A Simple and Cost-Effective TLC-Densitometric Method for the Quantitative Determination of Acetylsalicylic Acid and Ascorbic Acid in Combined Effervescent Tablets
Abstract
:1. Introduction
2. Results and Discussion
2.1. Validation of TLC Method
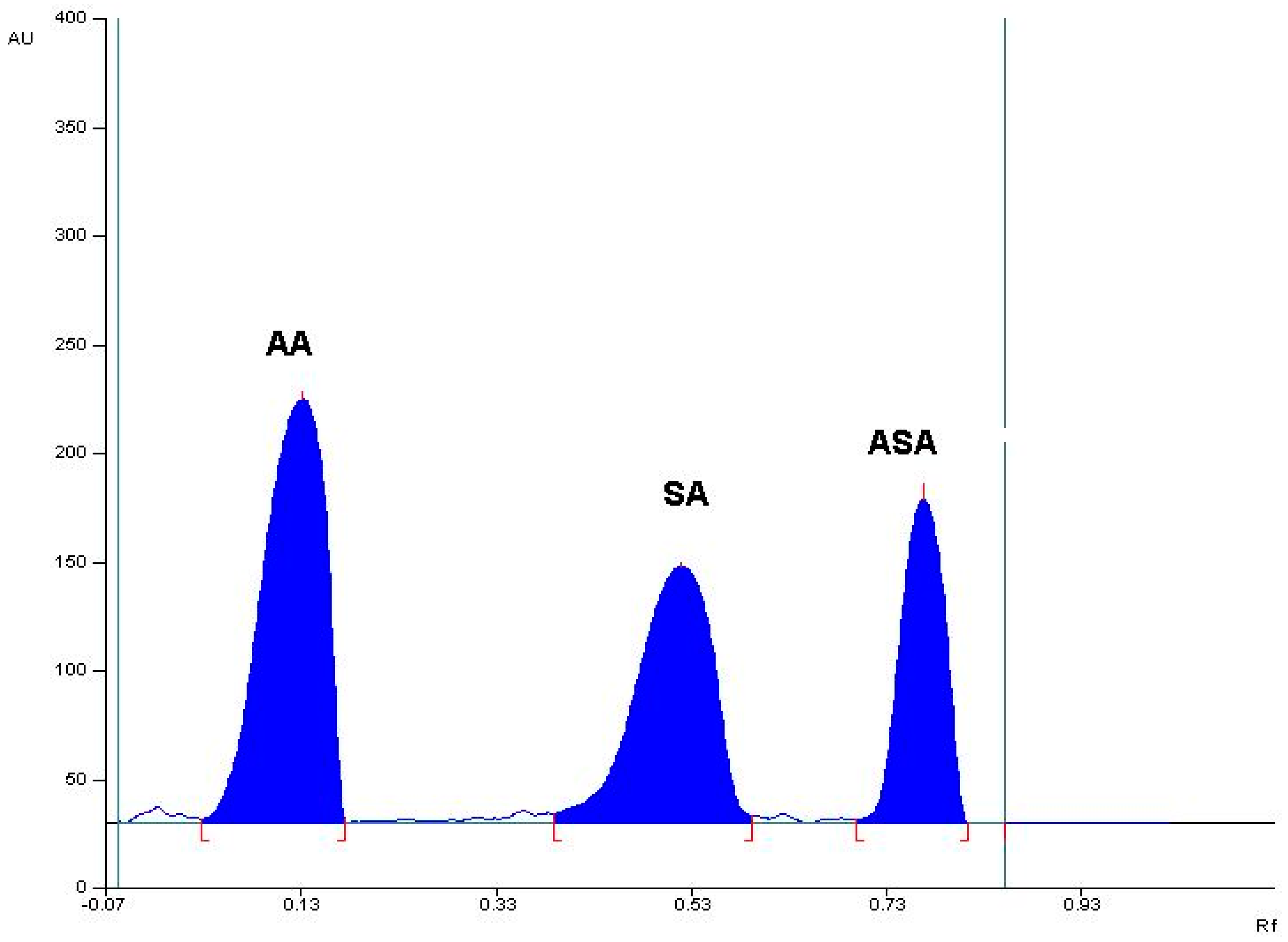
2.1.1. Specificity
- mobile phase B: chloroform:ethanol 96%:water (3:6:1.5, v/v/v) [24]
- mobile phase B1: chloroform:ethanol 96% (3:6, v/v)
- mobile phase B2: chloroform:ethanol (5:5, v/v)
- mobile phase B3: chloroform:ethanol 96%:glacial acetic acid (5:5:0.03, v/v/v)
- mobile phase B4: chloroform:ethanol 96%:glacial acetic acid (5:4:0.03, v/v/v)
- mobile phase C: methylene chloride:ethyl acetate:ethanol 96% (5:5:1, v/v/v) [33]
- mobile phase E: n-hexane:acetone:glacial acetic acid (5:5:0.09, v/v/v)
- mobile phase F: n-hexane:acetone:glacial acetic acid (5:5:0.5, v/v/v)
- mobile phase G: acetone:n-hexane:glacial acetic acid (5:5:0.06, v/v/v)
- mobile phase G1: n-hexane-acetone-glacial acetic acid in the following volume composition: 7 mL:2 mL:1 drop [8].
2.1.2. Accuracy
2.1.3. Calibration and Range
2.1.4. Precision
2.1.5. Limit of Detection (LOD) and Limit of quantification (LOQ) Based on the Calibration Curves
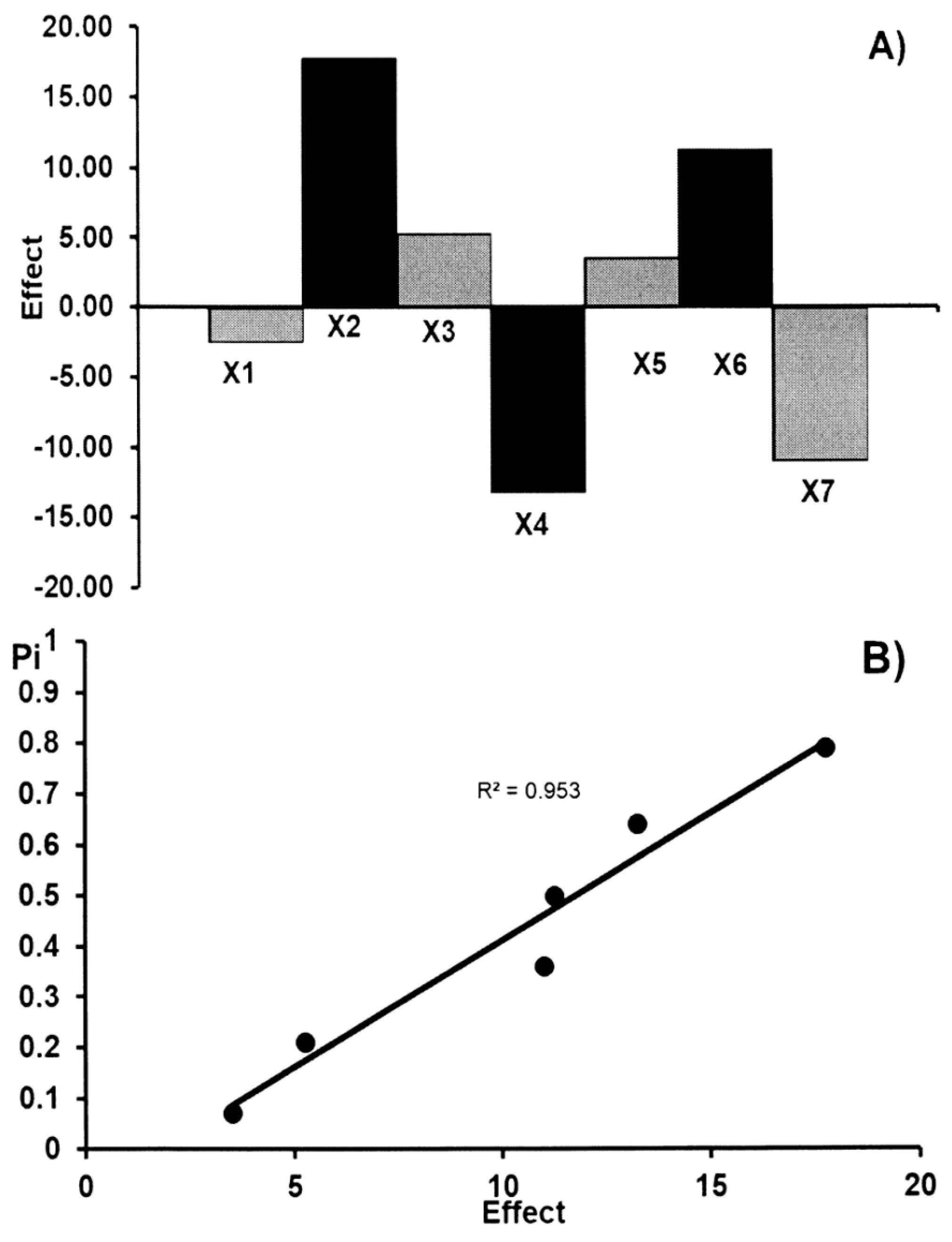
2.1.6. Robustness
2.1.7. Analysis of Acetylsalicylic Acid and Ascorbic Acid in Commercial Effervescent Tablets
2.2. Comparison with the Indirect Iodometric Method
3. Materials and Methods
3.1. Apparatus
3.2. Chemicals
3.3. Pharmaceutical Preparation
3.4. Preparation Sample of Tablets
3.5. Preparation of Standard Solutions
3.6. Thin Layer Chromatography
3.7. Densitometric and Spectro-Densitometric Study
3.8. Validation of the NP-TLC Method
3.8.1. Specificity
3.8.2. Linearity and Range
3.8.3. Accuracy
3.8.4. Precision
3.8.5. Limit of Detection and Limit of Quantification Based on the Calibration Curves
3.8.6. Robustness
3.9. Comparing with the Indirect Iodometric Method
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Disclosure
References
- Wabaidur, S.M.; Alothman, Z.A.; Khan, M.R. A rapid method for the simultaneous determination of L- ascorbic acid and acetylsalicylic acid in aspirin C effervescent tablet by ultra performance liquid chromatography-tandem mass spectrometry. Spectrochim. Acta A Mol. Biomol. Spetrosc. 2013, 108, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kokot, Z.; Burda, K. Simultaneous determination of salicylic acid and acetylsalicylic acid in aspirin delayed-release tablet formulations by second-derivative UV spectrophotometry. J. Pharm. Biomed. Anal. 1998, 18, 871–875. [Google Scholar] [CrossRef]
