Abstract
Pseudoaspidinol is a phloroglucinol derivative with Antifungal activity and is a major active component of Dryopteris fragrans. In our previous work, we studied the total synthesis of pseudoaspidinol belonging to a phloroglucinol derivative and investigated its antifungal activity as well as its intermediates. However, the results showed these compounds have low antifungal activity. In this study, in order to increase antifungal activities of phloroglucinol derivatives, we introduced antifungal pharmacophore allylamine into the methylphloroglucinol. Meanwhile, we remained C1–C4 acyl group in C-6 position of methylphloroglucinol using pseudoaspidinol as the lead compound to obtain novel phloroglucinol derivatives, synthesized 17 compounds, and evaluated antifungal activities on Trichophyton rubrum and Trichophyton mentagrophytes in vitro. Molecular docking verified their ability to combine the protein binding site. The results indicated that most of the compounds had strong antifungal activity, in which compound 17 were found to be the most active on Trichophyton rubrum with Minimum Inhibitory Concentration (MIC) of 3.05 μg/mL and of Trichophyton mentagrophytes with MIC of 5.13 μg/mL. Docking results showed that compounds had a nice combination with the protein binding site. These researches could lay the foundation for developing antifungal agents of clinical value.
1. Introduction
Fungi are widely distributed in nature and frequently present as pathogens in the animal and plant kingdoms [1]. In recent decades, despite progress in antifungal therapy, infectious diseases caused by a variety of clinically significant species of fungi remain a major global health concern, due to the development of antifungal drug resistance [2,3]. However, resistance to several antifungal agents among a variety of clinically significant species of fungi is becoming an increasingly major global problem and is reaching an alarming level on a global scale [4]. Hence, searching for efficient, nontoxic or low toxic chemotherapeutic agents with potent broad-spectrum antifungal activity is an important goal in new drug research [5,6]. Currently, the main targets of the antifungal agents are the cytochrome P450 sterol 14a-demethylase (CYP51), squalene epoxidase (SE) and β-1,3-glucan synthase, in which azole and triazole drugs are CYP51 inhibitors widely used as fungal antibiotics and antimycobacterial activity, spinosins are β-1,3-glucan synthase inhibitors and allylamine agents act on squalene epoxidase (SE) [7,8,9,10].
Dryopteris fragrans is mainly used as a folk medicine at present. It has been found that the main active compounds from Dryopteris fragrans have a significant effect on a variety of skin diseases caused by fungi [11,12]. Our group isolated a variety of phloroglucinols from the plant and activity experiment showed that the phloroglucinols had strong antifungal activity such as the minimal inhibition concentration (MIC) values activity of aspidin BB against some clinical S. aureus was ranged from 15.63 mg/mL to 62.5 mg/mL [13,14,15,16]. In addition, in our current research, phlorglucinols, especially 2-methyl-6-acyl phloroglucinol derivatives. Pseudoaspidinol belongs to phlorglucinols and shows good antifungal activity and its key intermediate methyl phlorglucinol can be used to generate diverse compounds. In our previous study, a preliminary structure-activity study found that the hydroxyl group of methyl phloroglucinol and the butyryl group at the C-4 position are the active groups of the compound, and C-4 or C-6 is the active site. Introduction of different groups at this position may alter its properties and biological activity. In order to increase antifungal activity of phloroglucinol derivatives, in this study, we introduced antifungal pharmacophore allylamine into the methyl phloroglucinol and remained C1–C4 acyl group in C-6 position using pseudoaspidinol as lead compound to obtain novel phloroglucinol derivatives 4–17 (Figure 1).
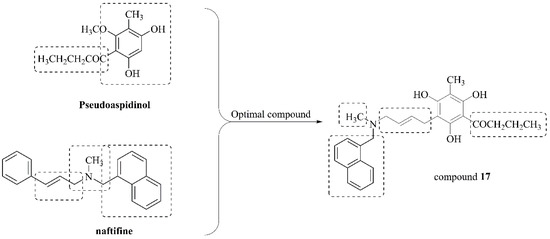
Figure 1.
Design of compounds.
We found in the literature that the main antifungal target of allylamine antifungal drugs is squalene oxidase, especially the representative drug naftifine [17]. It is particularly noteworthy that naftifine is the first marketed drug for allylamine antifungal drugs and is not replaceable in antifungal action. Unfortunately, the widespread use of allylamine has led to the development of severe drug resistance, which has significantly reduced their efficacy [18]. These compounds were synthesized and evaluated for their antifungal activity against Trichophyton rubrum and Trichophyton mentagrophytes in vitro. Molecular docking simulations were conducted to confirm the mechanism of action between the compound and squalene epoxidase (SE).
2. Results and Discussions
2.1. Chemistry
The synthetic route of compounds was demonstrated in Scheme 1. Methyl phloroglucinol (3) was synthesized using 1,3,5-hydroxybenzene (1) as raw material through Vilsmeier–Haack reaction and reduction reaction. The formyl chloride, acetyl chloride, propionyl chloride and butyryl chloride are introduced into the C-4 position of methyl phloroglucinol under the catalysis of aluminum trichloride and nitrobenzene to obtain the compound 4–7. In addition, we are at normal temperature conditions. The 4-chloro-2-butenamine hydrochloride is introduced into the benzene ring of the compound 3 to obtain the compound 8. Compound 8 reacted with the above four acid chloride to make compound 9–12 by Friedel–Crafts reaction using AlCl3 as catalyst under mild reaction conditions. On the one hand, compound 12 is achieved in DMF by adding potassium carbonate and methyl iodide to give compounds 13 and 14 under reflux. On the other hand, the methylnaphthalene group of compounds 15–16 were obtained by reacting a compound 12, 1-Chloromethyl naphthalene in the presence of K2CO3 and potassium iodide in ethanol. Finally, 1-chloromethylnaphthalene is introduced into a compound 12 to give the final product compound 17.
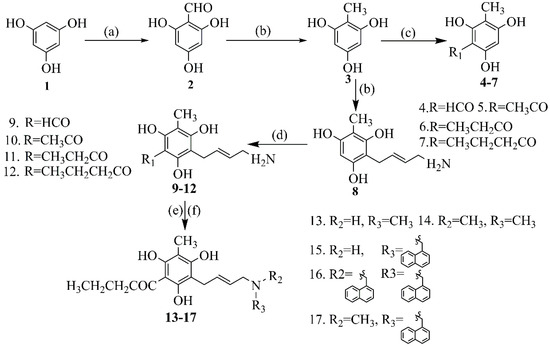
Scheme 1.
