Exploiting the Electronic Tuneability of Carboranes as Supports for Frustrated Lewis Pairs
Abstract
1. Introduction
2. Results and Discussion
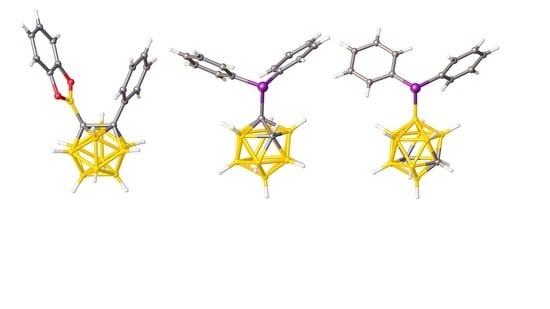
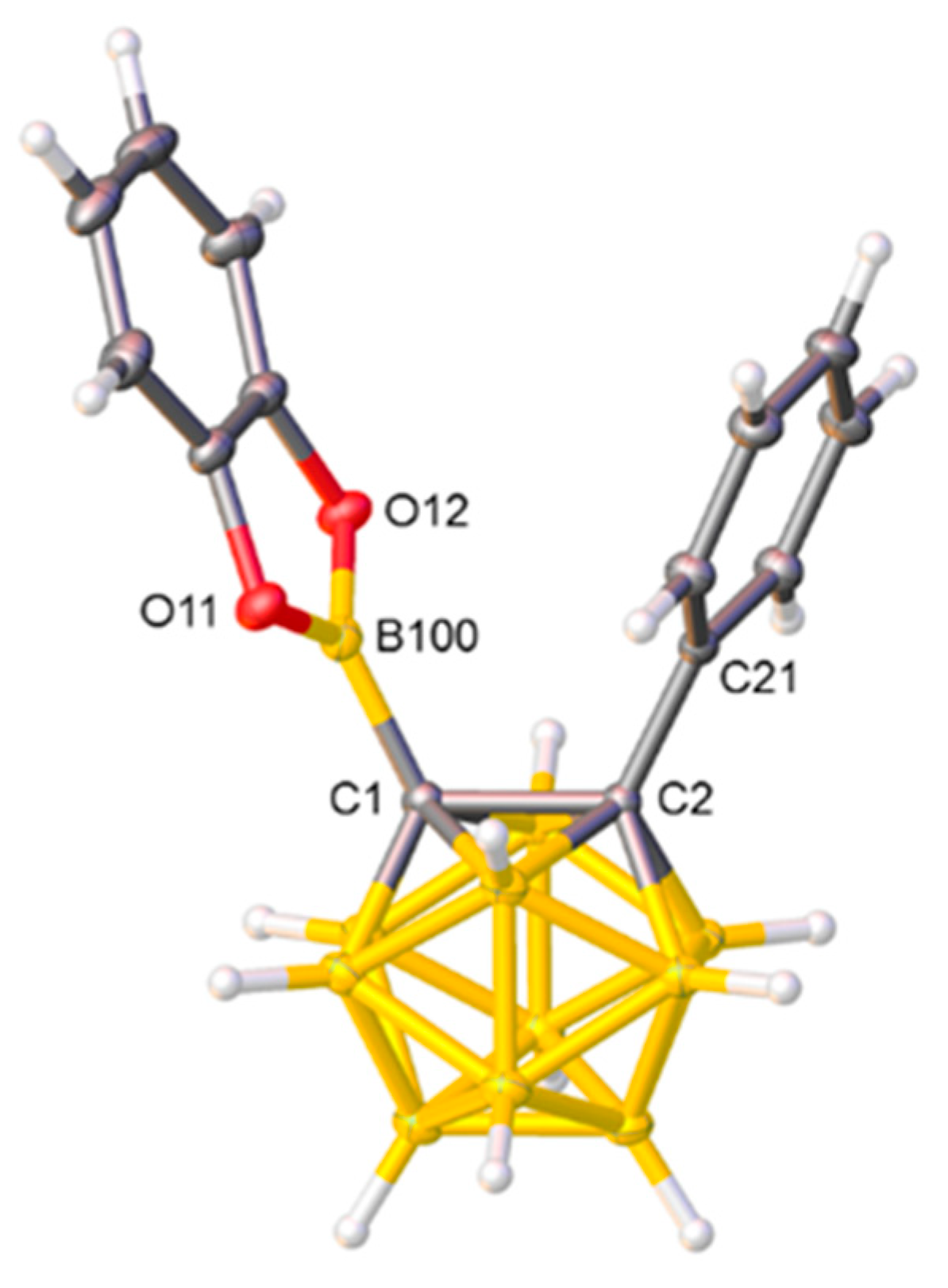
2.1. Synthesis and Characterization of Compound 1
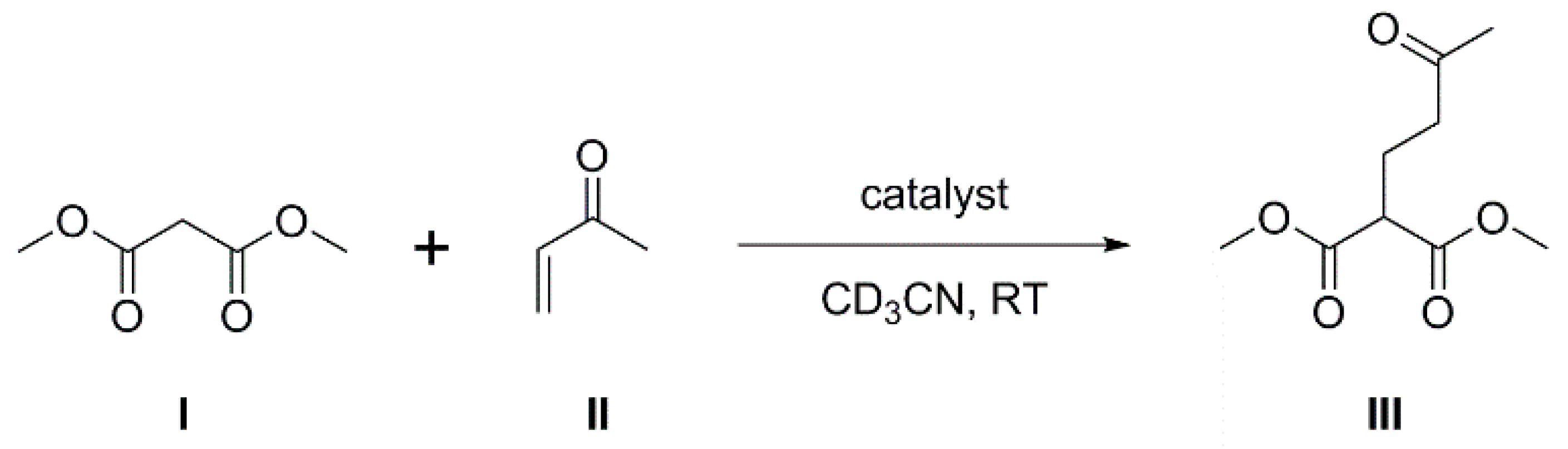
2.2. Carborane-Supported Components of Intermolecular FLPs to Catalyze Michael Addition
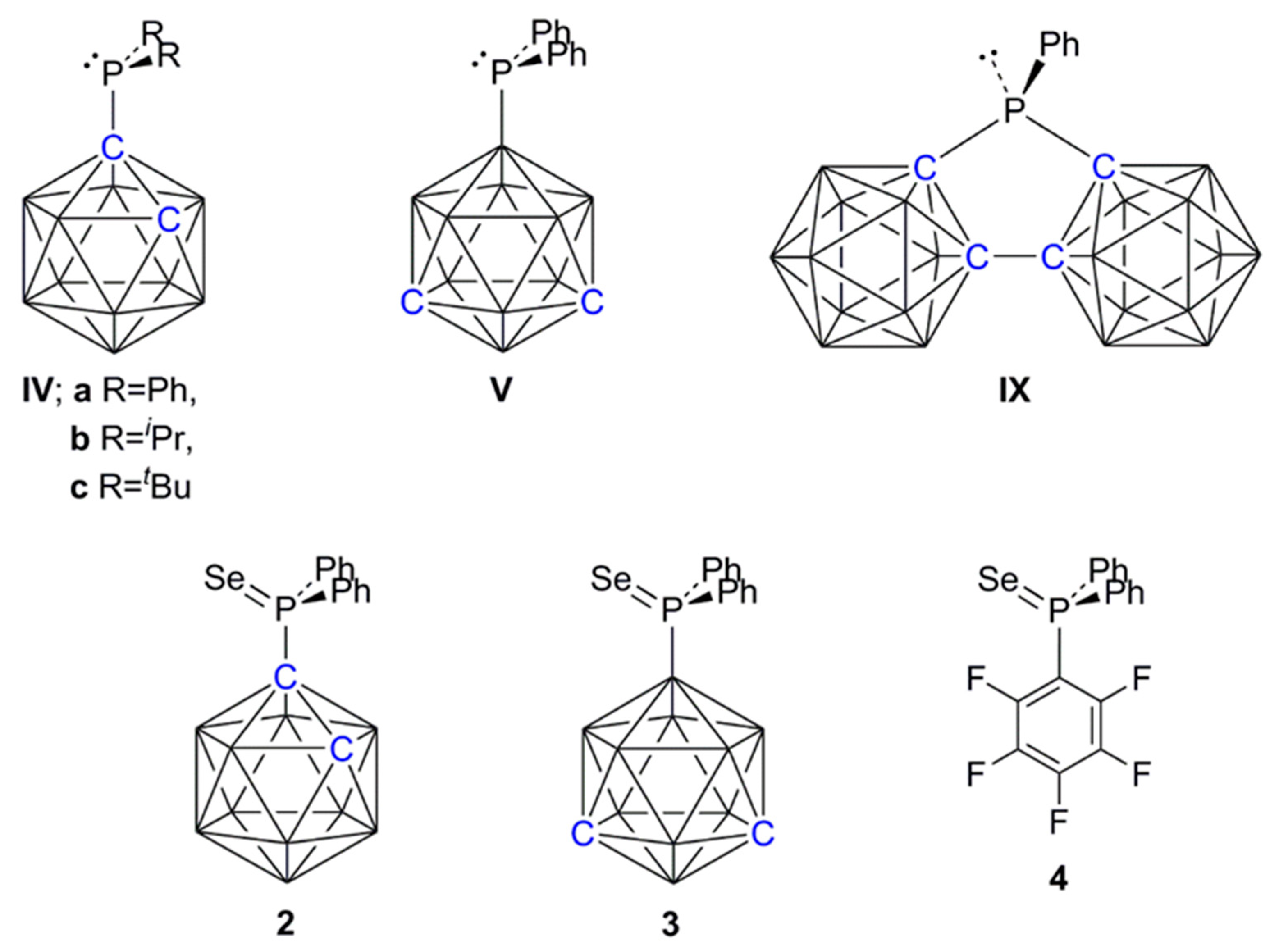
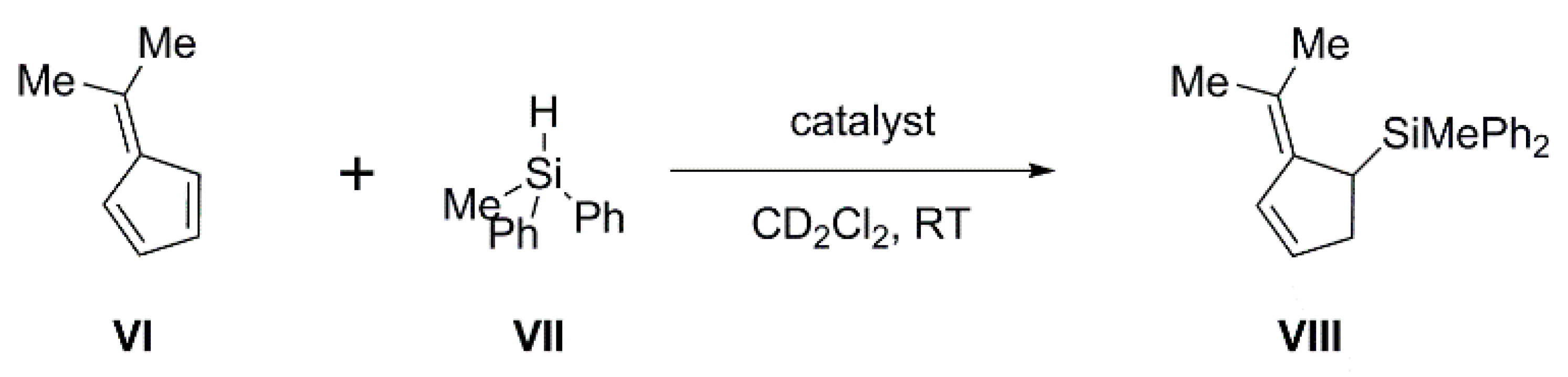
2.3. Carborane-Supported Components of Intermolecular FLPs to Catalyze Hydrosilylation
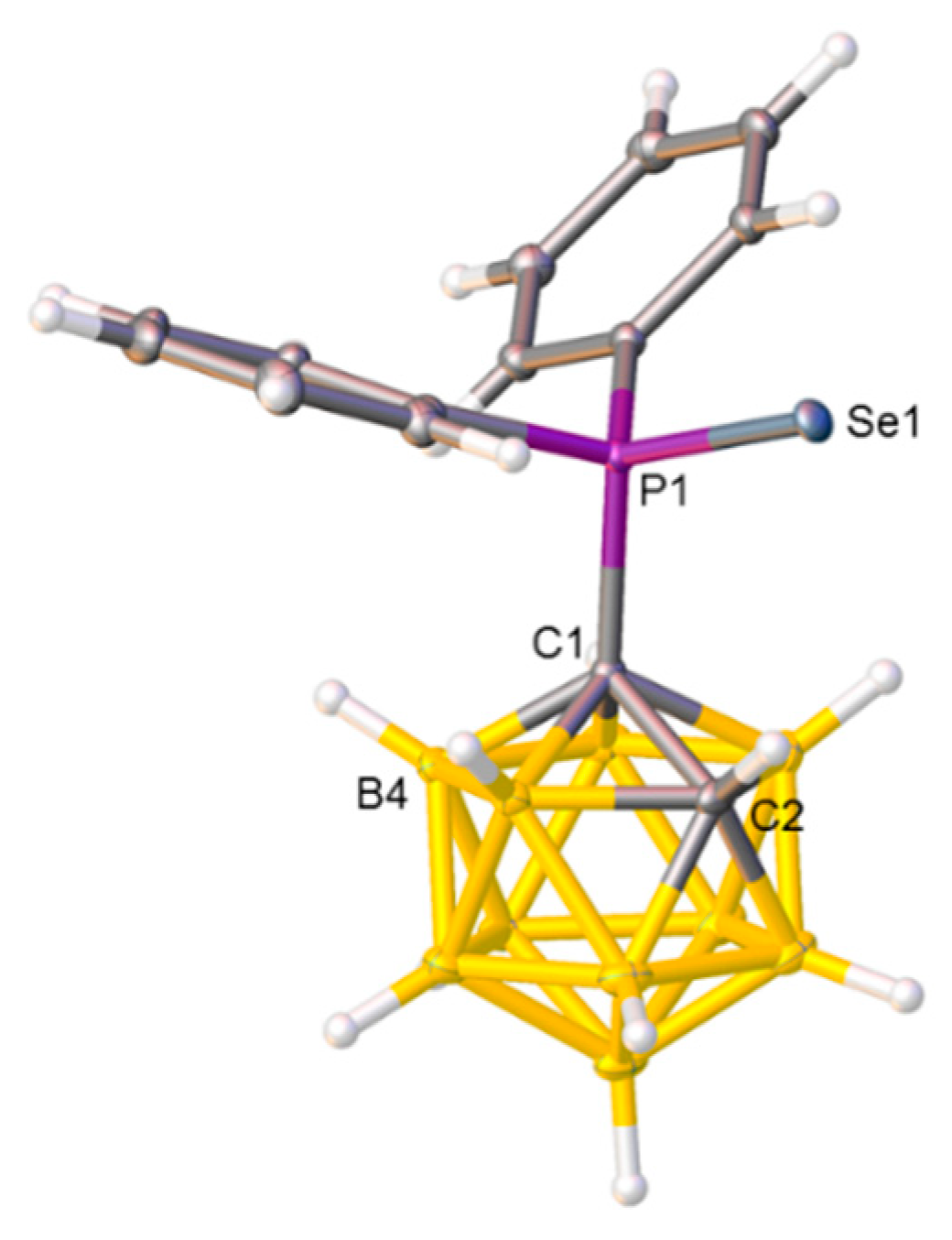
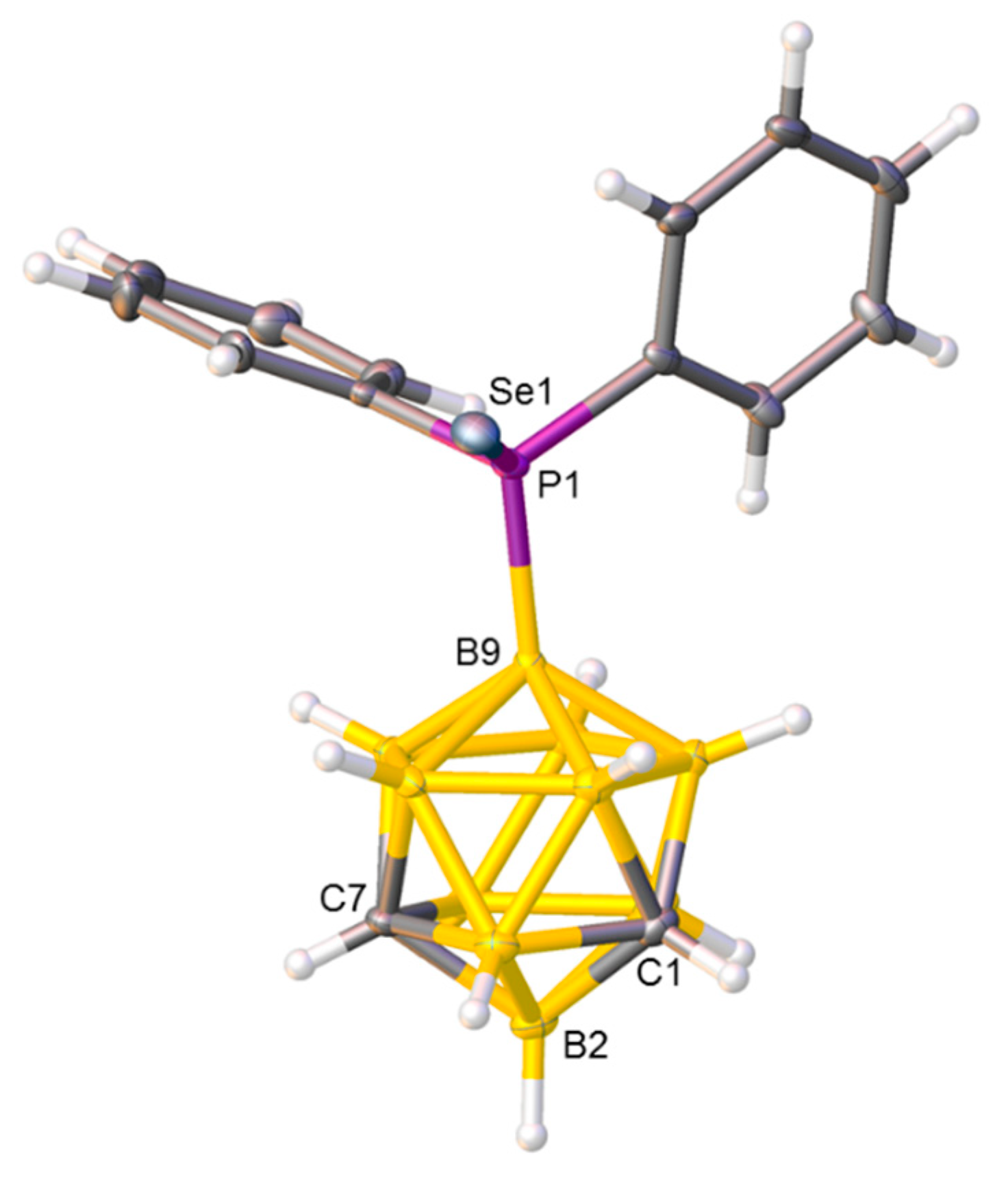
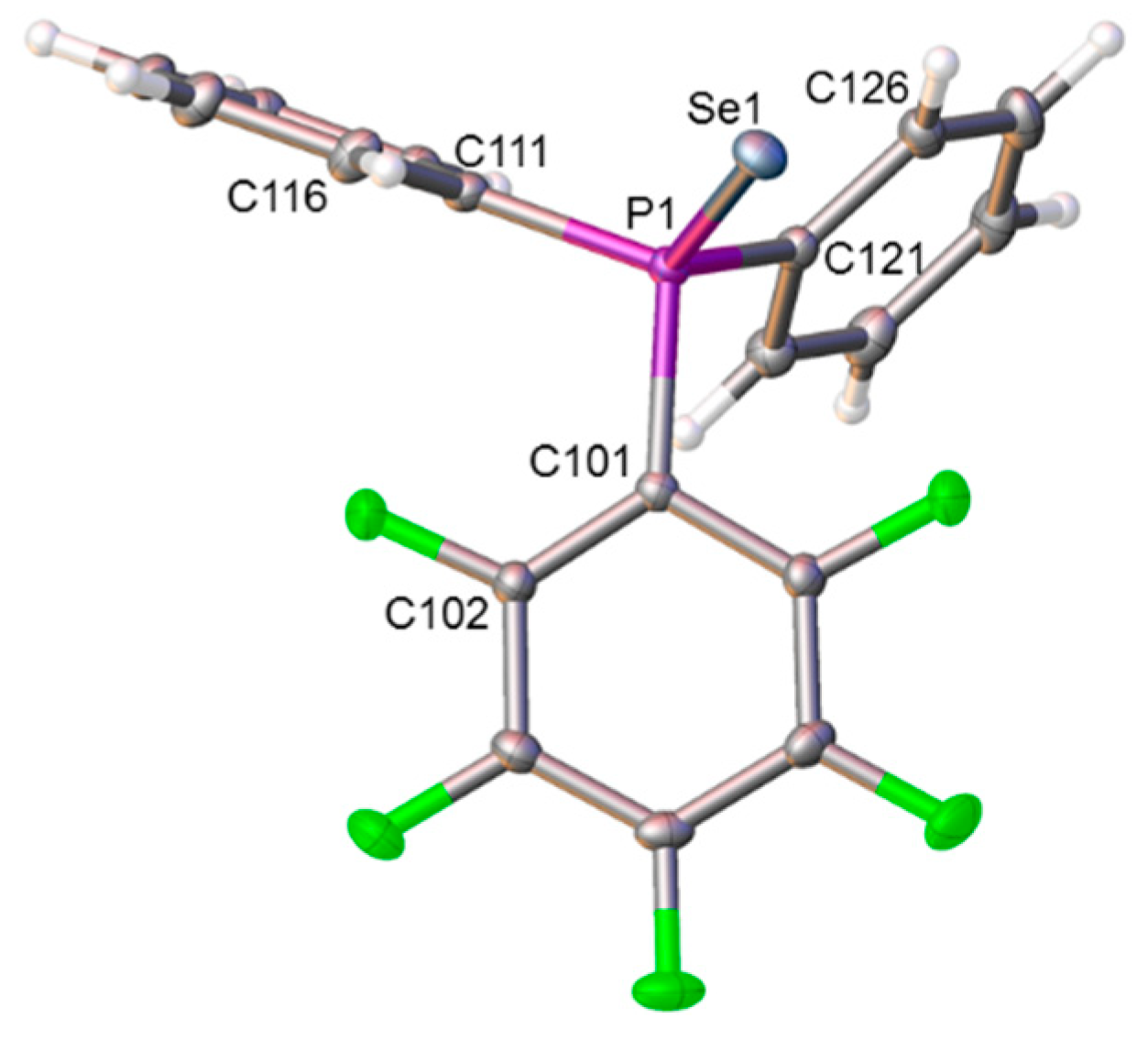
2.4. Synthesis and Characterization of Phosphineselenides 2, 3 and 4
3. Experimental Section
3.1. General Considerations
3.1.1. Synthesis and Characterization of [1-Bcat-2-Ph-closo-1,2-C2B10H10] (1)
3.1.2. General Synthesis and Characterization of Phosphine Selenides 2, 3, and 4
3.2. Crystallographic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Welch, G.C.; San Juan, R.R.; Masuda, J.D.; Stephan, D.W. Reversible, Metal-Free hydrogen activation. Science 2006, 314, 1124–1126. [Google Scholar] [CrossRef]