- Navalón, A.; Blanc, R.; del Olmo, M.; Vilchez, J.L. Simultaneous determination of naproxen, salicylic acid and acetylsalicylic acid by spectrofluorimetry using partial least-squares (PLS) multivariate calibration. Talanta 1999, 48, 469–475. [Google Scholar] [CrossRef]
- Franeta, J.T.; Agbaba, D.; Eric, S.; Pavkov, S.P.; Aleksic, M.B.; Vladimirov, S.D. HPLC assay of acetylsalicylic acid, paracetamol, caffeine and phenobarbital in tablets. IL Farm. 2002, 57, 709–713. [Google Scholar] [CrossRef]
- Bocheńska, P.; Pyka, A. Determination of acetylsalicylic acid in pharmaceutical drugs by TLC with densitometric detection in UV. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 1346–1363. [Google Scholar]
- Patel, B.R.; Shankar, B.M. Simultaneous estimation of acetylsalicylic acid and clopidogrel bisulfate in pure powder and tablet formulations by high-performance column liquid chromatography and high-performance thin-layer chromatography. J. AOAC Int. 2008, 91, 750–755. [Google Scholar] [PubMed]
- Franeta, J.T.; Agbaba, D.; Eric, S.; Pavkov, S.P.; Vladimirov, S.D.; Aleksic, M.B. Quantitative analysis of analgoantipyretics in dosage form using planar chromatography. J. Pharm. Biomed. Anal. 2001, 24, 1169–1173. [Google Scholar] [CrossRef]
- Bocheńska, P.; Pyka, A. Use of TLC for the quantitative determination of acetylsalicylic acid, caffeine, and ethoxybenzamide in combined tablets. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 2405–2421. [Google Scholar]
- Polish Pharmaceutical Society. Polish Pharmacopoeia; Polish Pharmaceutical Society: Warsaw, Poland, 2002. (In Polish) [Google Scholar]
- United States Pharmacopeial Convention. The United States Pharmacopoeia, 34th ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2011. [Google Scholar]
- Hobl, E.L.; Jilma, B.; Ebner, J.; Schmid, W.R. Simultaneous determination of acetylsalicylic acid and salicylic acid in human plasma by isocratic high-pressure liquid chromatography with post-column hydrolysis and fluorescence detection. Biomed. Chromatogr. 2013, 27, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Pirola, R.; Bareggi, S.R.; De Benedittis, G. Determination of acetylsalicylic acid and salicylic acid in skin and plasma by high-performance liquid chromatography. J. Chromatogr. B 1998, 705, 309–315. [Google Scholar] [CrossRef]
