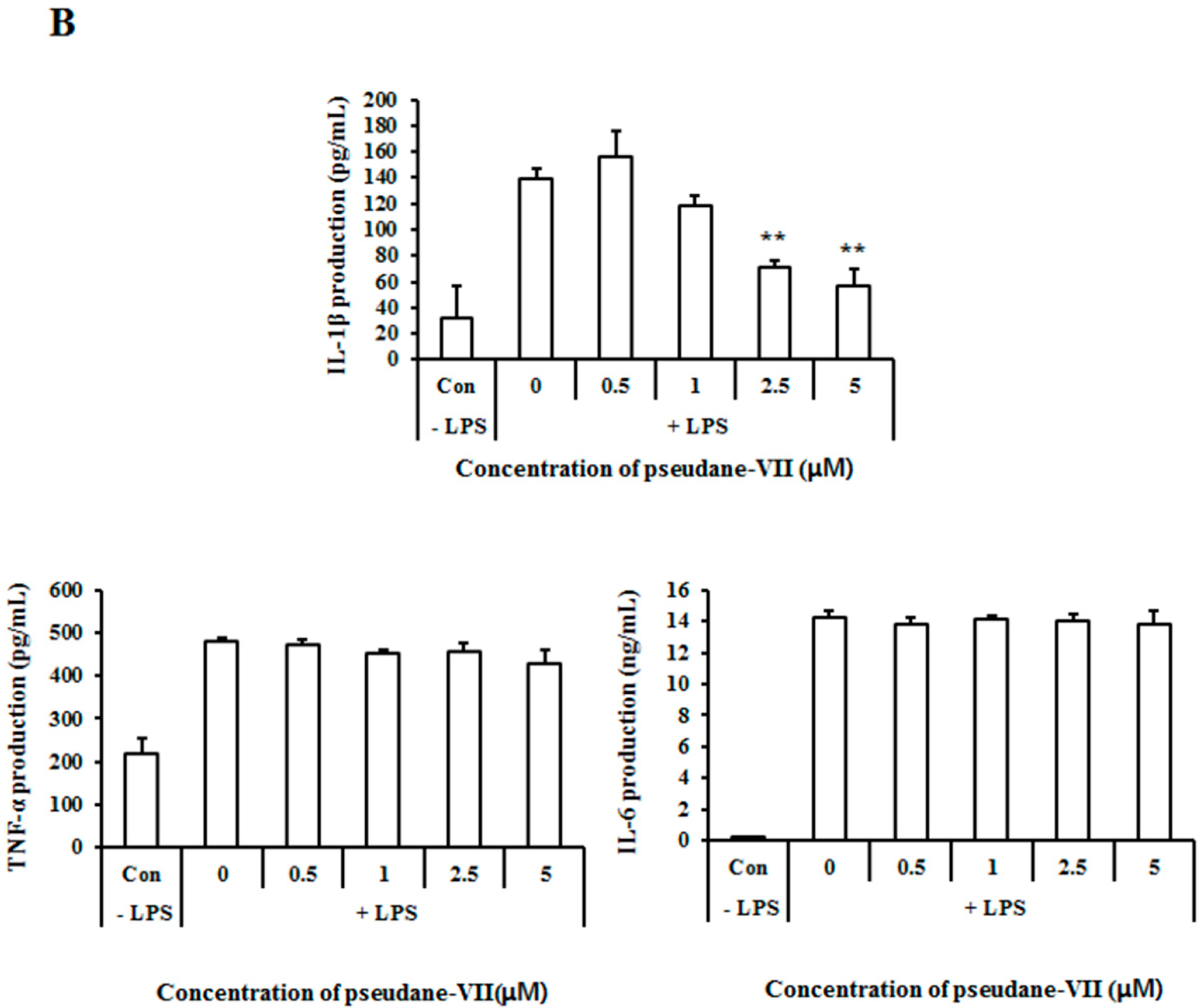
2.3. Pseudane-VII Inhibits mRNA Expression and Protein Production of the Pro-Inflammatory FactorIL-1β
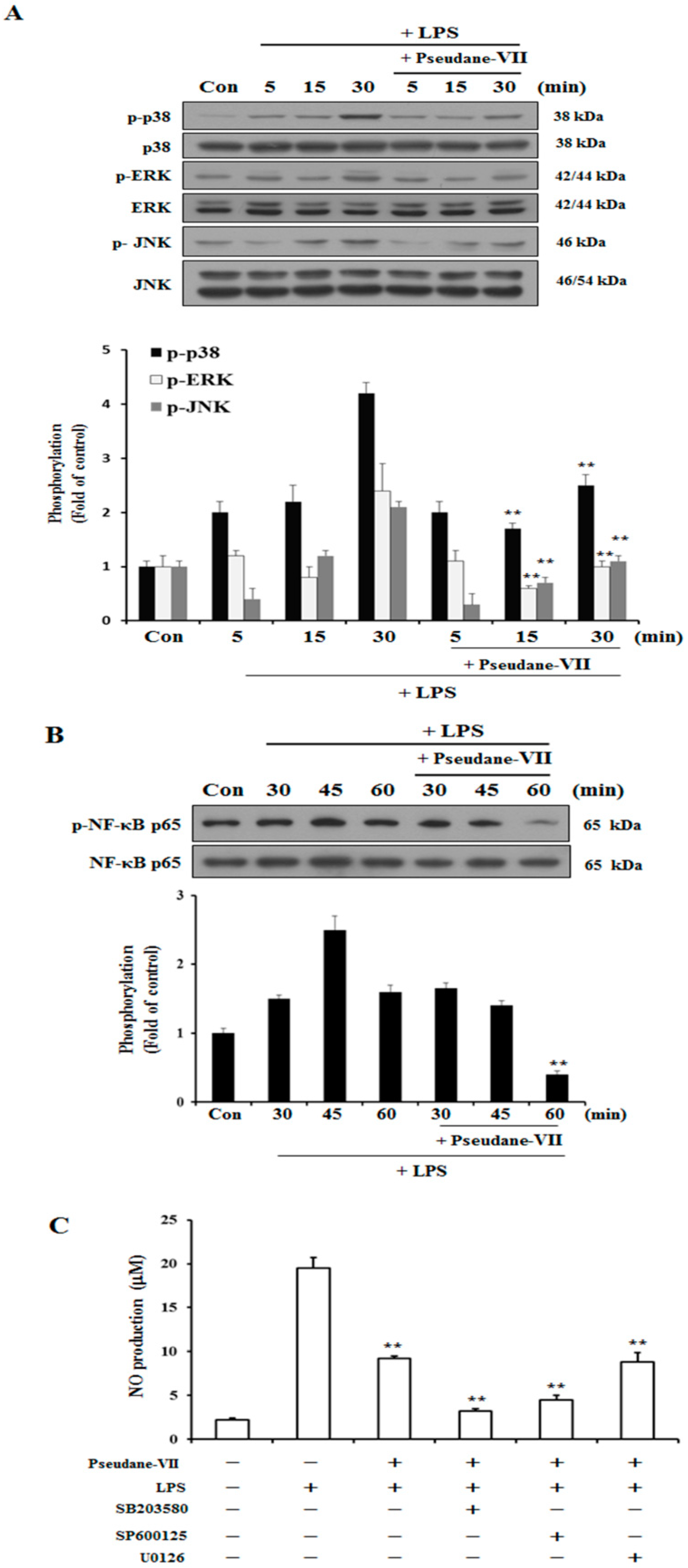
2.4. Pseudane-VII Suppresses MAPK and NF-κB Phosphorylation
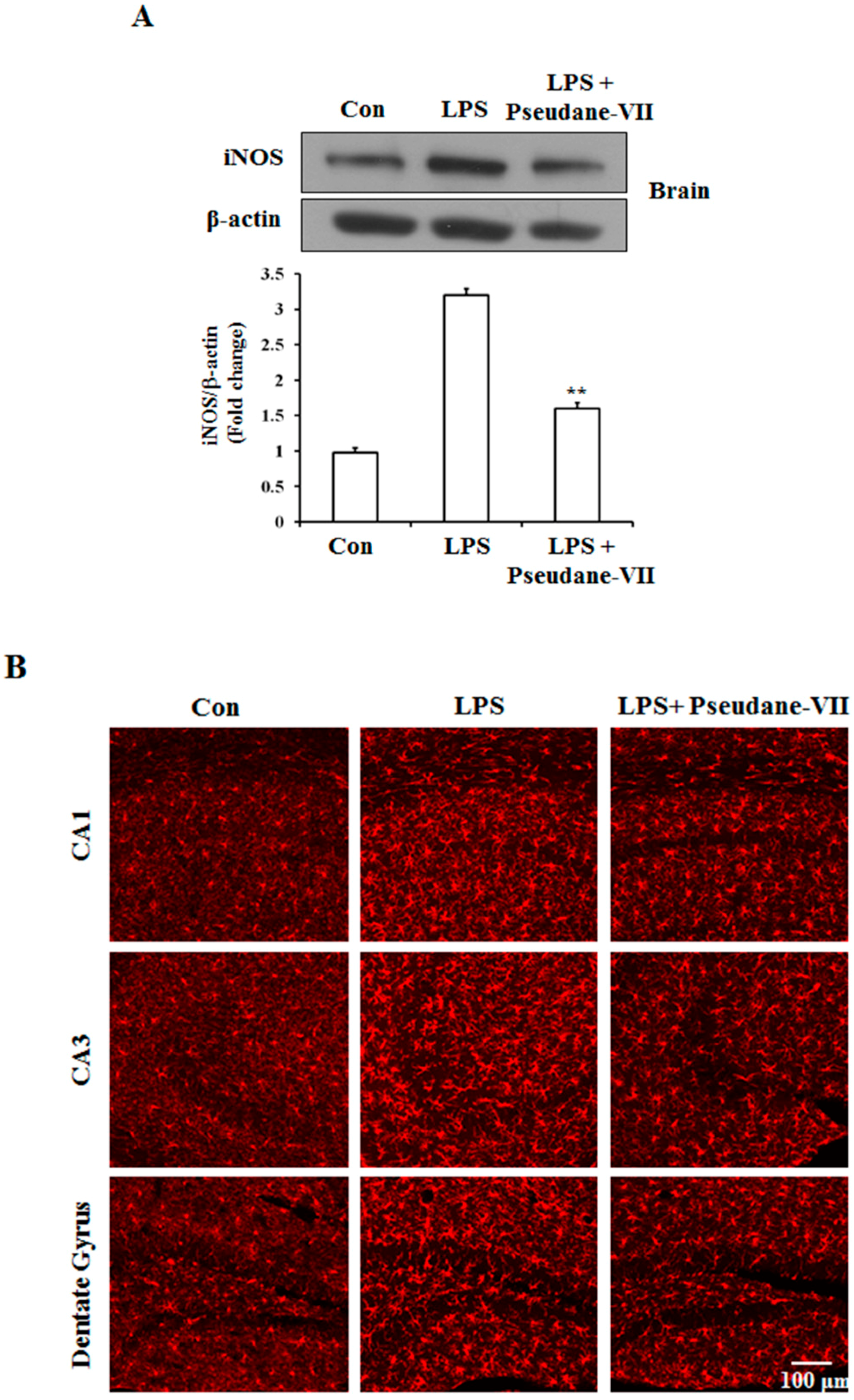
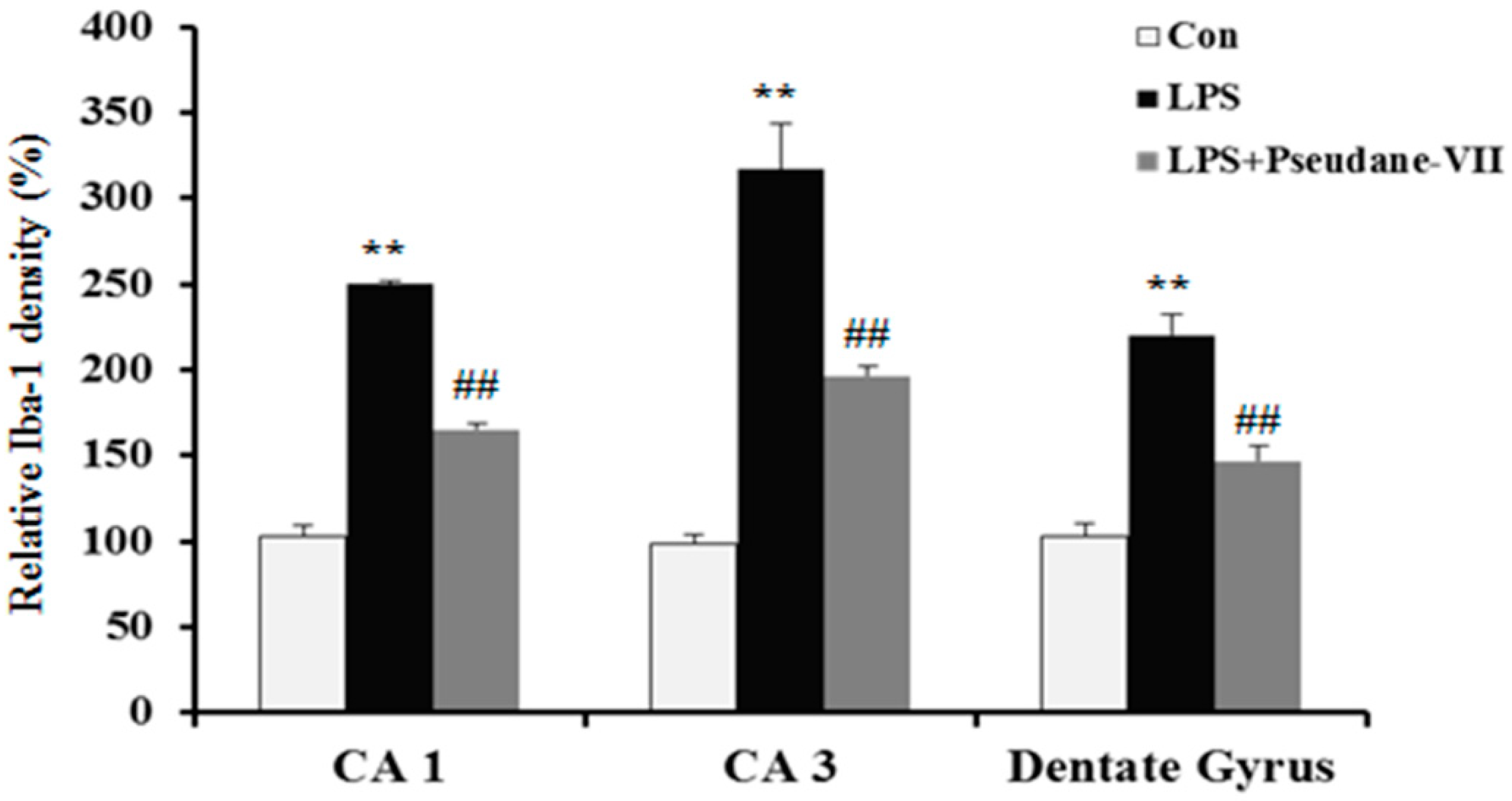
2.5. Pseudane-VII Ameliorates LPS-Induced iNOS Expression in Brain Tissue
3. Discussion
4. Material and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Treatment
4.3. Cytotoxicity Assay
4.4. NO Assay
4.5. RNA Isolation and RT-PCR
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Western Blot Analysis
4.8. In Vivo Experiment
4.9. Immunohistochemistry
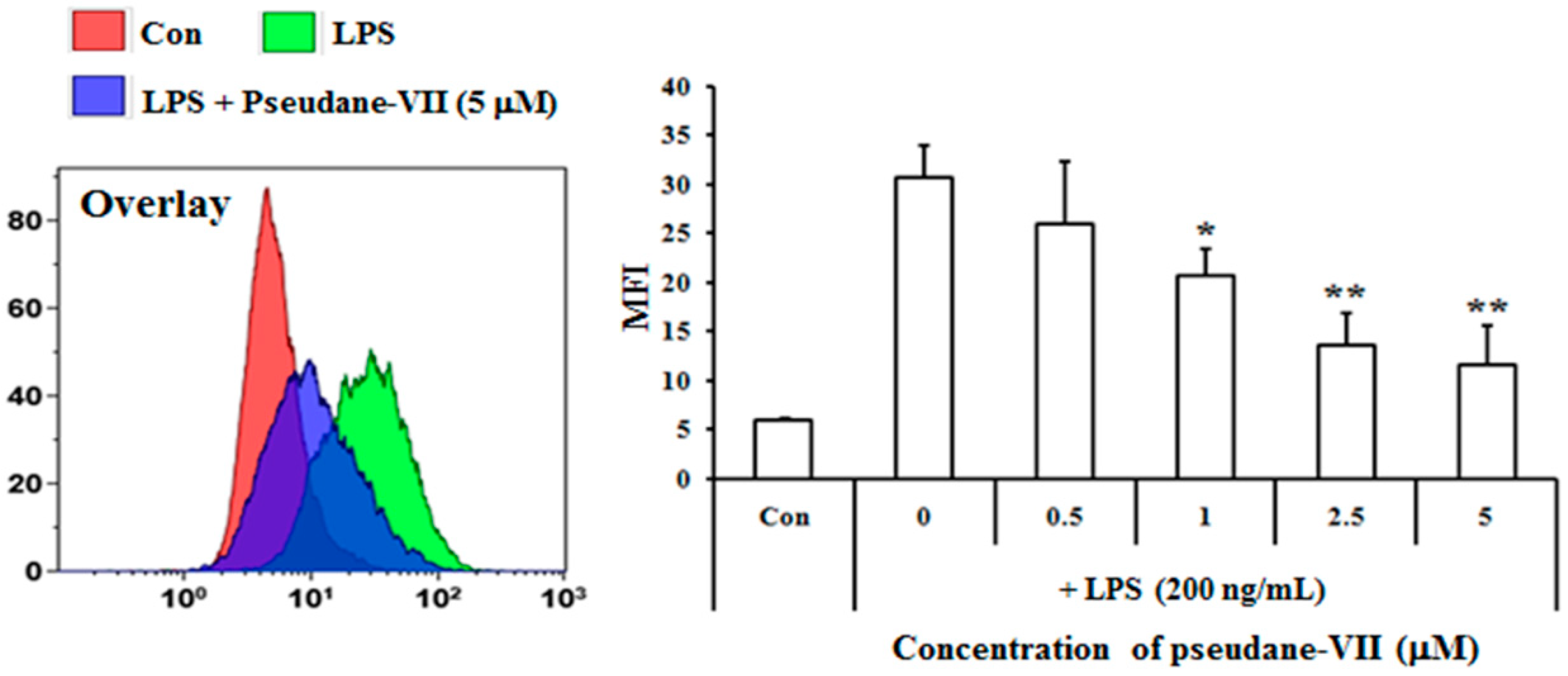
4.10. Intracellular ROS Analysis
4.11. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [PubMed]
- De Vries, H.E.; Kuiper, J.; de Boer, A.G.; Van Berkel, T.J.; Breimer, D.D. The blood-brain barrier in neuroinflammatory diseases. Pharmacol. Rev. 1997, 49, 143–155. [Google Scholar] [PubMed]