- Stephan, D.W. “Frustrated Lewis pairs”: a concept for new reactivity and catalysis. Org. Biomol. Chem. 2008, 6, 1535–1539. [Google Scholar] [CrossRef]
- Stephan, D.W. Frustrated Lewis pairs: a new strategy to small molecule activation and hydrogenation catalysis. Dalton Trans. 2009, 3129–3136. [Google Scholar] [CrossRef]
- Stephan, D.W.; Erker, G. Frustrated Lewis pairs: Metal-Free hydrogen activation and more. Angew. Chem. Int. Ed. 2010, 49, 46–76. [Google Scholar] [CrossRef]
- Stephan, D.W. “Frustrated Lewis pair” hydrogenations. Org. Biomol. Chem. 2012, 10, 5740–5746. [Google Scholar] [CrossRef]
- Stephan, D.W.; Erker, G. Frustrated Lewis pair chemistry of carbon, nitrogen and sulfur oxides. Chem. Sci. 2014, 5, 2625–2641. [Google Scholar] [CrossRef]
- Stephan, D.W. Frustrated Lewis pairs from concept to catalysis. Acc. Chem. Res. 2015, 48, 306–316. [Google Scholar] [CrossRef]
- Stephan, D.W. Frustrated Lewis pairs. J. Am. Chem. Soc. 2015, 137, 10018–10032. [Google Scholar] [CrossRef]
- Stephan, D.W. The broadening reach of frustrated Lewis pair chemistry. Science 2016, 354, 1248–1256. [Google Scholar] [CrossRef]