- Rubak, P.; Hardlei, F.T.; Wurtz, M.; Kristensen, D.S.; Hvas, A.M. Low-dose acetylsalicylic acid therapy monitored with ultra high performance liquid chromatography. Clin. Biochem. 2013, 46, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Ballesteros, E. Gas chromatography-mass spectrometry determination of pharmacologically active substances in urine and blood samples by use of a continuous solid-phase extraction system and microwave-assisted derivatization. J. Chromatogr. B 2012, 891–892, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.H.; Jensen, M.E.; Bjørnsdottir, I. Assay of acetylsalicylic acid and three of its metabolites in human plasma and urine using non-aqueous capillary electrophoresis with reversed electroosmotic flow. J. Pharm. Biomed. Anal. 1998, 17, 1155–1160. [Google Scholar] [CrossRef]
- Krzek, J.; Starek, M.; Kwiecien, M. Simultaneous determination of guaiamar carbamate and acetylsalicylic acid by a chromatographic-densitometric method. Acta Pol. Pharm. Drug Res. 1998, 55, 429–434. [Google Scholar]
- Rzeszutko, W.; Somogyi, E.; Woltyńska, H. Application of cooper(II) chloride to indirect iodometric determination of the ascorbic acid in selected pharmaceuticals. Acta Pol. Pharm. Drug Res. 1996, 53, 79–80. [Google Scholar]
- Fathi, M.R.; Elahi, R.; Hashemi, P. Kinetic spectrophotometric determination of ascorbic acid in pharmaceutical formulations. Anal. Chem. 2005, 50, 1069–1076. [Google Scholar]
- Srividya, K.; Balasubramanian, N. Sensitive spectrophotometric determination of ascorbic acid in pharmaceutical samples and fruit extracts. Anal. Chem. 1999, 44, 689–696. [Google Scholar]
- Kukoc-Modum, L.; Biocic, M.; Radić, N. Indirect method for spectrophotometric determination of ascorbic acid in pharmaceutical preparations with 2,4,6-tripyridyl-s-triazine by flow-injection analysis. Talanta 2012, 96, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Noroozifar, M.; Khorasani-Motlagh, M.; Akhavan, K. Atomic absorption spectrometry for the automatic indirect determination of ascorbic acid based on the reduction of manganese dioxide. Anal. Sci. 2005, 21, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Şahin, M.; Özcan, L.; Usta, B.; Şahin, Y. Determination of ascorbic acid by polypyrrole potentiometric detector in ion chromatography. Biosens. Bioelectron. 2009, 24, 3492–3497. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.Z.; Frank, L.E. Development and implementation of an HPLC-ECD method for analysis of vitamin C in plasma using single column and automatic alternating dual column regeneration. Pract. Lab. Med. 2016, 6, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Jarzębiński, J.; Ługowska, E. Application of densitometric measurements for the determination of active ingredients in drugs. Acta Pol. Pharm. Drug Res. 1989, 46, 160–164. (In Polish) [Google Scholar]
- Aburjai, T.; Amro, B.I.; Aiedeh, K.; Abuirjeie, M.; Al-Khalil, S. Second derivative ultraviolet spectrophotometry and HPTLC for the simultaneous determination of vitamin C and dipyrone. Pharmazie 2000, 55, 751–754. [Google Scholar] [PubMed]
- Romeu-Nadal, M.; Morera-Pons, S.; Castellote, A.I.; López-Sabater, M.C. Rapid high-performance liquid chromatographic method for Vitamin C determination in human milk versus an enzymatic method. J. Chromatogr. B 2006, 830, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, A.; Blomhoff, R.; Gundersen, E.T. High-throughput analysis of Vitamin C in human plasma with the use of HPLC with monolithic column and UV-detection. J. Chromatogr. B 2005, 824, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Hatambeygi, N.; Abedi, G.; Talebi, M. Method development and validation for optimised separation of salicylic, acetylsalicylic and ascorbic acid in pharmaceutical formulations by hydrophilic interaction chromatography and response surface methodology. J. Chromatogr. A 2011, 1218, 5995–6003. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Alothman, Z.A.; Naushad, M.; Ghfar, A.A.; Wabaidur, S.M. Simultaneous analysis of vitamin C and aspirin in Aspirin C effervescent tablets by high performance liquid chromatography- photodiode array detector. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 2454–2461. [Google Scholar]
- ICH. ICH Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology, Q2(R1). 2005. Available online: http://www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html (accessed on 5 March 2018).
- Ferenczi Fodor, K.; Renger, B.; Végh, Z. The frustrated reviewer–recurrent failures in manuscripts describing validation of quantitative TLC/HPTLC procedures for analysis of pharmaceuticals. J. Planar Chromatogr. Mod. TLC 2010, 23, 173–179. [Google Scholar] [CrossRef]