Synthesis of phloroglucinol derivatives. (a) POCl3, DMF, EtoAc. (b) NaBH3CN, THF, PH 4. (c) AlCl3, CS2, reflux. (d) AlCl3, CH2Cl2, rt. (e) CH3I, K2CO3, DMF, reflux. (f) PEG-400, K2CO3, KI, EtOH, reflux.
In our previous studies, the reduction of aldehyde groups on the benzene ring to methyl groups mostly uses NaBH3CN as a catalyst and tetrahydrofuran as a solvent, but this reduction reaction is sensitive to pH, and the reaction at room temperature requires at least 10 h [1]. In the subsequent synthesis optimization, we used zinc powder as a catalyst, diethyl ether and ethyl acetate as a common solvent. This improvement makes the whole reaction not need to adjust pH, and the normal temperature reaction can be completely finished within one hour—even the yield increased from 60% to 90%. It is well known that Friedel–Crafts reaction is part of the most important reactions. In the past, when butyryl group was introduced into methyl phloroglucinol, if only AlCl3 and a single solvent carbon disulfide were unreactive, the addition of nitrobenzene had a momentous influence on the reaction. In this study, there are hydroxyl groups on both sides of the C-4 position of methyl phloroglucinol. This electron donating group will offset the passivation of the acyl ring to the benzene ring. The second acyl group is submitted at the C-6 position in the reaction, so the reaction yield is low. It is worth mentioning that after the introduction of an acyl group on the benzene ring of the compound 4–7, the acyl group as the electron withdrawing group was completely inert to Friedel-Crafting. Subsequently, we introduce 4-chloro-2-butenamine hydrochloride into the C-6 position of compound 2 to obtain compound 8, then carry out acylation reaction to obtain compound 9–12. Surprisingly, the synthesis of compound 9–12 did not require the addition of nitrobenzene, and the solvent was changed from carbon disulfide to dichloromethane. This reaction stilled be and the yield was partially improved. This change is more in line with the contemporary concept of green chemistry. Compound 12 has a lone pair of electrons on the nitrogen atom, so it is easy to methylate, then the ratio of methyl iodide and DMF is changed to obtain compounds 13 and 14. After exploring various methods, we add PEG-400 and potassium iodide, enhancing the activity of 1-chloromethylnaphthalene and introducing a methylnaphthalene into the nitrogen atom of compound 12 under mild normal temperature conditions to obtain a secondary amine compound 15 and a tertiary amine compound 16. In the final synthesis of compound 17, methylnaphthalene is more likely to be included onto compound 13 than when a methyl group is introduced on compound 15. Since the introduction of a methyl group by a secondary amine usually requires refluxed reaction, this causes instability of the naphthalene and excessive impurities.
2.2. Evaluation of Antifungal Activity
The antifungal results of all synthetic compounds, pseudoaspidinol and Naftifine were shown in Table 1. The reading of the minimum inhibitory concentration results complied with by observing the growth effect of the two fungal strains on the 96-well plate (seen in Figure 2). Pseudoaspidinol exhibited strong activity against T. mentagrophyte and T. rubrum, with IC50 values of 21.06 μg/mL and 19.89 μg/mL, respectively. Compound 3 exhibited poor activity against T. mentagrophytes and T. rubrum. Subsequently, the activity of the compound 4–7 showed that only the compound 6 and the compound 7 had an antifungal effect. The antifungal activity of compound 6 was 62.5 μg/mL, while the IC50 of compound 7 against T. rubrum was 39.89 μg/mL. Activity of against T. mentagrophytes was similar. Disappointingly, Compound 8 was lower active against both fungi. The activity of Compound 12 was not optimized compared to the activity of Compound 7, but the activity of Compound 12 was decreased by nearly 1/3 compared with Compound 6, and the activity against both fungi was 39.24 μg/mL and 39.17 μg/mL, respectively. Surprisingly, the activity of Compound 9 was practically four times lower than that of Compound 4. Compounds 15 and 17 showed the best activity against Trichophyton rubrum and T. mentagrophyte, and the antifungal effect was much higher than that of the lead compound pseudomicinol.

Table 1.
Antifungal activities of compounds on Trichophyton rubrum (Tr) and Trichophyton mentagrophytes (Tm).
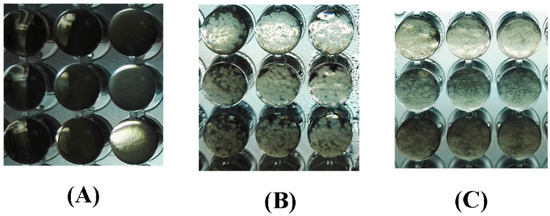
Figure 2.
(A) Complete antifungal effect. (B) Growth effect of fungal strains: Trichophyton rubrun. (C) Growth effect of fungal strains: Trichophyton mentagrophytes.
The results showed that the butyryl group at the C-4 position of methyl phloroglucinol may be a pharmacophore of pseudomicinol. Subsequently, it was found that most of the antifungal activity results of the compound 9–12 after the para-introduction of the acyl group after the introduction of the allylamine group at the C-6 position of the methyl phloroglucinol skeleton was optimized (Figure 3A,B). This clearly indicates that the allylamine group may not be suitable as a single antifungal group, but it has some antifungal optimization effects. In addition, the naphthylmethyl group of N in allylamine can improve the antifungal activity. Obviously, structurally modified compounds 15 and 17 exhibited excellent activity against Trichophyton rubrum and T. mentagrophyte, compared to pseudomicinol (Figure 3C,D). From the optimal compound 17, it can be assumed that the antifungal key pharmacophore of the allylamine antifungal agent such as Naftifine is methylnaphthalene. Further, research work on structure-activity relationship is currently under investigation and will be reported in due course.
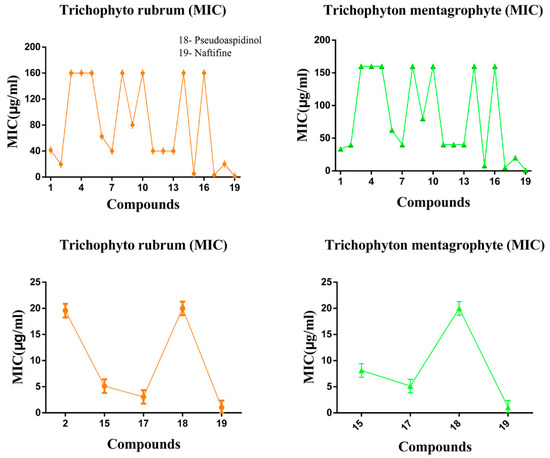
Figure 3.
