Identification of Ubiquinones in Honey: A New View on Their Potential Contribution to Honey’s Antioxidant State
Abstract
1. Introduction
2. Results and Discussion
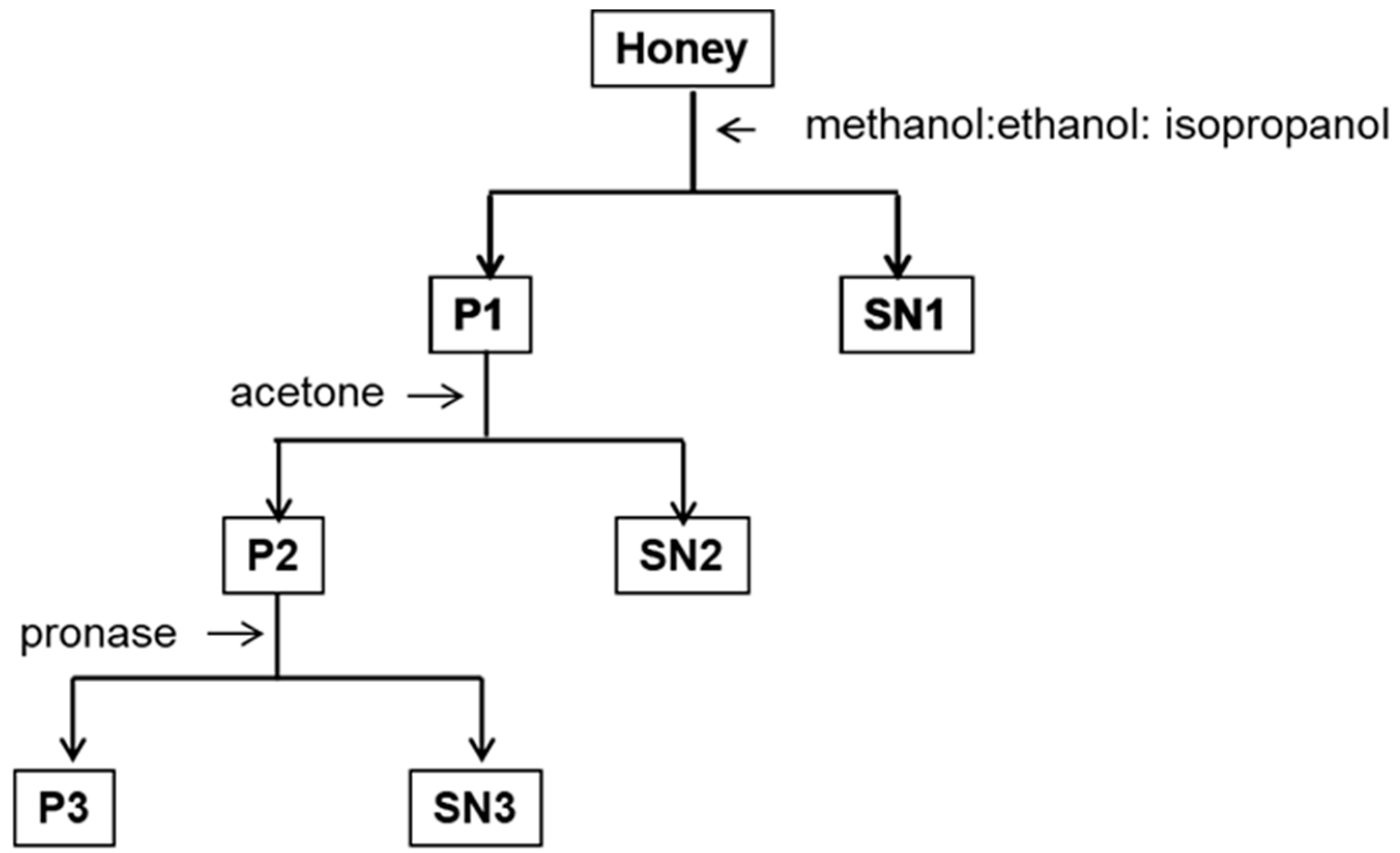
2.1. Extraction and Separation of Ubiquinone-Like Compounds