- Hachuła, U.; Zawisza, B.; Winkler, W. Application of the chromatographic and spectrophotometric method for the analytical control of L-ascorbic acid in the pharmaceutical and plant material. Ann. Natl. Inst. Hyg. 2000, 51, 71–77. (In Polish) [Google Scholar]
- El Sadek, M.; El Shanawany, A.; Aboul Khier, A. Determination of the components of analgesic mixture using high-performance thin-layer chromatography. Analyst 1990, 115, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Gołkiewicz, W.; Bartos, A. Determination of trace amounts of salicylic acid and acetylsalicylic acid in Polopiryna and other drugs containing acetylsalicylic acid. Acta Pol. Pharm. 1993, 50, 143–147. (In Polish) [Google Scholar] [PubMed]
- Nagy Turák, A.; Végh, Z.; Ferenczi Fodor, K. Validaton of the quantitative planar chromatographic analysis of drug substances. III. Robustness testing in OPLC. J. Planar Chromatogr. Mod. TLC 1995, 8, 188–193. [Google Scholar]
- Ferenczi Fodor, K.; Nagy Turák, A.; Végh, Z. Validation and monitoring of quantitative thin layer chromatographic purity tests for bulk drug substances. J. Planar Chromatogr. Mod. TLC 1995, 8, 349–356. [Google Scholar]
- Hendix, C.D. What every technologist should know about experiment design. Chem. Tech. 1979, 9, 167–174. [Google Scholar]
- Pyka, A.; Wiatr, E.; Kwiska, K.; Gurak, D. Validation thin layer chromatography for the determination of naproxen in tablets and comparison with a pharmacopeil method. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 829–847. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Method Characteristic | Results | |
|---|---|---|
| Specificity | Specific | |
| Range (μg/spot) | 1.50–13.50 | |
| Linearity | A = 370.9 (±310.4) + 1944.8 (±36.8)·x n = 9; r = 0.999; s = 427.3; F = 2797.0; p < 0.0001 | |
| Limit of detection (LOD) (μg/spot) | 0.25 | |
| Limit of quantification (LOQ) (μg/spot) | 0.75 | |
| For Tablets | ||
| Accuracy and Precision | ||
| Accuracy (n = 6) | ||
| for 50% standard added (n = 6) | R = 100.3%; CV = 2.59% | |
| for 100% standard added (n = 6) | R = 99.3%; CV = 2.62% | |
| for 150% standard added (n = 6) | R = 100.0%; CV = 1.81% | |
| Quantity of Precision (CV, (%)) n = 3 | Ascorbic Acid | |
| Interday | 12.80 μg/spot | 1.78 |
| 7.36 μg/spot | 1.38 | |
| 2.56 μg/spot | 2.61 | |
| Intraday | 12.80 μg/spot | 1.77 |
| 7.36 μg/spot | 1.68 | |
| 2.56 μg/spot | 2.94 | |
| Robustness | Robust | |
| Method Characteristic | Results | |
|---|---|---|
| Specificity | Specific | |
| Range (μg/spot) | 1.50–9.00 | |
