Synthesis, Experimental and Density Functional Theory (DFT) Studies on Solubility of Camptothecin Derivatives
Abstract
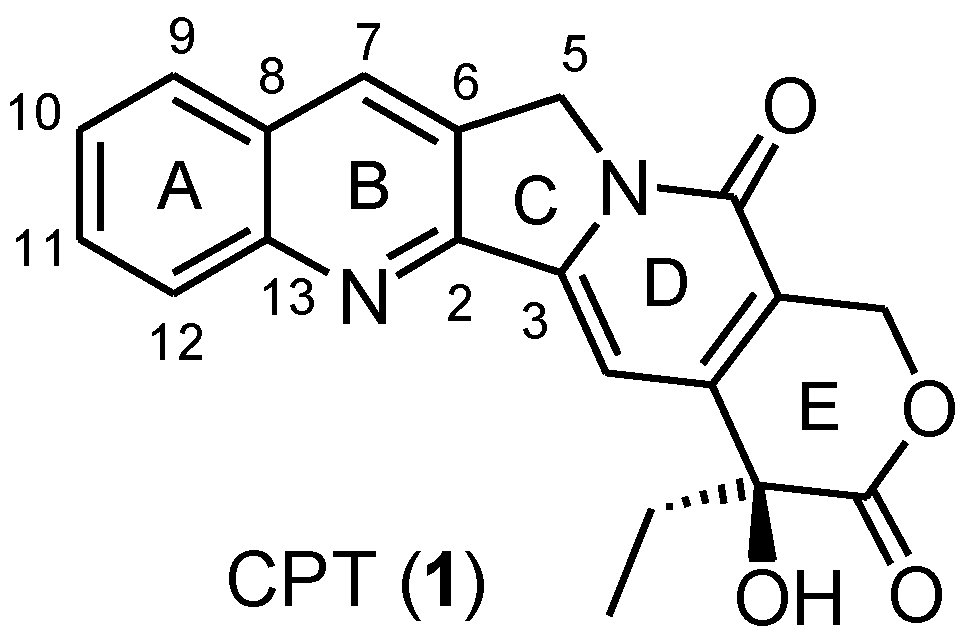
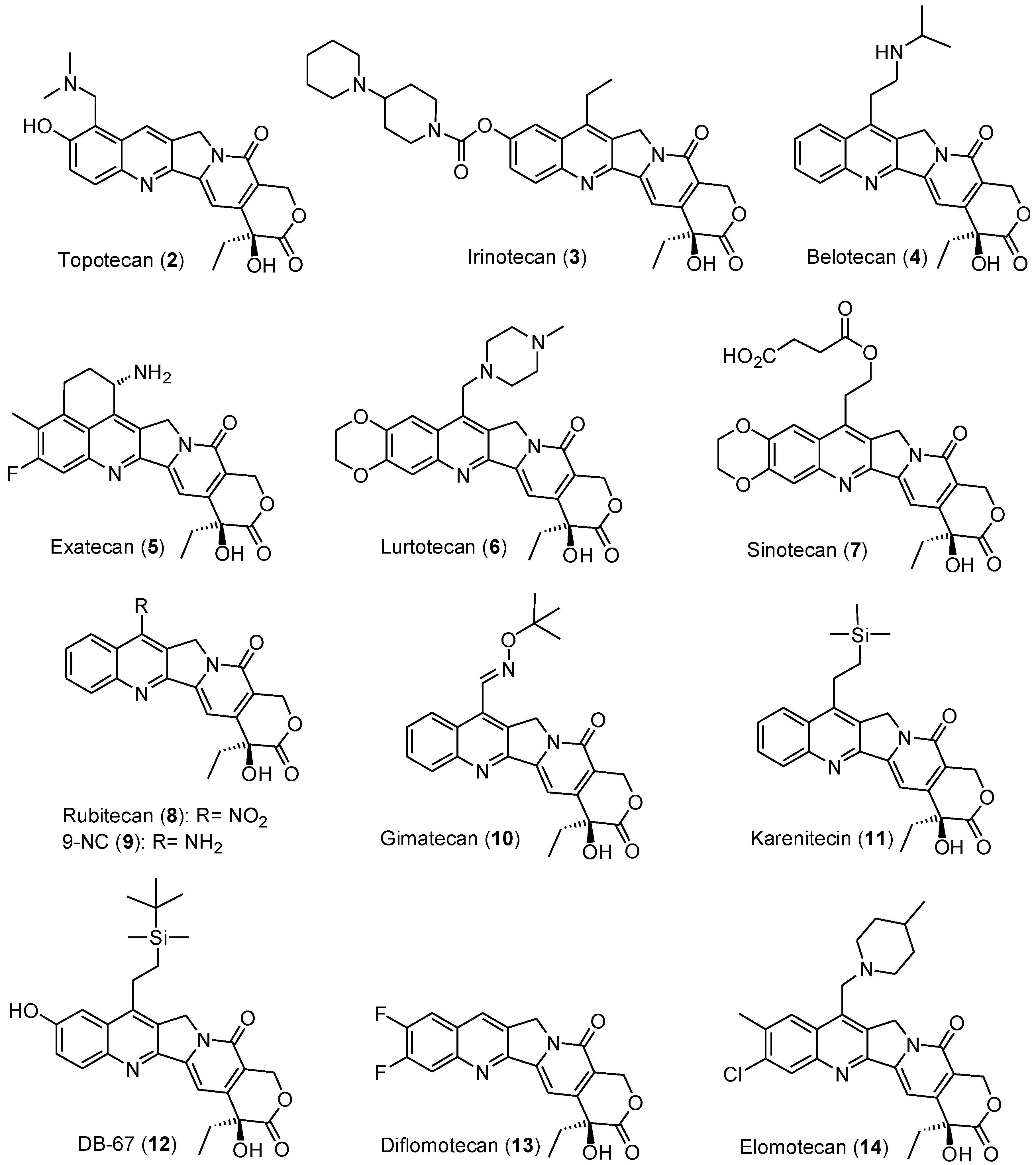
:1. Introduction
2. Results and Discussion
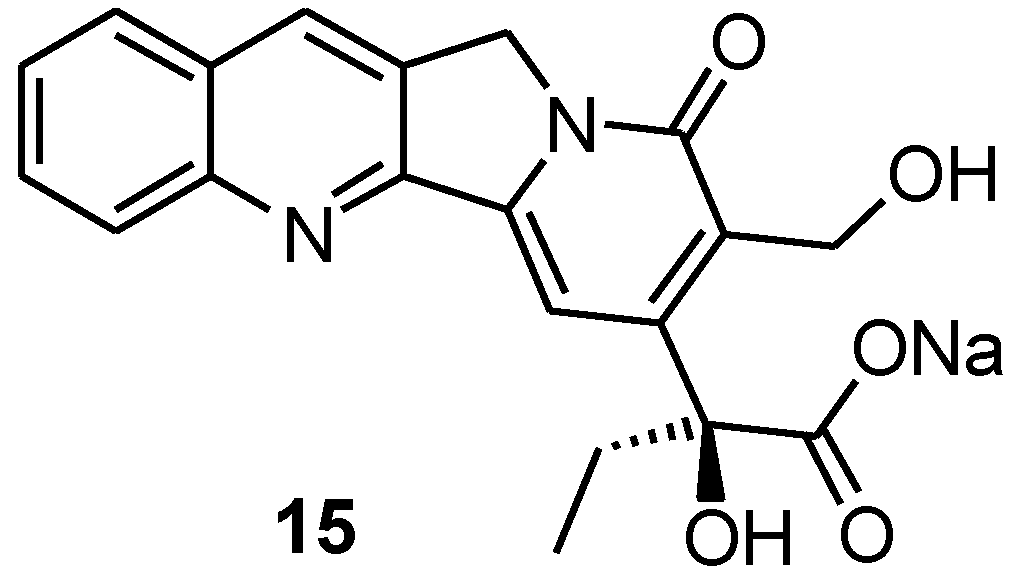
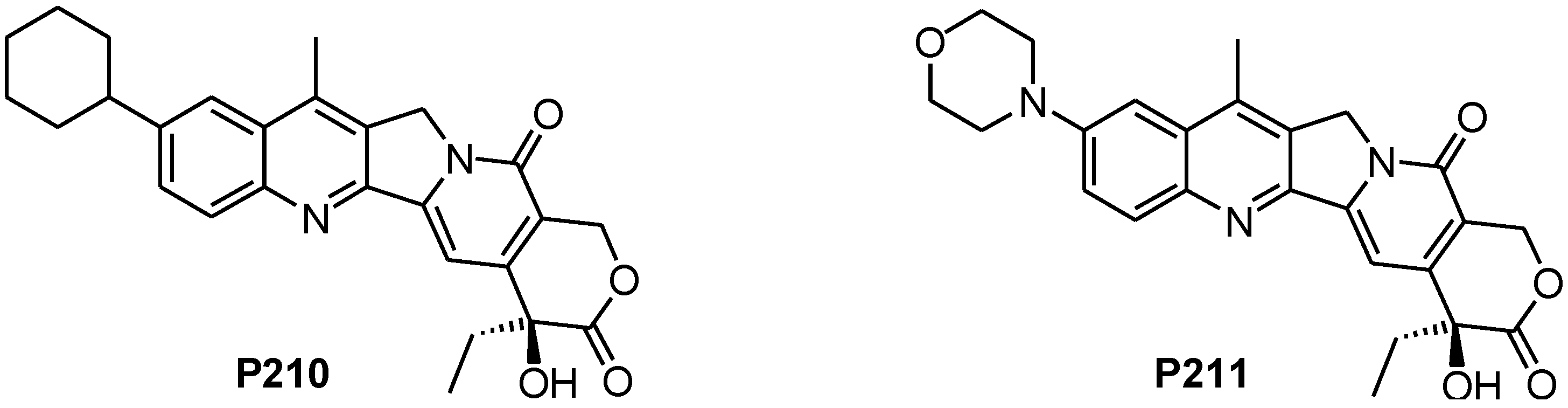
2.1. Synthesis of CPT Derivatives P210 and P211
2.2. The Difference in Solubility between P210 and P211
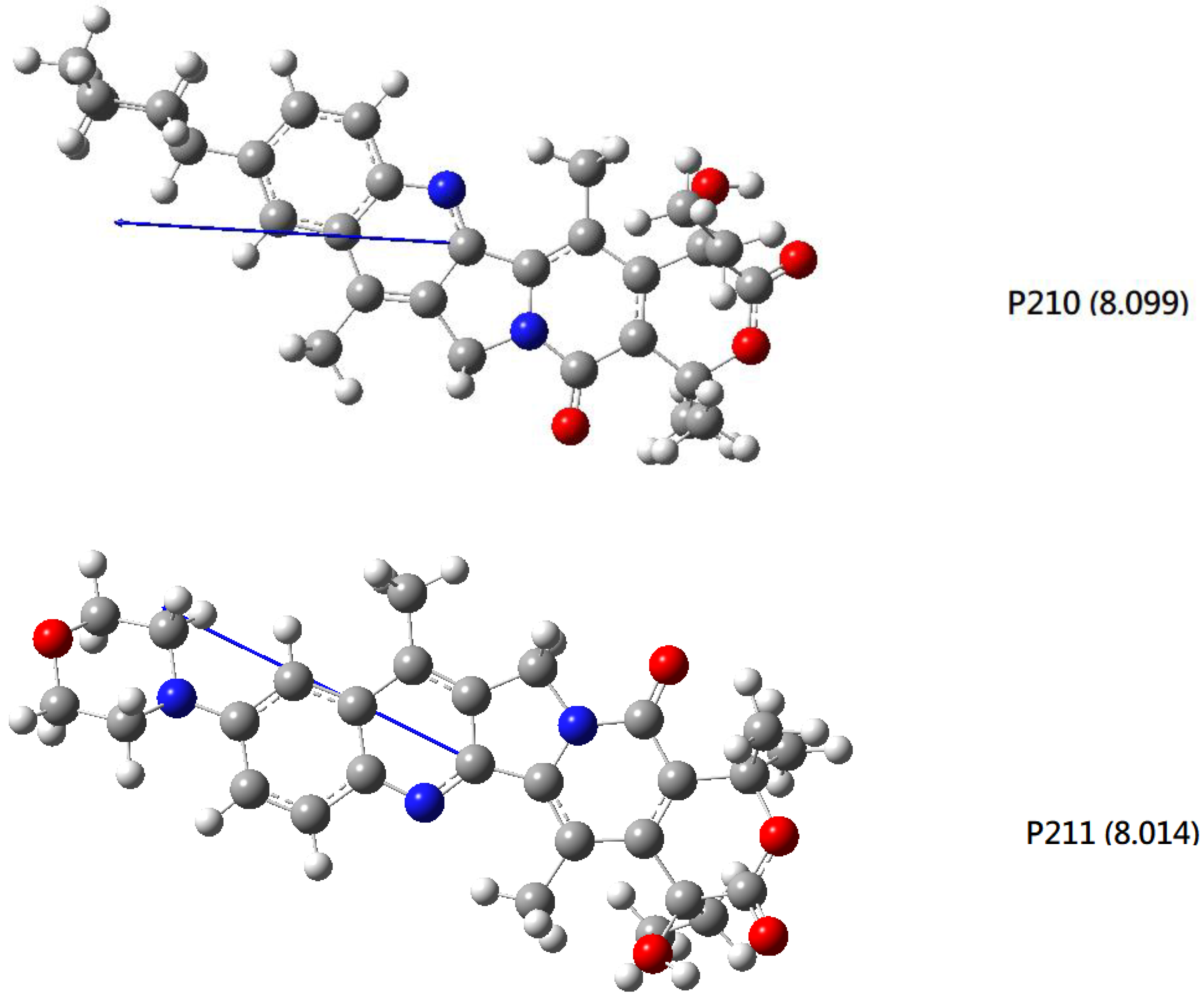
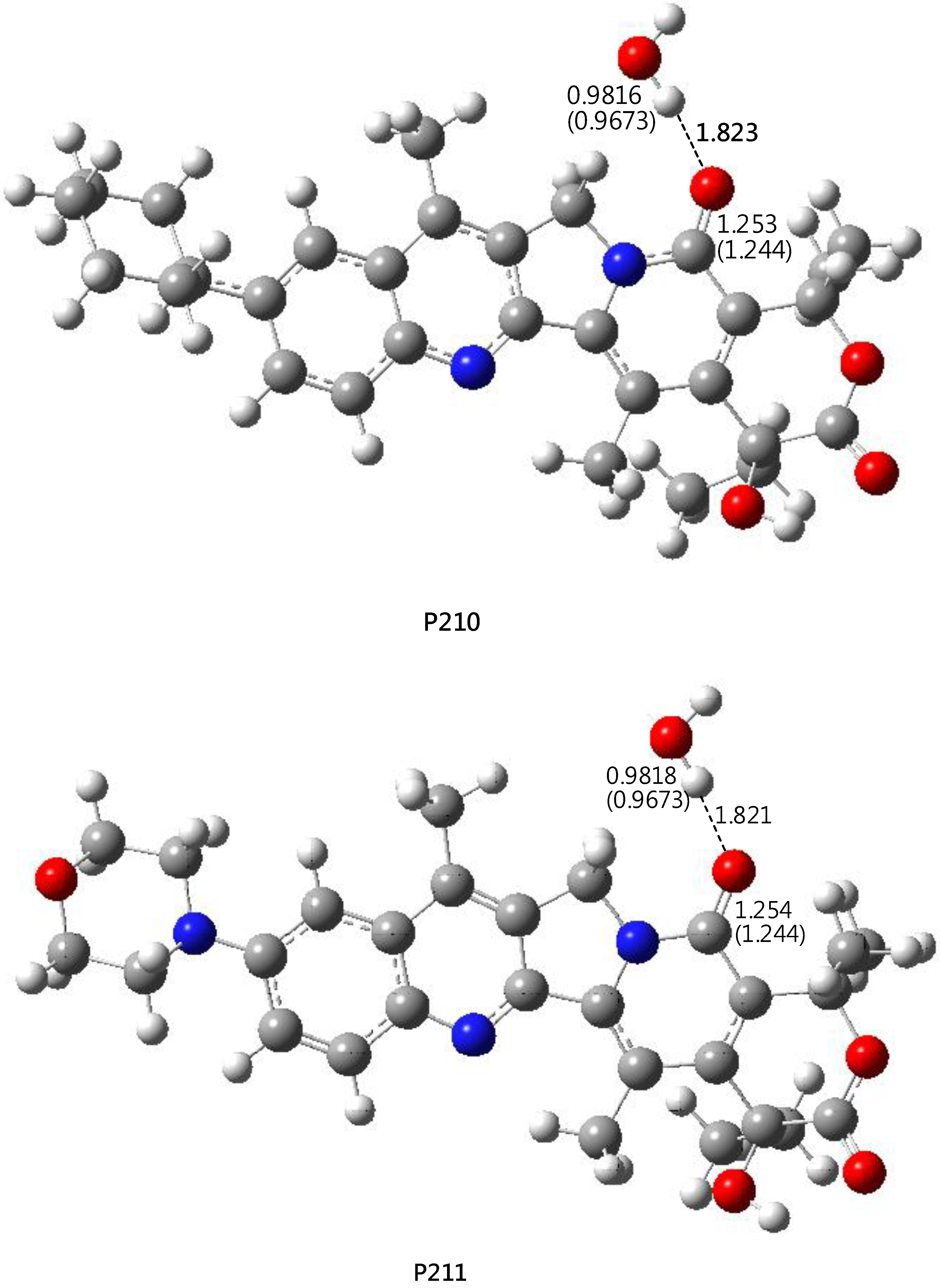
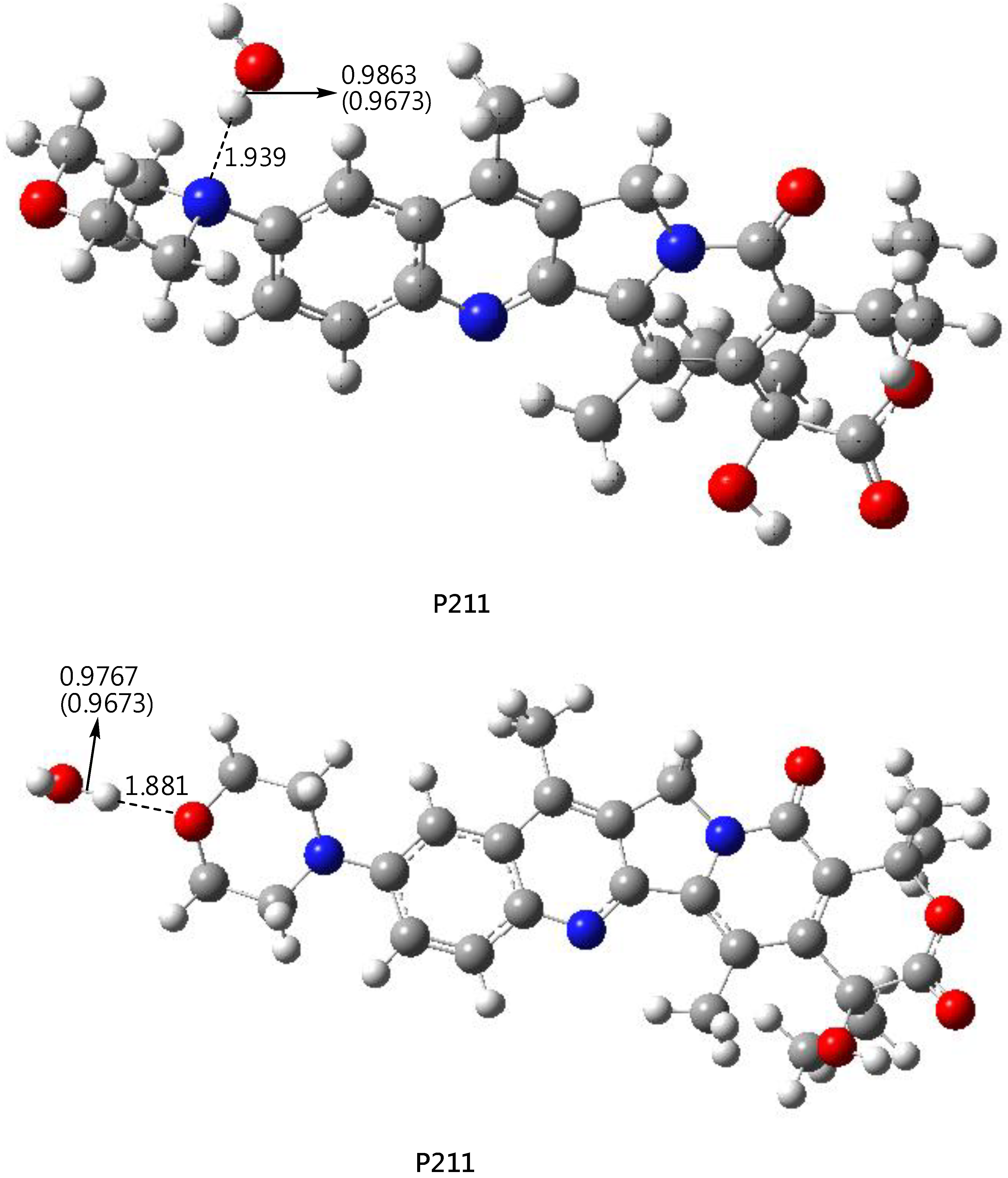
2.3. DFT-B3LYP Study of P210 (P211) and Their Hydrated Complexes
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 1-(2-Amino-5-cyclohexylphenyl)ethanone (8)
3.3. General Procedure for the Condensation of 16 with 17
3.4. The Solubility Test
3.5. DFT-B3LYP Study of P210 (P211) and Their Hydrated Complexes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wall, M.E.; Wani, M.C.; Cook, C.E.; Palmer, K.H.; McPhail, A.T.; Sim, G.A. Plant antitumor agents I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 1966, 88, 3888–3890. [Google Scholar] [CrossRef]