- Grimes, R.N. Carboranes, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Zheng, Z.; Diaz, M.; Knobler, C.B.; Hawthorne, M.F. A mercuracarborand characterized by B-Hg-B bonds: Synthesis and structure of cyclo-[(t-BuMe2Si)2C2B10H8Hg]3. J. Am. Chem. Soc. 1995, 117, 12338–12339. [Google Scholar] [CrossRef]
- Spokoyny, A.M.; Machan, C.W.; Clingerman, D.J.; Rosen, M.S.; Wiester, M.J.; Kennedy, R.D.; Stern, C.L.; Sarjeant, A.A.; Mirkin, C.A. A coordination chemistry dichotomy for icosahedral carborane-based ligands. Nat. Chem. 2011, 3, 590–596. [Google Scholar] [CrossRef]
- Spokoyny, A.M.; Lewis, C.D.; Teverovskiy, G.; Buchwald, S.L. Extremely electron-rich, boron-functionalized, icosahedral carborane-based phosphinoboranes. Organometallics 2012, 31, 8478–8481. [Google Scholar] [CrossRef]
- Janoušek, Z.; Lehmann, U.; Častulik, J.; Cisařová, I.; Michl, J. Li+-Induced σ-Bond metathesis: Aryl for methyl exchange on boron in a methylated monocarbadodecaborate anion. J. Am. Chem. Soc. 2004, 126, 4060–4061. [Google Scholar] [CrossRef]
- Svidlov, S.V.; Voloshin, Y.Z.; Yurgina, N.S.; Potapova, T.V.; Belyy, A.Y.; Ananyev, I.V.; Bubnov, Y.N. Synthesis, structure, and reactivity of C-isopropyl-ortho-carborane organoboron derivatives. Russ. Chem. Bull. Int. Ed. 2014, 63, 2343–2350. [Google Scholar] [CrossRef]