| Linearity | A = 2547.7 (±292.4) + 1532.7 (±50.0)·x n = 6; r = 0.9979; s = 314.0; F = 937.9; p < 0.0001 | |
| Limit of detection (LOD) (μg/spot) | 0.20 | |
| Limit of quantification (LOQ) (μg/spot) | 0.61 | |
| For Tablets | ||
| Accuracy and Precision | ||
| Accuracy (n = 6) | ||
| for 50% standard added (n = 6) | R = 99.0%; CV = 1.45% | |
| for 100% standard added (n = 6) | R = 99.2%; CV = 1.57% | |
| for 150% standard added (n = 6) | R = 99.8%; CV = 1.21% | |
| Quantity of Precision (CV, (%)) n = 3 | Acetylsalicylic Acid | |
| Interday | 8.00 μg/spot | 0.78 |
| 4.80 μg/spot | 0.84 | |
| 1.60 μg/spot | 1.73 | |
| Intraday | 8.00 μg/spot | 1.70 |
| 4.80 μg/spot | 1.56 | |
| 1.60 μg/spot | 1.92 | |
| Robustness | Robust | |
| Symbol | Factors | Method Condition | Levels | |
|---|---|---|---|---|
| + | − | |||
| X1 | Chamber type | Twin Trough, 20 × 20 cm | Twin Trough, 20 × 20 cm | Twin Trough, 20 × 10 cm |
| X2 | Sorbent type (E. Merck, #) | Al sheet (1.05570) | Al sheet (1.05570) | Al sheet (1.05554) |
| X3 | Temperature of plate activation (°C) | 120 | 130 | 110 |
| X4 | Extraction time (min) | 30 | 31 | 29 |
| X5 | Volume of chloroform (mL) | 5.0 | 5.1 | 4.9 |
| X6 | Volume of ethanol (mL) | 4.0 | 4.1 | 3.9 |
| X7 | Saturation time of the chamber (°C) | 20 | 22 | 18 |
| Experiment No | X1 | X2 | X3 | X4 | X5 | X6 | X7 | Active Substance a Content (yi) (mg/tablet) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ASA | AA | ||||||||||
| 1 | + | + | + | + | + | + | + | 503.5 | 207.2 | ||
| 2 | + | + | − | + | − | − | − | 494.5 | 182.2 | ||
| 3 | + | − | + | − | − | + | − | 506.5 | 200.4 | ||
| 4 | + | − | − | − | + | − | + | 482.5 | 199.6 | ||
| 5 | − | + | + | − | + | - | - | 519.0 | 207.0 | ||
| 6 | − | + | - | - | - | + | + | 510.5 | 204.0 | ||
| 7 | − | − | + | + | - | - | + | 473.5 | 192.2 | ||
| 8 | − | − | - | + | + | + | - | 494.0 | 198.2 | ||
| Size of effect | ASA | 2.500 | 17.750 | 5.250 | 13.250 | 3.500 | 11.250 | 11.000 | |||
| AA | 3.000 | 2.500 | 5.700 | –7.800 | 8.300 | 7.200 | 3.800 | ||||
| The label claim (mg) | 500 | 200 | |||||||||
| Average amount (mg) | 498.0 | 198.8 | |||||||||
| Variance | 224.1 | 69.6 | |||||||||
| Standard devitation (SD) | 15.0 | 8.3 | |||||||||
| Coefficient of variation (CV, %) | 3.01 | 4.18 | |||||||||
| Active Substance a Determined by | |||
|---|---|---|---|
| NP-TLC with Densitometry | Indirect Iodometric Method | ||
| ASA | AA | AA | |