- Liu, L.F.; Desai, S.D.; Li, T.-K.; Mao, Y.; Sun, M.; Sim, S.-P. Mechanism of Action of Camptothecin. Ann. N. Y. Acad. Sci. 2000, 922, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, Y.H.; Hertzberg, R.; Hecht, S.; Liu, L.F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J. Biol. Chem. 1985, 260, 14873–14878. [Google Scholar] [PubMed]
- Potmesil, M.; Pinedo, H. Camptothecins: New Anticancer Agents; CRC: Boca Raton, FL, USA, 1995; pp. 9–20. ISBN 978-0849347641. [Google Scholar]
- Onishi, H.; Machida, Y. Macromolecular and nanotechnological modification of camptothecin and its analogs to improve the efficacy. Curr. Drug Discov. Technol. 2005, 2, 169–183. [Google Scholar] [CrossRef]
- Houghton, P.J.; Cheshire, P.J.; Myers, L.; Stewart, C.F.; Synold, T.W.; Houghton, J.A. Evaluation of 9-dimethylaminomethyl-10-hydroxycamptothecin against xenografts derived from adult and childhood solid tumors. Cancer Chemother. Pharmacol. 1992, 31, 229–239. [Google Scholar] [CrossRef]
- Kunimoto, T.; Nitta, K.; Tanaka, T.; Uehara, N.; Baba, H.; Takeuchi, M.; Yokokura, T.; Sawada, S. Antitumor activity of 7-ethyl-10-[4-(1-piperidino)-1-piperidino] carbonyloxycamptothecin, a novel water soluble derivative of camptothecin, against murine tumors. Cancer Res. 1987, 7, 5944–5948. [Google Scholar]
- Ahn, S.K.; Choi, N.S.; Jeong, B.S.; Kim, K.K.; Journ, D.J.; Kim, J.K. Practical synthesis of (S)-7-(2-isopropylamino)ethylcamptothecin hydrochloride, potent topoisomerase I inhibitor. J. Heterocycl. Chem. 2000, 37, 1141–1144. [Google Scholar] [CrossRef]
- Slichenmyer, W.J.; Rowinsky, E.K.; Donehower, R.C.; Kaufmann, S.H. The current status of camptothecin analogues as antitumor agents. J. Natl. Cancer Inst. 1993, 85, 271–290. [Google Scholar] [CrossRef]
- Schultz, A.G. Camptothecin. Chem. Rev. 1973, 73, 385–405. [Google Scholar] [CrossRef]
- Wall, M.E.; Wani, M.C. Camptothecin and taxol: From discovery to clinic. J. Ethnopharmacol. 1996, 51, 239–254. [Google Scholar] [CrossRef]
- Leary, O.J.; Muggia, F.M. Camptothecins: A review of their development and schedules of administration. Eur. J. Cancer 1998, 34, 1500–1508. [Google Scholar] [CrossRef]