- Perry, V.H.; Teeling, J. Microglia and macrophages of the central nervous system: The contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol. 2013, 35, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Q.; Li, D.D.; Huang, C.; Lu, D.S.; Zhang, C.; Zhou, S.Y.; Liu, J.; Zhang, F. Icariin Reduces Dopaminergic Neuronal Loss and Microglia-Mediated Inflammation in Vivo and in Vitro. Front. Mol. Neurosci. 2017, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Loane, D.J.; Byrnes, K.R. Role of microglia in neurotrauma. Neurotherapeutics 2010, 7, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Prow, N.A.; Irani, D.N. The inflammatory cytokine, interleukin-1 beta, mediates loss of astroglial glutamate transport and drives excitotoxic motor neuron injury in the spinal cord during acute viral encephalomyelitis. J. Neurochem. 2008, 105, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Xu, Y.; Lv, D.; Zhang, L. Rosiglitazone ameliorates astrocyte over-activation and inflammatory cytokine release induced by global cerebral ischemia/reperfusion. Exp. Ther. Med. 2016, 11, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Bachstetter, A.D.; Van Eldik, L.J. The p38 MAP Kinase Family as Regulators of Proinflammatory Cytokine Production in Degenerative Diseases of the CNS. Aging Dis. 2010, 1, 199–211. [Google Scholar]