| Number of analysis | 10 | 10 | 10 |
| 487.8 | 204.6 | 203.2 | |
| 478.2 | 203.4 | 202.8 | |
| 469.8 | 201.6 | 202.0 | |
| 491.6 | 205.8 | 205.8 | |
| 476.3 | 201.8 | 201.6 | |
| 465.7 | 199.6 | 198.4 | |
| 502.2 | 205.6 | 200.6 | |
| 498.1 | 203.8 | 198.0 | |
| 489.2 | 201.8 | 198.2 | |
| 495.3 | 203.8 | 202.2 | |
| The label claim (mg) | 500 | 200 | 200 |
| Average amount (mg) | 485.4 | 203.2 | 201.3 |
| Minimum amount (mg) | 465.7 | 199.6 | 198.0 |
| Maximum amount (mg) | 502.2 | 205.8 | 205.8 |
| Variance | 151.8 | 3.85 | 6.32 |
| Standard deviation (SD) | 12.3 | 1.96 | 2.51 |
| Coefficient of variation (CV, %) | 2.09% | 0.96 | 1.25 |
| Confidence interval of arithmetic mean with confidence level equal 95% | μ = 485.4 ± 8.8 | μ = 203.2 ± 1.4 | μ = 201.3 ± 1.8 |
| Amount of active substance (%) in relation to the label claim | 97.1% | 101.6% | 100.7% |
| TLC-Densitometric Method Compared with the Indirect Iodometric Method Determination of Ascorbic Acid | |||
| t-test | t calculated | 1.89 | |
| t(95%.18) tabulated | 2.101 | ||
| F-test | F calculated | 1.64 | |
| F(95%.f1 = f2 = 9) tabulated | 3.18 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyka-Pająk, A.; Dołowy, M.; Parys, W.; Bober, K.; Janikowska, G. A Simple and Cost-Effective TLC-Densitometric Method for the Quantitative Determination of Acetylsalicylic Acid and Ascorbic Acid in Combined Effervescent Tablets. Molecules 2018, 23, 3115. https://doi.org/10.3390/molecules23123115
Pyka-Pająk A, Dołowy M, Parys W, Bober K, Janikowska G. A Simple and Cost-Effective TLC-Densitometric Method for the Quantitative Determination of Acetylsalicylic Acid and Ascorbic Acid in Combined Effervescent Tablets. Molecules. 2018; 23(12):3115. https://doi.org/10.3390/molecules23123115
Chicago/Turabian StylePyka-Pająk, Alina, Małgorzata Dołowy, Wioletta Parys, Katarzyna Bober, and Grażyna Janikowska. 2018. "A Simple and Cost-Effective TLC-Densitometric Method for the Quantitative Determination of Acetylsalicylic Acid and Ascorbic Acid in Combined Effervescent Tablets" Molecules 23, no. 12: 3115. https://doi.org/10.3390/molecules23123115
APA StylePyka-Pająk, A., Dołowy, M., Parys, W., Bober, K., & Janikowska, G. (2018). A Simple and Cost-Effective TLC-Densitometric Method for the Quantitative Determination of Acetylsalicylic Acid and Ascorbic Acid in Combined Effervescent Tablets. Molecules, 23(12), 3115. https://doi.org/10.3390/molecules23123115