- Oberlies, N.H.; Kroll, D.J. Camptothecin and taxol: Historic achievements in natural products research. J. Nat. Prod. 2004, 67, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Pizzolato, J.F.; Saltz, L.B. The camptothecins. Lancet 2003, 361, 2235–2242. [Google Scholar] [CrossRef]
- Lorence, A.; Nessler, C.L. Camptothecin, over four decades of surprising findings. Phytochem. 2004, 65, 2735–2749. [Google Scholar] [CrossRef] [PubMed]
- Zunino, F.; Dallavalle, S.; Laccabue, D.; Beretta, G.; Merlinib, L.; Pratesi, G. Current status and perspectives in the development of camptothecins. Curr. Pharm. Design 2002, 8, 2505–2520. [Google Scholar] [CrossRef]
- Thomas, C.J.; Rahier, N.J.; Hecht, S.M. Camptothecin: Current perspectives. Bioorg. Med. Chem. 2004, 12, 1585–1604. [Google Scholar] [CrossRef] [PubMed]
- Driver, R.W.; Yang, L.X. Synthesis and pharmacology of new camptothecin drugs. Mini. Rev. Med. Chem. 2005, 5, 425–439. [Google Scholar] [CrossRef]
- Lerchen, H.G. Milestones in camptothecin research. Drugs Future 2002, 27, 869–878. [Google Scholar] [CrossRef]