- Heeb, S.; Fletcher, M.P.; Chhabra, S.R.; Diggle, S.P.; Williams, P.; Camara, M. Quinolones: From antibiotics to autoinducers. FEMS Microbiol. Rev. 2011, 35, 247–274. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.E.; Jung, I.; Lee, J.S.; Na, J.Y.; Kim, W.J.; Kim, Y.O.; Park, Y.D. Pseudane-VII Isolated from Pseudoalteromonas sp. M2 Ameliorates LPS-Induced Inflammatory Response In Vitro and In Vivo. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Kim, Y.O.; Kim, J.H.; Nam, B.H.; Kim, D.G.; An, C.M.; Lee, J.S.; Kim, P.S.; Lee, H.M.; Oh, J.S.; et al. Liquid Chromatography-Mass Spectrometry-Based Rapid Secondary-Metabolite Profiling of Marine Pseudoalteromonas sp. M2. Mar. Drugs 2016, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.D.; Liu, Z.H.; Zhang, L.F.; Yao, J.N.; Wang, C.F. Sanggenon C induces apoptosis of colon cancer cells via inhibition of NO production, iNOS expression and ROS activation of the mitochondrial pathway. Oncol. Rep. 2017, 38, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.F.; Kodadek, T. The Immune System and Neuroinflammation as Potential Sources of Blood-Based Biomarkers for Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease. ACS Chem. Neurosci. 