Activities of all compounds against Trichophyton rubrun and Trichophyton mentagrophytes. (A) The antifungal results of all compounds against Trichophyton rubrun. (B) The antifungal results of all compounds against Trichophyton mentagrophytes. (C) The strong antifungal results of compounds against Trichophyton rubrun. (D) The strong antifungal results of compounds against Trichophyton mentagrophytes.
2.3. Docking Studies
Currently, the main targets of the antifungal agents are the cytochrome P450 sterol 14a-demethylase (CYP51), squalene epoxidase (SE) and β-1,3-glucansynthase. The mechanism of molecular docking was targeted at the positive drug, Naftifine. Docking experiments select SE (PDB ID: 2AIB) to perform molecular docking since allylamine antifungal agents mainly acts on SE [7,8,9,10]. The docking score of compounds is shown in Table 2. The results show compounds 4–13 had higher scores than pseudoaspidinol in accord with the results of antifungal activities and compound 8–17 demonstrate better score and antifungal activities, and the 3D interactions of compounds 9–13 with 2AIB are shown in Figure 4. As can be seen from the diagram, compounds formed the hydrogen bonding interactions of allylamine NH with TYR 47 and phenolic hydroxyl with MET 35 and TYR 33, hydrophobic interaction of allyl with LEU 41, MET 59 and ALA 88, and Pi-Pi interaction of naphthalene ring with LYS 94. Compound 17 shows the best antifungal activity owing to Pi-Pi interaction of a naphthalene ring and hydrophobic interactions of N–CH3 with ILE 60 (seen in Figure 5). In order to understand the binding mode in a dynamic environment, molecular dynamics simulation was executed for the compound 17 with the crystal structure of 2AIB. Compound 17 exhibited a stable binding conformation throughout the MD run as confirmed through the ligand Root Mean Squared Deviation (RMSD), and the ligand tends to stable during 15–35 ps (RMSD < 0.4 Å) (Figure 6).

Table 2.
Docking results of synthesized compounds.
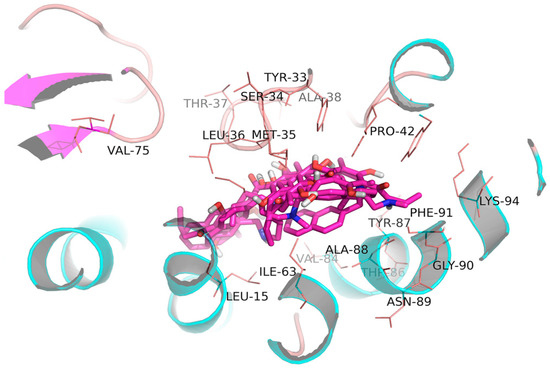
Figure 4.
Binding modes of compounds 13–17 with 2AIB. The structural framework of compounds was marked with amaranth color. Protein skeleton of 2AIB was marked with green color. The atoms involved in the path are identified as colored stick. (Oxygen (orange), nitrogen (blue), hydrogen in hydroxyl (white)).
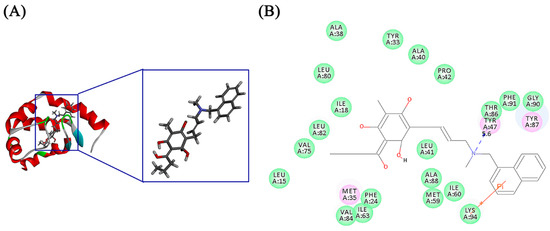
Figure 5.
(A) Optimal binding modes of compound 17 in squalene epoxidase (PDB ID 2AIB); (B) 2D interaction plot of 2AIB and compound 17. The structural framework of compound 17 was marked with green color. Protein skeleton of 2AIB was marked with red color. The atoms involved in the path are identified as colored stick. (Oxygen (red), nitrogen (blue), hydrogen (white)).
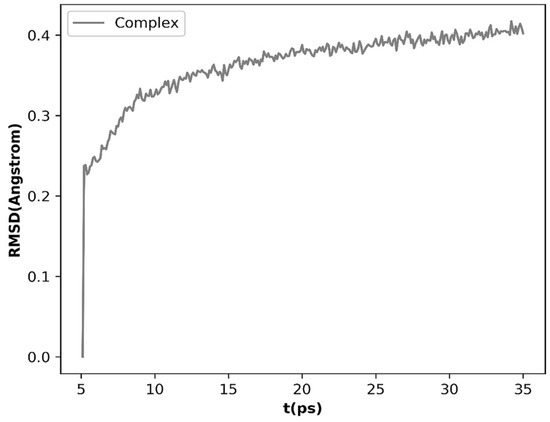
Figure 6.
Plots of RMSD for all of the backbone atoms (Å) vs. simulation time (ps) for 2AIB in complex with compound 17.
3. Materials and Methods
3.1. Synthesis of Compounds
The solvents and reagents used throughout this study were purchased from commercial vendors and were dried and purified by conventional methods prior to use. Nuclear magnetic resonance (NMR) spectra was recorded on a Bruker AC-400P spectrometer (Bruker, Karlsruhe, Germany) with TMS as the internal standard and was analyzed by MestReNova (Mestrelab Research, Santiago de Compostela, Coruña, Spain) [19]. Infrared spectra were recorded using potassium bromide disks on a Hitachi 270-50 spectrophotometer (Hitachi Limited, Tokyo, Japan) and ESI mass spectra were made on an API-3000 LC-MS spectrometer (Applied Biosystems, Shanghai, China). Thin-Layer Chromatography (TLC) analysis was performed on silica gel 60 F254 silica plates (Merck KGaA, Darmstadt, Germany). 1H-NMR, 13C-NMR and MS of compounds 2–17 were found in Supplementary Materials.
Synthesis of3. To the solution of 1-formyl-2,4,6-trihydroxy benzene (2) (1.54 g, 10 mmol) in tetrahydrofuran (20 mL) we added methyl orange (1–2 drops) followed by the addition of sodium cyanoborohydride (1.90 g, 30 mmol). The pH of the reaction mixture was maintained at 4.0, throughout the reaction, by addition of 1 N HCl. The reaction mixture was stirred at room temperature for 12 h prior to extraction with ethyl acetate (30 mL × 4). The ethyl acetate layer was washed with brine solution (30 mL × 3) and finally dried over Na2SO4. Solvent was evaporated to get the crude product, which was purified by column chromatography with hexane/EtOAc (50:50 v/v).