- Cheng, R.; Qiu, Z.; Xie, Z. Iridium-catalysed regioselective borylation of carboranes via direct B-H activation. Nat. Commun. 2017, 8, 14827. [Google Scholar] [CrossRef]
- Weber, L.; Kahlert, J.; Brockhinke, R.; Böhling, L.; Brockhinke, A.; Stammler, H.-G.; Neumann, B.; Harder, R.A.; Fox, M.A. Luminescence properties of C-diazaborolyl-ortho-carboranes as donor–acceptor systems. Chem. Eur. J. 2012, 18, 8347–8357. [Google Scholar] [CrossRef]
- Weber, L.; Kahlert, J.; Böhling, L.; Brockhinke, A.; Stammler, H.-G.; Neumann, B.; Harder, R.A.; Low, P.J.; Fox, M.A. Electrochemical and spectroelectrochemical studies of C-benzodiazaborolyl-ortho-carboranes. Dalton Trans. 2013, 42, 2266–2281. [Google Scholar] [CrossRef]
- Gimbert, C.; Lumbierres, M.; Marchi, C.; Moreno-Mañas, M.; Sebastián, R.M.; Vallribera, A. Michael additions catalyzed by phosphines. An overlooked synthetic method. Tetrahedron 2005, 61, 8598–8605. [Google Scholar] [CrossRef]
- Gómez-Bengoa, E.; Cuerva, J.M.; Mateo, C.; Echavarren, A.M. Michael reaction of stabilized carbon nucleophiles catalyzed by [RuH2(PPh3)4]. J. Am. Chem. Soc. 1996, 118, 8553–8565. [Google Scholar] [CrossRef]
- Saidi, M.R.; Azizi, N.; Akbari, E.; Ebrahimi, F. LiCO4/Et3N: Highly efficient and active catalyst for selective Michael addition of active methylene compounds under solvent-free condition. J. Mol. Cat. A: Chem. 2008, 292, 44–48. [Google Scholar] [CrossRef]