2016, 7, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Filiou, M.D.; Arefin, A.S.; Moscato, P.; Graeber, M.B. ‘Neuroinflammation’ differs categorically from inflammation: Transcriptomes of Alzheimer’s disease, Parkinson’s disease, schizophrenia and inflammatory diseases compared. Neurogenetics 2014, 15, 201–212. [Google Scholar] [CrossRef]
- Moore, A.H.; Bigbee, M.J.; Boynton, G.E.; Wakeham, C.M.; Rosenheim, H.M.; Staral, C.J.; Morrissey, J.L.; Hund, A.K. Non-Steroidal Anti-Inflammatory Drugs in Alzheimer’s Disease and Parkinson’s Disease: Reconsidering the Role of Neuroinflammation. Pharmaceuticals 2010, 3, 1812–1841. [Google Scholar] [CrossRef]
- Boje, K.M.; Arora, P.K. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992, 587, 250–256. [Google Scholar] [CrossRef]
- McGeer, P.L.; Kawamata, T.; Walker, D.G.; Akiyama, H.; Tooyama, I.; McGeer, E.G. Microglia in degenerative neurological disease. Glia 1993, 7, 84–92. [Google Scholar] [CrossRef]
- Stojakovic, A.; Paz-Filho, G.; Arcos-Burgos, M.; Licinio, J.; Wong, M.L.; Mastronardi, C.A. Role of the IL-1 Pathway in Dopaminergic Neurodegeneration and Decreased Voluntary Movement. Mol. Neurobiol. 2017, 54, 4486–4495. [Google Scholar] [CrossRef]
- Kim, S.H.; Smith, C.J.; Van Eldik, L.J. Importance of MAPK pathways for microglial pro-inflammatory cytokine IL-1 beta production. Neurobiol. Aging 2004, 25, 431–439. [Google Scholar] [CrossRef]
- Jana, M.; Dasgupta, S.; Saha, R.N.; Liu, X.; Pahan, K. Induction of tumor necrosis factor-alpha (TNF-alpha) by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J. Neurochem. 2003, 86, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Wilms, H.; Rosenstiel, P.; Sievers, J.; Deuschl, G.; Zecca, L.; Lucius, R. Activation of microglia by human neuromelanin is NF-kappaB dependent and involves p38 mitogen-activated protein kinase: Implications for Parkinson’s disease. FASEB J. 2003, 17, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Suter, M.R. p38 MAPK, microglial signaling, and neuropathic pain. Mol. Pain 2007, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Akama, K.T.; Albanese, C.; Pestell, R.G.; Van Eldik, L.J. Amyloid beta-peptide stimulates nitric oxide production in astrocytes through an NFkappaB-dependent mechanism. Proc. Natl. Acad. Sci. USA 1998, 95, 5795–5800. [Google Scholar] [CrossRef] [PubMed]