Synthesis of7. 2,4,6-trihydroxytoluene (2 g, 14.28 mmol) was placed in a 250 mL three-necked flask, carbon disulfide (10 mL) and anhydrous aluminum trichloride powder (5.7 g, 42.84 mmol) were added, and nitrobenzene (6 mL) was added with stirring, and heated to reflux. After 0.5 h, then butyryl chloride (1.68 g, 15.7 mmol) was slowly added dropwise, and the mixture was refluxed for 1.5 h, cooled to room temperature, and poured into a mixture of ice water (10 mL of concentrated hydrochloric acid, 70 g of ice water) with stirring, and distilled for 30 min. The mixture was filtered while hot, and the yellow needle-like crystals were precipitated in the filtrate. After sufficient cooling, the mixture was filtered, and the remaining oil was repeatedly subjected to hot extraction several times. The combined crystals were dried to obtain 2-methyl-6-butyrylphloroglucol.
Synthesis of8. At room temperature, 2-methylbenzene-1,3,5-triol (421.04 mg, 3 mmol) and anhydrous aluminum trichloride powder (799.98 mg, 6 mmol) were slowly added to a solution of methylene chloride (20 mL), stirred evenly and refluxed for 1 h. Simultaneously, 4-chloro-2-butenamine hydrochloride (468.41 mg, 3.31 mmol) was dissolved in a 10% (by mass) aqueous sodium hydroxide solution(10 mL), stirred for 10 min to neutralize the hydrochloride, extracted three times(15 mL) with methylene chloride and dried over anhydrous sodium sulfate. The neutralized solution of 4-chloro-2-butenamine in methylene chloride was slowly added to a flask containing 2-methylbenzene-1,3,5-triol at 0 °C. At room temperature, the reaction was stirred until complete. The solvent was recovered, dissolved in deionized water, extracted with ethyl acetate five times (20 mL), dried over anhydrous sodium sulfate and the ethyl acetate removed under reduced pressure to obtain the crude product as a yellow solid. This crude product was isolated by silica gel column chromatography (hexane/EtOAc 1:1 v/v) to obtain compound 8.
Synthesis of11. A mixture of 8 (627.62 mg, 3 mmol) and aluminum chloride (1.201 g, 9 mmol) in 20 mL of methylene chloride was added to propionyl chloride (305 mg, 3.3 mmol). The reaction mixture was stirred at 42 °C and monitored by TLC. Once the reaction was complete (5 h), the mixture was poured onto ice water (70.00 g) and hydrochloric acid (2.00 g) was added. The solvent was recovered, dissolved in deionized water, extracted with ethyl acetate five times (20 mL), dried over anhydrous sodium sulfate and the ethyl acetate removed under reduced pressure to obtain the crude product. The residue was subsequently purified by silica gel column chromatography (hexane/EtOAc 3:2 v/v) to give a pure product.
Synthesis of13. The intermediate of compound 12 (558.30 mg, 2 mmol) was then dissolved in dry DMF (20 mL), followed by the addition of K2CO3 (2.66 g, 12 mmol) and Me2SO4 (2.51 g, 12 mmol). The reaction mixture was refluxed for 4 h, after which the mixture was filtered to obtain compound 13.
Synthesis of15. PEG-400 (5 d) and potassium carbonate were added to a mixture of compound 12 (1.40 g, 5 mmol) in EtOH (15 mL). The solution of 1-Chloromethyl naphthalene (1.77 g, 10 mmol) and potassium iodide (1.60 g, 10 mmol) in EtOH (5 mL) was then added dropwise. The reaction was stirred at room temperature for 36 h and neutralized with a saturated solution of NaHCO3. Following extraction with dichloromethane, the organic layer was washed with brine, dried over anhydrous sodium sulfate, concentrated, and purified by column chromatography (methyl alcohol/dichloromethane, 1:20 v/v, Rf = 0.57) to obtain the intermediary of compound 15.
2,4,6-Trihydroxybenzaldehyde (2). Yield: 82%. Yellow power. m.p.: 195.4–198.3 °C. 1H-NMR (400 MHz, DMSO): δ(ppm) 11.46 (s, 2H), 10.66 (s, 1H), 9.93 (s, 1H), 5.79 (s, 2H), 2.50 (dt, J = 3.5, 1.7 Hz, 1H). 13C-NMR (101 MHz, DMSO): δ(ppm) 192.4 (s), 167.5 (s), 165.7 (s), 159.3 (s), 102.9 (s), 94.9 (s), 87.9 (s). EI-MS: 153.36 [M − H]−. Anal. calcd for C7H6O4 (154.03): C, (54.34) 54.55; H, (3.91) 3.92; O, (41.75) 41.52.
2-Methylbenzene-1,3,5-triol (3). Yield: 78%. Yellow power. m.p.: 213.4–216.5 °C. 1H-NMR (400 MHz, DMSO): δ(ppm) 8.79 (s, 2H), 8.66 (s, 1H), 5.77 (s, 2H), 2.50 (s, 3H). 13C-NMR (101 MHz, DMSO): δ(ppm) 166.4 (s), 153.5 (d, J = 7.8 Hz), 103.1 (s), 94.0 (s), 86.3 (s), 8.2 (s). EI-MS: 139.52 [M − H]−. Anal. calcd for C7H8O3 (140.05): C, 60.00 (59.97); H, 5.75 (5.73); O, 34.25 (34.30).
2,4,6-Trihydroxy-3-methylbenzaldehyde (4). Yield: 58%. White solid. m.p.: 223.7–224.5 °C. 1H-NMR (400 MHz, DMSO): δ(ppm) 8.79 (s, 2H), 8.66 (s, 1H), 5.77 (s, 2H), 2.50 (s, 3H). 13C-NMR (101 MHz, DMSO): δ(ppm) 192.3 (s), 166.0 (s), 165.2 (s), 164.9 (s), 102.5 (s), 101.9 (s), 94.6 (s), 8.4 (s). EI-MS: 169.52 [M + H]+. Anal. calcd for C8H8O4 (168.04): C, 57.14 (57.08); H, 4.80 (4.83); O, 38.06 (38.09).
1-(2,4,6-Trihydroxy-3-methylphenyl)ethan-1-one (5). Yield: 76%. Yellow power. m.p.: 206.9–208.1 °C. 1H-NMR (400 MHz, DMSO): δ(ppm) 10.50 (s, 1H), 10.25 (s, 1H), 6.00 (s, 1H), 2.53 (s, 3H), 1.82 (s, 3H). 13C-NMR (101 MHz, DMSO): δ(ppm) 205.0 (s), 164.4 (d, J = 2.9 Hz), 163.1 (s), 104.0 (s), 102.8 (s), 94.3 (s), 28.6 (s), 7.8 (s). EI-MS: 183.90 [M + H]+. Anal. calcd for C9H10O4 (182.06): C, 59.34 (59.23); H, 5.53 (5.59); O, 35.13 (35.18).