- Baslé, O.; Porcel, S.; Ladeira, S.; Bouhadir, G.; Bourissou, D. Phosphine-boronates: efficient bifunctional organocatalysts for Michael addition. Chem. Commun. 2012, 48, 4495–4497. [Google Scholar] [CrossRef]
- Tamke, S.; Daniliuc, C.-G.; Paradies, J. Frustrated Lewis pair catalyzed hydrosilylation and hydrosilane mediated hydrogenation of fulvenes. Org. Biomol. Chem. 2014, 12, 9139–9144. [Google Scholar] [CrossRef]
- Riley, L.E.; Krämer, T.; McMullin, C.L.; Ellis, D.; Rosair, G.M.; Sivaev, I.B.; Welch, A.J. Large, weakly basic bis(carboranyl)phosphines: an experimental and computational study. Dalton Trans. 2017, 46, 5218–5228. [Google Scholar] [CrossRef]
- Allen, D.W.; Taylor, B.F. The chemistry of heteroarylphosphorus compounds. Part 15. Phosphorus-31 nuclear magnetic resonance studies of the donor properties of heteroarylphosphines towards selenium and platinum(II). J. C. S. Dalton 1982, 51–54. [Google Scholar] [CrossRef]
- Beckmann, U.; Süslüyan, D.; Kunz, P.C. Is the 1JPSe coupling constant a reliable probe for the basicity of phosphines? A 31P NMR study. phosphorus. Sulfur Silicon Relat. Elem. 2011, 186, 2061–2070. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Kivekäs, R.; Teixidor, F.; Viñas, C.; Nuñez, R. 1-Diphenylphosphino-1,2-dicarba-closo-dodecaborane(12) at 153 K. Acta Cryst. 1995, C51, 1868–1870. [Google Scholar]
- Nuñez, R.; Viñas, C.; Teixidor, F.; Sillanpää, R.; Kivekäs, R. Contribution of the o-carboranyl fragment to the chemical stability and the 31P-NMR chemical shift in closo-carboranylphosphines. Crystal structure of bis(1-yl-2-methyl-1,2-dicarba-closo-dodecaborane)phenylphosphine. J. Organometal. Chem. 1999, 592, 22–28. [Google Scholar] [CrossRef]
- Fey, N.; Haddow, M.F.; Mistry, R.; Norman, N.C.; Orpen, A.G.; Reynolds, T.J.; Pringle, P.G. Regioselective B-Cyclometalation of a bulky o-carboranyl phosphine and the unexpected formation of a dirhodium(ii) complex. Organometallics 2012, 31, 2907–2913. [Google Scholar] [CrossRef]
- Fein, M.M.; Grafstein, D.; Paustian, J.E.; Bobinski, J.; Lichstein, B.M.; Mayes, N.; Schwartz, N.N.; Cohen, M.S. Carboranes. II. The preparation of 1- and 1,2-substituted carboranes. Inorg. Chem. 1963, 2, 1115–1119. [Google Scholar] [CrossRef]