- Sriram, D.; Yogeeswari, P.; Thirumurugan, R.; Bal, T.R. Camptothecin and its analogues: A review on their chemotherapeutic potential. Nat. Prod. Res. 2005, 19, 393–412. [Google Scholar] [CrossRef]
- Gottlieb, J.A.; Guarino, A.M.; Call, J.B.; Oliverio, V.T.; Block, J.B. Preliminary pharmacologic and clinical evaluation of camptothecin sodium (NSC-100880). Cancer Chemother. Rep. 1970, 54, 461–479. [Google Scholar]
- Muggia, F.M.; Creaven, P.J.; Jansen, H.H.; Cohen, M.N.; Selawry, D.S. Phase I clinical trials of weekly and daily treatment with camptothecin (NSC 100880). Correlation with clinical studies. Cancer Chemother. Rep. 1972, 56, 515–521. [Google Scholar] [PubMed]
- Moertel, C.G.; Schutt, A.J.; Reitemeier, R.J.; Hahn, R.G. Phase II study of camptothecin (NSC-100880) in the treatment of advanced gastrointestinal cancer. Cancer Chemother. Rep. 1972, 56, 95–101. [Google Scholar] [PubMed]
- Ishikawa, M.; Hashimoto, Y. Improvement in aqueous solubility in small molecule drug discovery programs by disruption of molecular planarity and symmetry. J. Med. Chem. 2011, 54, 1539–1554. [Google Scholar] [CrossRef] [PubMed]