1-(2,4,6-Trihydroxy-3-methylphenyl)propan-1-one (6). 1H-NMR (400 MHz, DMSO): δ(ppm) 10.48 (s, 1H), 10.20 (s, 1H), 6.00 (s, 1H), 2.99 (q, J = 7.2 Hz, 2H), 1.82 (s, 3H), 1.04 (t, J = 7.2 Hz, 3H). 13C-NMR (101 MHz, DMSO): δ(ppm) 206.1 (s), 163.7 (s), 162.7 (s), 160.2 (s), 103.8 (s), 101.8 (s), 94.4 (s), 36.8 (s), 9.3 (s), 7.7 (s). EI-MS: 197.93 [M + H]+. Anal. calcd for C10H12O4 (196.07): C, 61.22 (61.31); H, 6.17 (5.20); O, 32.62 (33.58).
1-(2,4,6-Trihydroxy-3-methylphenyl)butan-1-one (7). Yield: 68%. Yellow power. m.p.: 162.0–164.2 °C. 1H-NMR (400 MHz, DMSO): δ(ppm) 14.01 (s, 1H), 10.52 (s, 1H), 10.26 (s, 1H), 6.01 (s, 1H), 2.97 (t, J = 7.3 Hz, 2H), 1.84 (s, 3H), 1.60 (dd, J = 14.7, 7.3 Hz, 2H), 0.91 (t, J = 7.4 Hz, 3H). 13C-NMR (101 MHz, DMSO): δ(ppm) 205.1 (s), 163.4 (s), 162.3 (s), 159.7 (s), 103.5 (s), 101.4 (s), 94.0 (s), 45.1 (s), 17.8 (s), 13.9 (s), 7.3 (s). EI-MS: 211.53 [M + H]+. Anal. calcd for C11H14O4 (210.09): C, 62.85 (62.79); H, 6.71 (6.83); O, 30.44 (30.38).
(E)-2-(4-Aminobut-2-en-1-yl)-4- methylbenzene-1,3,5-triol (8). Yield: 95%. 1H-NMR (400 MHz, CDCl3): δ(ppm) 7.01 (s, 1H), 6.48 (s, 1H), 6.33–6.28 (m, 1H), 4.12 (dd, J = 14.3, 7.1 Hz, 1H), 2.24 (d, J = 34.0 Hz, 2H), 2.09 (d, J = 38.0 Hz, 2H), 1.57 (s, 3H). 13C-NMR (101 MHz, DMSO): δ(ppm) 156.5 (s), 155.6 (s), 153.2 (s), 129.6 (s), 123.0 (s), 107.2 (s), 101.7 (s), 94.5 (s), 46.6 (s), 25.4 (s), 8.4 (s). EI-MS: 209.56 [M − H]−. Anal. calcd for C11H15NO3 (209.11): C, 63.14 (63.12); H, 7.23 (7.24); N, 6.69 (6.71); O, 22.94 (22.93).
(E)-3-(4-Aminobut-2-en-1-yl)-2,4,6-trihydroxy-5-methylbenzaldehyde (9). Yield: 53%. 1H-NMR (400 MHz, DMSO): δ(ppm) 9.15 (s, 1H), 8.53 (s, 1H), 8.12 (s, 1H), 6.22 (dt, J = 24.2, 10.0, 1.5 Hz, 1H), 5.71 (dt, J = 24.1, 9.9, 1.6 Hz, 1H), 4.78 (s, 1H), 3.39–3.24 (m, 4H), 2.16 (s, 3H), 1.46 (s, 2H). 13C-NMR (101 MHz, DMSO): δ(ppm) 193.2 (s), 161.9 (s),156.8 (s), 155.9 (s), 129.6 (s), 123.0 (s), 106.4 (s), 101.2 (s), 94.5 (s), 46.6 (s), 24.8 (s), 8.4 (s). EI-MS: 236.21 [M − H]−. Anal. calcd for C12H15NO4 (237.10): C, 60.75 (60.73); H, 6.37 (6.38); N, 5.90 (5.89); O, 26.97 (27.00).
(E)-1-(3-(4-Aminobut-2-en-1-yl)-2,4,6-trihydroxy-5-methylphenyl)ethan-1-one (10). Yield: 58%. 1H-NMR (400 MHz, DMSO): δ(ppm) 9.36 (s, 1H), 7.02 (s, 1H), 6.55 (s, 1H),5.65 (dt, J = 10Hz, 1H), 5.42 (dt, J = 9 Hz, 1H), 3.35 (d, J = 3Hz, 2H), 3.23 (d, J = 3 Hz, 2H), 2.32 (d, J = 4Hz, 6H), 1.26 (s, 2H). 13C-NMR (101 MHz, DMSO): δ(ppm) 205.7 (s), 162.1 (s), 161.6 (s), 161.2 (s), 129.6 (s), 123.0 (s), 107.0 (s), 106.6 (s), 104.5 (s), 46.6 (s), 28.6 (s), 24.8 (s), 8.1 (s). EI-MS: 251.73 [M + H]+. Anal. calcd for C13H17NO4 (251.12): C, 62.14 (61.13); H, 6.82 (6.83); N, 5.57 (5.56); O, 25.47 (27.48).
(E)-1-(3-(4-Aminobut-2-en-1-yl)-2,4,6-trihydroxy-5-methylphenyl)propan-1-one (11). Yield: 76%. Yellow power. m.p.: 155.6–157.4 °C. 1H-NMR (400 MHz, DMSO): δ(ppm) 8.65 (s, H), 8.53 (s, 1H), 5.83 (dt, J = 20.9, 12.3 Hz,1H), 5.57 (dt, J = 20.9, 9.1 Hz,1H), 4.25 (s, 1H), 3.64 (d, 2H), 3.62 (d, 2H), 2.64 (q, J = 8.4 Hz, 2H), 2.50 (s, 3H), 1.31 (t, J =8.5 Hz, 3H), 1.79 (s, 3H). 13C-NMR (101 MHz, DMSO): δ(ppm) 208.4 (s), 162.8 (s), 161.4 (d, J = 11.0 Hz), 129.6 (s), 123.0 (s), 106.6 (s), 106.1 (s), 102.8 (s), 46.6 (s), 35.5 (s), 24.8 (s), 8.4 (s), 8.1 (s). EI-MS: 265.55 [M + H]+. Anal. calcd for C14H19NO4 (265.13): C, 63.38 (63.39); H, 7.22 (7.23); N, 5.28 (5.27); O, 24.12 (24.11).