- John, G.R.; Lee, S.C.; Brosnan, C.F. Cytokines: Powerful regulators of glial cell activation. Neuroscientist 2003, 9, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Meffert, M.K. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006, 13, 852–860. [Google Scholar] [CrossRef]
- Kwakowsky, A.; Milne, M.R.; Waldvogel, H.J.; Faull, R.L. Effect of Estradiol on Neurotrophin Receptors in Basal Forebrain Cholinergic Neurons: Relevance for Alzheimer’s Disease. Int. J. Mol. Sci. 2016, 17, 2122. [Google Scholar] [CrossRef]
- Kwakowsky, A.; Potapov, K.; Kim, S.; Peppercorn, K.; Tate, W.P.; Abraham, I.M. Treatment of beta amyloid 1-42 (Abeta(1-42))-induced basal forebrain cholinergic damage by a non-classical estrogen signaling activator in vivo. Sci. Rep. 2016, 6, 21101. [Google Scholar] [CrossRef]
- Jahrling, J.B.; Hernandez, C.M.; Denner, L.; Dineley, K.T. PPARgamma recruitment to active ERK during memory consolidation is required for Alzheimer’s disease-related cognitive enhancement. J. Neurosci. 2014, 34, 4054–4063. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.E.; Jung, I.; Na, J.Y.; Lee, Y.; Lee, J.; Lee, J.S.; Lee, J.S. Pseudane-VII Regulates LPS-Induced Neuroinflammation in Brain Microglia Cells through the Inhibition of iNOS Expression. Molecules 2018, 23, 3196. https://doi.org/10.3390/molecules23123196
Kim ME, Jung I, Na JY, Lee Y, Lee J, Lee JS, Lee JS. Pseudane-VII Regulates LPS-Induced Neuroinflammation in Brain Microglia Cells through the Inhibition of iNOS Expression. Molecules. 2018; 23(12):3196. https://doi.org/10.3390/molecules23123196
Chicago/Turabian StyleKim, Mi Eun, Inae Jung, Ju Yong Na, Yujeong Lee, Jaewon Lee, Jong Suk Lee, and Jun Sik Lee. 2018. "Pseudane-VII Regulates LPS-Induced Neuroinflammation in Brain Microglia Cells through the Inhibition of iNOS Expression" Molecules 23, no. 12: 3196. https://doi.org/10.3390/molecules23123196
APA StyleKim, M. E., Jung, I., Na, J. Y., Lee, Y., Lee, J., Lee, J. S., & Lee, J. S. (2018). Pseudane-VII Regulates LPS-Induced Neuroinflammation in Brain Microglia Cells through the Inhibition of iNOS Expression. Molecules, 23(12), 3196. https://doi.org/10.3390/molecules23123196