- Burgin, A.B., Jr.; Feese, M.D.; Staker, B.L.; Stewart, L. Crystallographic insight into the mechanism of drug-induced topoisomerase I DNA damage. In Camptothecins in Cancer Therapy; Adams, V.R., Ed.; Springer: New York, NY, USA, 2005; pp. 23–38. ISBN 978-1-59259-866-3. [Google Scholar]
- Sugimori, M.; Ejima, A.; Ohsuki, S.; Uoto, K.; Mitsui, I.; Matsumoto, K.; Kawato, Y.; Yasuoka, M.; Sato, K.; Tagawa, H.; et al. Antitumor Agents. 7. Synthesis and Antitumor Activity of Novel Hexacyclic Camptothecin Analogues. J. Med. Chem. 1994, 37, 3033–3039. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, W.; Zuo, S.; Liu, L.; Zhang, X.; Wang, J. Direct Exchange of a Ketone Methyl or Aryl Group to Another Aryl Group through CC Bond Activation Assisted by Rhodium Chelation. Angew. Chem. Int. Ed. 2012, 51, 12334–12338. [Google Scholar] [CrossRef] [PubMed]
- Zazza, C.; Coletta, A.; Sanna, N.; Chillemi, G.; Mancini, G.; Desideri, A. Solvent Effects on the Valence UV-Vis Absorption Spectra of Topotecan Anticancer Drug in Aqueous Solution at Room Temperature: A Nanoseconds Time-Scale TD-DFT/MD Computational Study. J. Phys. Chem. B 2010, 114, 6770–6778. [Google Scholar] [CrossRef] [PubMed]