(E)-1-(3-(4-Aminobut-2-en-1-yl)-2,4,6-trihydroxy-5-methylphenyl)butan-1-one (12). Yield: 82%. White power. m.p.: 98.5–102.3 °C. 1H-NMR (400 MHz, DMSO): δ(ppm) 9.86 (s, 1H), 6.14 (dt, J = 24.3, 12.4 Hz, 1H), 5.71 (dt, J = 21.4, 11.4 Hz, 1H), 4.84 (s, 1H), 4.04 (s, 1H), 3.31 (m, J = 12.6, 4.1 Hz, 4H), 2.96 (t, J = 9.2 Hz, 2H), 2.16 (s, 3H), 1.60 (m, 2H), 1.43 (d, J = 65.2 Hz, 2H), 0.94 (t, J = 13.1 Hz, 3H). 13C-NMR (101 MHz, DMSO): δ(ppm) 205.5 (s), 163.8 (s), 162.7 (s), 160.1 (s), 129.6 (s), 123.0 (s), 104.0 (s), 101.8 (s), 94.4 (s), 45.5 (s), 41.8 (s), 24.8 (s), 18.3 (s), 14.3 (s), 7.7 (s). EI-MS: 279.57 [M + H]+. Anal. calcd for C15H21NO4 (279.15): C, 64.50 (64.49); H, 7.58 (7.59); N, 5.01 (4.99); O, 22.91 (22.93).
(E)-1-(2,4,6-Trihydroxy-3-methyl-5-(4-(methylamino)but-2-en-1-yl)phenyl)butan-1-one (13). Yield: 49%. 1H-NMR (400 MHz, DMSO): δ(ppm) 9.55 (s, 1H), 6.02 (dt, J = 11.2, 9.8 Hz, 1H), 5.71 (dt, J = 11.2, 9.84 Hz, 1H), 5.41 (s, 1H), 4.08 (s, 1H), 3.97 (s, 3H), 3.41–3.31 (m, 4H), 2.96 (t, J = 8.5 Hz, 2H), 2.16 (s, 3H), 1.67–1.47 (m, 2H), 1.34 (s, 1H), 0.94 (t, J = 10.48 Hz, 3H). 13C-NMR (101 MHz, DMSO): δ(ppm) 207.8 (s), 163.1 (s), 161.6 (d, J = 1.6 Hz), 127.3 (s), 124.6 (s), 106.7 (s), 106.5 (s), 103.4 (s), 55.4 (s), 41.8 (s), 36.2 (s), 24.8 (s), 19.6 (s), 13.5 (s), 8.1 (s). EI-MS: 293.81 [M + H]+. Anal. calcd for C16H23NO4 (293.16): C, 65.51 (65.50); H, 7.90 (7.91); N, 4.77 (4.76); O, 21.81 (21.83).
(E)-1-(3-[4-(Dimethylamino)but-2-en-1-yl]-2,4,6-trihydroxy-5-methylphenyl)butan-1-one (14). Yield: 51%. 1H-NMR (400 MHz, DMSO): δ(ppm) 7.75 (s, H), 6.04 (dt, J = 21.2, 11.3 Hz, 1H), 5.67 (dt, J = 18.2, 12.0, 9.04 Hz, 1H), 5.55 (s, 1H), 4.80 (s, 1H), 3.33 (dd, J = 9.8, 1.4 Hz, 2H), 3.08–2.90 (m, 4H), 2.75 (s, 6H), 2.16 (s, 3H), 1.68–1.45 (m, 2H), 0.94 (t, J = 10.5 Hz, 3H). 13C-NMR (101 MHz, DMSO): δ(ppm) 208.4 (s), 161.9 (d, J = 1.6 Hz), 159.3 (s), 157.7 (s), 131.7 (s), 126.8 (s), 111.8 (s), 106.5 (s), 106.5 (s), 49.1 (s), 46.3 (s), 45.4 (s), 25.4 (s), 17.1 (s), 13.8 (s), 7.4 (s). EI-MS: 307.57 [M + H]+. Anal. calcd for C17H25NO4 (307.18): C, 66.43 (66.42); H, 8.20 (8.21); N, 4.56 (4.58); O, 20.82 (20.79).
(E)-1-(2,4,6-Trihydroxy-3-methyl-5-(4-((naphthalen-2-ylmethyl)amino)but-2-en-1-yl)phenyl)butan-1-one (15). Yield: 32%. 1H-NMR (400 MHz, DMSO): δ(ppm) 9.46 (s, 1H), 8.05–7.91 (m, 2H), 7.69 (dt, J = 11.8, 2.3 Hz, 1H), 7.56 (dt, J = 12.0, 2.4 Hz, 1H), 7.29 (dd, J = 18, 14.48, 7.28 Hz, 2H), 6.98 (dd, J = 12.0, 2.5 Hz, 1H), 6.22 (dt, J = 22.8, 9.9, 1.4 Hz, 1H), 5.78 (dt, J = 24.2, 10.0, 1.6 Hz, 1H), 5.01 (s, 1H), 4.20 (s, 2H), 3.66 (s, 1H), 3.49 (dd, J = 9.8, 1.6 Hz, 1H), 3.40 (dd, J = 9.84, 1.6 Hz, 1H), 3.33 (dd, J = 9.84, 1.4 Hz, 2H), 2.96 (t, J = 12.8 Hz, 2H), 2.16 (s, 4H), 1.69–1.45 (m, 2H), 0.94 (t, J = 10.6 Hz, 3H). 13C-NMR (101 MHz, DMSO): δ(ppm) 161.8 (s), 159.1 (s), 157.7 (s), 133.9 (s), 133.7 (s), 131.1 (s), 129.9 (s), 129.5 (s), 129.1 (s), 128.4 (s), 127.1 (d, J = 7.6 Hz), 125.9 (s), 124.3 (s), 111.0 (s), 106.8 (s), 106.1 (d, J = 34.1 Hz), 51.2 (s), 49.1 (s), 44.9 (s), 25.4 (s), 17.5 (s), 13.7 (s), 7.8 (s). EI-MS: 419.53 [M − H]−. Anal. calcd for C26H29NO4 (419.21): C, 74.44 (74.42); H, 6.97 (6.98); N, 3.34 (3.35); O, 15.25 (15.25).