- Brain, P.T.; Cowie, J.; Donohue, D.J.; Hnyk, D.; Rankin, D.W.H.; Reed, D.; Reid, B.D.; Robertson, H.E.; Welch, A.J.; Hofmann, M.; et al. 1-Phenyl-1, 2-dicarba-closo-dodecaborane, 1-Ph-1, 2-closo-C2B10H11. Synthesis, characterization, and structure as determined in the gas phase by electron diffraction, in the crystalline phase at 199 K by X-ray diffraction, and by ab initio computations. Inorg. Chem. 1996, 35, 1701–1708. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- McAnaw, A.; Scott, G.; Elrick, L.; Rosair, G.M.; Welch, A.J. The VCD method‚A simple and reliable way to distinguish cage C and B atoms in (hetero)carborane structures determined crystallographically. Dalton Trans. 2013, 42, 645–664. [Google Scholar] [CrossRef]
- McAnaw, A.; Lopez, M.E.; Ellis, D.; Rosair, G.M.; Welch, A.J. Asymmetric 1,8/13,2,x-M2C2B10 14-vertex metallacarboranes by direct electrophilic insertion reactions; the VCD and BHD methods in critical analysis of cage C atom positions. Dalton Trans. 2014, 43, 5095–5105. [Google Scholar] [CrossRef]
- Welch, A.J. What can we learn from the crystal structures of metallacarboranes? Crystals 2017, 7, 234. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds not available. |
| Entry | Catalyst(s) | Time (h) | Yield (%) 2 |
|---|---|---|---|
| 1 | PPh3 | 6 | 43 |
| 2 | PPh3 | 24 | 64 |
| 3 | 1/PPh33 | 6 | 56 |
| 4 | 1/PPh3 | 24 | 76 |
| 5 | V | 6 | 85 |
| 6 | V | 24 | 92 |