- Sanna, N.; Chillemi, G.; Gontrani, L.; Grandi, A.; Mancini, G.; Castelli, S.; Zagotto, G.; Zazza, C.; Vincenzo Barone, V.; Desideri, A. UV-Vis Spectra of the Anticancer Campothecin Family Drugs in Aqueous Solution: Specific Spectroscopic Signatures Unraveled by a Combined Computational and Experimental Study. J. Phys. Chem. B 2009, 113, 5369–5375. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr.; Hay, P.J. Modern Theoretical Chemistry; Schaefer, H.F., III, Ed.; Plenum: New York, NT, USA, 1977; pp. 1–27. ISBN 978-1-4757-0887-5. [Google Scholar]
Sample Availability: Samples of the compounds 16b, P210, and P211 are available from the authors. |

| P210 | P211 | |
|---|---|---|
| DMSO | 10.6 sparingly soluble | 7.2 slightly soluble |
| DMAC | 49.5 soluble | 13.2 sparingly soluble |
| 5% DMSO + 95% normal saline | 0.10 very slightly soluble | 0.10 very slightly soluble |
| 5% DMAC + 95% normal saline | 0.10 very slightly soluble | 0.10 very slightly soluble |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, C.-H.; Chang, C.-C.; Weng, Y.-L.; Chuang, T.-H. Synthesis, Experimental and Density Functional Theory (DFT) Studies on Solubility of Camptothecin Derivatives. Molecules 2018, 23, 3170. https://doi.org/10.3390/molecules23123170
Lai C-H, Chang C-C, Weng Y-L, Chuang T-H. Synthesis, Experimental and Density Functional Theory (DFT) Studies on Solubility of Camptothecin Derivatives. Molecules. 2018; 23(12):3170. https://doi.org/10.3390/molecules23123170
Chicago/Turabian StyleLai, Chin-Hung, Chia-Chin Chang, Yi-Lin Weng, and Ta-Hsien Chuang. 2018. "Synthesis, Experimental and Density Functional Theory (DFT) Studies on Solubility of Camptothecin Derivatives" Molecules 23, no. 12: 3170. https://doi.org/10.3390/molecules23123170
APA StyleLai, C.-H., Chang, C.-C., Weng, Y.-L., & Chuang, T.-H. (2018). Synthesis, Experimental and Density Functional Theory (DFT) Studies on Solubility of Camptothecin Derivatives. Molecules, 23(12), 3170. https://doi.org/10.3390/molecules23123170