(E)-1-(3-(4-(bis(Naphthalen-2-ylmethyl)amino)but-2-en-1-yl)-2,4,6-trihydroxy-5-methylphenyl)butan-1-one (16). Yield: 40%. 1H-NMR (400 MHz, DMSO): δ(ppm) 8.25–7.81 (m, 4H), 7.68 (dt, J = 12.0, 2.5 Hz, 2H), 7.55 (dt, J = 11.9, 2.4 Hz, 2H), 7.28 (dt, J = 17.9, 14.3, 7.2 Hz, 4H), 7.18 (s, 1H), 6.97 (dd, J = 12.0, 2.3 Hz, 2H), 6.92 (s, 1H), 6.25 (dt, J = 24.2, 9.9, 1.6 Hz, 1H), 5.72 (dt, J = 22.9, 9.8, 1.4 Hz, 1H), 4.68 (s, 1H), 4.09 (s, 4H), 3.32 (dd, J = 9.9, 1.5 Hz, 2H), 3.11–2.88 (m, 4H), 2.15 (s, 3H), 1.71–1.46 (m, 2H), 0.94 (t, J = 10.5 Hz, 3H). 13C-NMR (101 MHz, DMSO): δ(ppm) 208.8 (s), 161.6 (s), 159.2 (s), 157.5 (s), 138.0 (s), 137.2 (s), 133.4 (s), 132.1 (s), 131.9 (s), 130.1 (d, J = 26.4 Hz), 130.2–130.7 (m), 128.1 (d, J = 37.0 Hz), 128.2 (d, J = 13.1 Hz), 127.3 (d, J = 8.6 Hz), 126.4 (d, J = 29.7 Hz), 126.2 (d, J = 8.3 Hz), 126.1 (s), 125.7 (s), 124.4 (d, J = 22.4 Hz), 124.2 (s), 111.5 (s),106.5 (s), 106.3 (s), 60.2 (s), 53.4 (s), 49.5 (s), 46.5 (s), 25.7 (s), 17.6 (s), 13.8 (s), 7.5 (s).EI-MS: 559.53 [M − H]−. Anal. calcd for C37H37NO4 (559.27): C, 79.40 (79.39); H, 6.66 (6.68); N, 2.50 (2.49); O, 11.43 (11.44).
(E)-1-(2,4,6-Trihydroxy-3-methyl-5-(4-(methyl(naphthalen-2-ylmethyl)aminbut-2-en-1-yl)phenyl)butan-1-one (17). Yield: 75%. 1H-NMR (400 MHz, DMSO): δ(ppm) 8.47 (s, 1H), 8.05–7.89 (m, 2H), 7.69 (dt, J = 11.8, 2.3 Hz, 1H), 7.56 (dt, J = 11.9, 2.4 Hz, 1H), 7.28 (dd, J = 17.9, 14.3, 7.2 Hz, 2H), 6.98 (dd, J = 11.2, 2.4 Hz, 1H), 5.92 (dt, J = 21.8, 9.4, 1.3 Hz, 1H), 5.71 (dt, J = 24.2, 9.8, 1.3 Hz, 1H), 4.82 (s, 1H), 4.09 (s, 2H), 3.51 (s, 1H), 3.32 (dd, J = 9.7, 1.4Hz, 2H), 3.13–2.83 (m, 4H), 2.26 (s, 3H), 2.16 (s, 3H), 1.70–1.44 (m, 2H), 0.94 (t, J = 10.6 Hz, 3H). 13C-NMR (101 MHz, DMSO): δ(ppm) 207.0 (s), 162.5 (s), 162.0 (s), 136.5 (s), 136.3 (s), 133.4 (s), 132.5 (s), 131.7–131.0 (m), 127.6–129.2 (m), 128.5 (s), 126.9 (s), 106.7 (s), 106.1 (s), 103.4 (s), 60.3 (s), 58.7 (s), 47.8 (s), 47.5 (s), 24.9 (s), 19.7 (s), 13.6 (s), 8.1 (s). EI-MS: 433.30 [M − H]−. Anal. calcd for C27H31NO4 (433.23): C, 74.80 (74.79); H, 7.21 (7.19); N, 3.23 (3.22); O, 14.76 (14.80).
3.2. Evaluation of Antifungal Activity
The antifungal activity of the compounds was evaluated against two standard human pathogenic fungal strains, Trichophyton rubrum (CMCC (F) T1d) and Trichophyton mentagrophytes (CMCC (F) T5c), purchased from the Chinese Academy of Medical Sciences (Hospital for Pathology and Dermatolog, Science and Peking Union Medical College). A solution of Naftifine (1.0 mg/mL) was used as a positive control, while pseudo mallow phenol (1.0 mg/mL) was used as a comparison. The minimum inhibitory concentration (MIC) of the compound was determined by a micro-broth dilution method according to the method defined by the National Clinical Laboratory Standards Committee (NCCLS National Clinical Laboratory Standards Committee 2002) [20]. For the assay, the MIC was determined in accordance with Clinical and Laboratory Standards Institute (CLSI) method M38-A2, in which the test compound monomer stock solution (32 mg/mL) and the positive drug naftifine were diluted 100-fold with RPMI-1640 liquid medium, and the concentration of the solvent DMSO after dilution (v/v) less than 1% to reduce the interference of the solvent on the experimental results. Then, the diluted compound monomer stock solution was subjected to lateral dilution in RPMI-1640 liquid medium. A blank control and growth control were placed in sterile 96-well plates. Finally, the 96 well plate mixed with the bacterial liquid and the drug solution was incubated at 35 °C for 4 days at a constant temperature, and the results were visually observed. The results were read by two people. The lowest concentration of the drug-free wells at the lowest drug concentration was the minimum inhibitory concentration (MIC). Each strain was tested three times.
3.3. Docking Studies
Docking studies of all derivatives were implemented in the program AutoDock 4.2.6 [21]. The X-ray crystallographic structure of SE (PDB ID: 2AIB) was taken from PDB (www.rcsb.org/pdb) and was invoked as a protein structure [22,23]. Beginning of docking, all the water and ligands were removed and the random hydrogen atoms were added. All the compounds were constructed using ChemDraw Ultra 14.0. Most parameters were default values except the calculation for the Lamarckian genetic algorithm (LGA). The grid box for docking size was set to 55 × 55 × 55 points in x, y and z directions. The docking experiment was implemented by AutoDock program. There were 100 multiples to independent docking runs, then each experiment was repeated 20 times, to obtain the best performance of the docking programs. Molecular dynamics of the complexes resulting from docking of compounds 13–17 were performed using Discovery Studio 3.5 Module Standard Dynamic Cascade (Sun Yat-sen University, Guangzhou, China). The CHARMm force field and the Moony–Rone charge were added to the composite, and then the H2O molecules and the NaCl molecules were added to make the NaCl concentration of the complex system 0.145, which was reached the osmotic pressure of the human body. After an initial default relaxation protocol, an MD production run was made with a time step of 2.0 ps [24].