| Entry | Catalyst(s) | Time (min) | Yield (%) 2 |
|---|---|---|---|
| 1 | B(C6F5)3/PPh2(C6F5) | 11 | 89 |
| 2 | B(C6F5)3/IVa 3 | 12 | 88 |
| 3 | B(C6F5)3/V 3 | 26 | 80 |
| 4 | B(C6F5)3/IX 3 | - | 0 4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benton, A.; Copeland, Z.; M. Mansell, S.; M. Rosair, G.; Welch, A.J. Exploiting the Electronic Tuneability of Carboranes as Supports for Frustrated Lewis Pairs. Molecules 2018, 23, 3099. https://doi.org/10.3390/molecules23123099
Benton A, Copeland Z, M. Mansell S, M. Rosair G, Welch AJ. Exploiting the Electronic Tuneability of Carboranes as Supports for Frustrated Lewis Pairs. Molecules. 2018; 23(12):3099. https://doi.org/10.3390/molecules23123099
Chicago/Turabian StyleBenton, Amanda, Zachariah Copeland, Stephen M. Mansell, Georgina M. Rosair, and Alan J. Welch. 2018. "Exploiting the Electronic Tuneability of Carboranes as Supports for Frustrated Lewis Pairs" Molecules 23, no. 12: 3099. https://doi.org/10.3390/molecules23123099
APA StyleBenton, A., Copeland, Z., M. Mansell, S., M. Rosair, G., & Welch, A. J. (2018). Exploiting the Electronic Tuneability of Carboranes as Supports for Frustrated Lewis Pairs. Molecules, 23(12), 3099. https://doi.org/10.3390/molecules23123099