4. Conclusions
In conclusion, our strategy to introduce antifungal pharmacophore allylamine into the methyl phloroglucinol and remain acyl group in the C-6 position using pseudoaspidinol as the lead compound to obtain novel phloroglucinol derivatives 4–17 was successful in leading to compounds, which showed inhibition of T. mentagrophytes and T. rubrum. Furthermore, the first basic efficacy analysis of Naftifine groups in the study. These compounds could be useful as lead compounds in developing novel antifungal agents.
Supplementary Materials
The following are available online, 1H-NMR, 13C-NMR and MS of compounds 2–17.
Author Contributions
L.Y. contributed to the conception and design of the experiments, analysis of the data and revised the paper, X.T. synthesized title compounds, and selected the data. Z.S. analyzed the data. Y.W. and P.S. performed the biological studies. J.G. performed the docking studies.
Funding
The work was supported by Special Innovation Project of Guangdong Education Department (Natural Science) (2017KTSCX107), Guangdong Natural Science Foundation (2015A03031356), Science and Technology Planning Project of Guangdong Province (2017ZC0199), Key Laboratory of New Drug Discovery and Evaluation of Ordinary Universities of Guangdong Province (2017KSYS002), Guangzhou Key Laboratory of Construction andAapplication of New Drug Screening Model Systems (201805010006), Guangdong Province Precise Medicine and Big Data Engineering Technology Research Center for Traditional Chinese Medicine, Guangdong Province Engineering Technology Center for Molecular Probes & Biomedical Imaging.
Acknowledgments
The authors are grateful to Tan and Sun Yet-Sen University for molecular docking experiment.
Conflicts of Interest
The authors declare no competing interests.
References
- Monk, B.C.; Perlin, D.S. Fungal plasma membrane proton pumps as promising new antifungal targets. CRC Crit. Rev. Microbiol. 1994, 20, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Tsui, C.; Kong, E.F.; Jabrarizk, M.A. Pathogenesis of Candida albicans biofilm. Pathog. Dis. 2016, 74, ftw018. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.K.; Garzan, A.; Garneautsodikova, S. Novel alkylated azoles as potent antifungal. Eur. J. Med. Chem. 2017, 133, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Araj, G.F.; Asmar, R.G.; Avedissian, A.Z. Candida profiles and antifungal resistance evolution over a decade in Lebanon. J. Infect. Dev. Ctries. 2015, 9, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z. The action target mechanism of antifungal agents and research advances of novel drugs. Chin. J. Pharm. Anal. 2015, 2, 193–202. [Google Scholar]
- Xie, H.W.; Yang, X.S.; Chao, T.L. Antifungal drugs and their mechanisms of action. Chin. J. Microecol. 2015, 27, 1477–1479. [Google Scholar]
- Sandra, G.A.; Nerea, J.; Elena, E.; Guillermo, Q. Postantifungal effect of caspofungin against the Candida albicans and Candida parapsilosis clades. Diagn. Microbiol. Infect. Dis. 2016, 86, 172–177. [Google Scholar]
- Yang, X.X.; Xu, Y. Cytochrome P450 Monooxygenases and Interaction of CYP125A18 with Azole Drugs in Rhodococcus sp. R04. Chin. J. Biochem. Mol. Biol. 2016, 32, 295–304. [Google Scholar]
- Huang, K.X.; Xia, L.; Zhang, Y.; Ding, X.; Zahn, J.A. Recent advances in the biochemistry of spinosyns. Appl. Microbiol. Biotechnol. 2009, 82, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Yoon, S.M.; Choi, E.J.; Lee, J. Terbinafine inhibits gap junctional intercellular communication. Toxicol. Appl. Pharmacol. 2016, 307, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.C.; Zhao, D.D.; Liu, Z.D. A New Human Cancer Cell Proliferation Inhibition Sesquiterpene, Dryofraterpene A, from Medicinal Plant Dryopteris fragrans (L.) Schott. Molecules 2017, 22, 180. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Fu, Y.J.; Luo, M. Preparative separation of dryofragin and aspidin BB from Dryopteris fragrans extracts by macroporous resin column chromatography. J. Pharm. Biomed. Anal. 2012, 61, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Guo, N.; Li, N. Investigation of antibacterial activity of aspidin BB against Propionibacterium acnes. Arch. Dermatol. Res. 2016, 308, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Gao, C.; Peng, X. Aspidin BB, a phloroglucinol derivative, exerts its antibacterial activity against Staphylococcus aureus by inducing the generation of reactive oxygen species. Res. Microbiol. 2014, 165, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.D.; Zhao, Q.S.; Liu, L. Compounds from Dryopteris fragrans (L.) Schott with cytotoxic activity. Molecules 2014, 19, 3345–3355. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Lin, P.; Du, W.; Wang, Y.; Tang, C.; Shen, Z. Preparation, Anti-dermatophyte Activity and Mechanism of Methylphloroglucinol Derivatives. Front. Microbiol. 2018, 9, 2262. [Google Scholar] [CrossRef] [PubMed]
- Karumuri, S.; Singh, P.K.; Shukla, P. In Silico Analog Design for Terbinafine Against Trichophyton rubrum: A Preliminary Study. Indian J. Microbiol. 2015, 55, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Favre, B.; Ghannoum, M.A.; Ryder, N.S. Biochemical characterization of terbinafine-resistant Trichophyton rubrum isolates. Med. Mycol. 2004, 42, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Castejón, D.; Fricke, P.; Cambero, M.I. Automatic 1H-NMR Screening of Fatty Acid Composition in Edible Oils. Nutrients 2016, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, O.; Silvestri, C.; Arzeni, D. In vitro activity of the protegrin IB-367 alone and in combination compared with conventional antifungal agents against dermatophytes. Mycoses 2014, 57, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Archer, M.; Martel, P. Crystal structures of the free and sterol-bound forms of beta-cinnamomin. Biochim. Biophys. Acta 2006, 1764, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Behrouz, S.; Rad, M.N.; Rostami, S. Design, synthesis, and biological activities of novel azole-bonded Formula Not Shown -hydroxypropyl oxime O-ethers. Mol. Divers. 2014, 18, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Montanari, S.; Bartolini, M.; Neviani, P. Multitarget Strategy to Address Alzheimer’s Disease: Design, Synthesis, Biological Evaluation, and Computational Studies of Coumarin-Based Derivatives. ChemMedChem 2015, 11, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 2–17 are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).