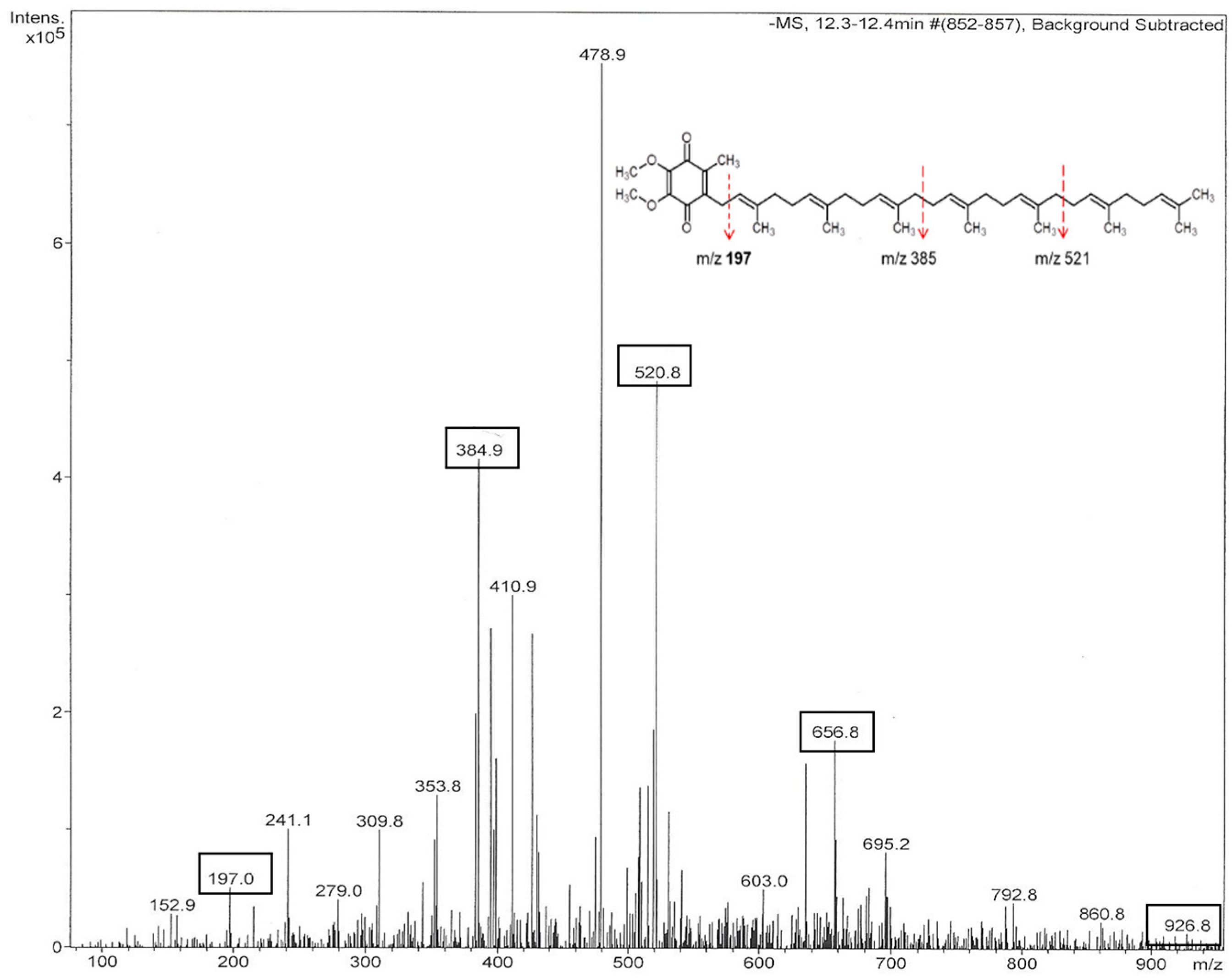
2.2. UHPLC-ESI-MS/MS Analysis and Identification of Ubiquinones
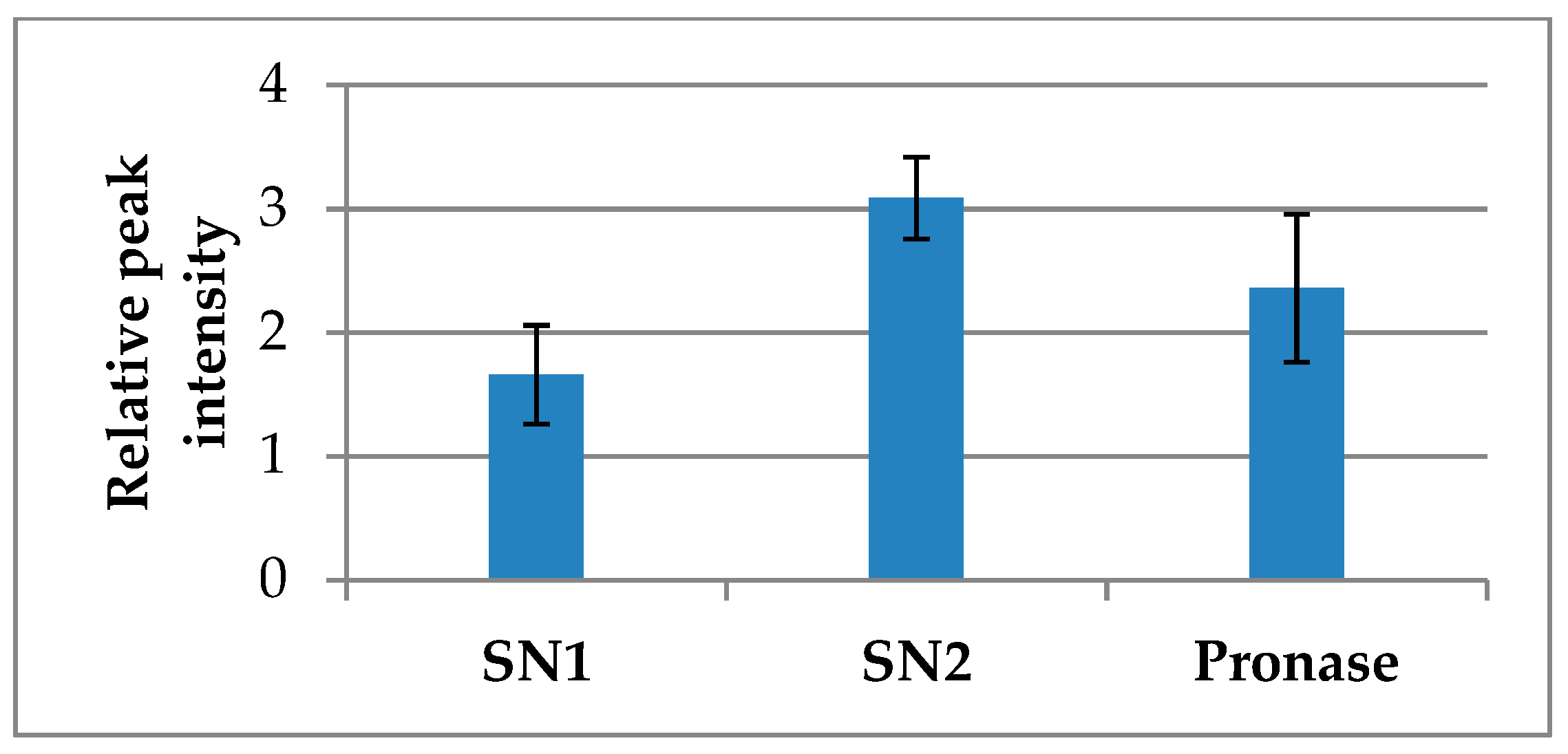
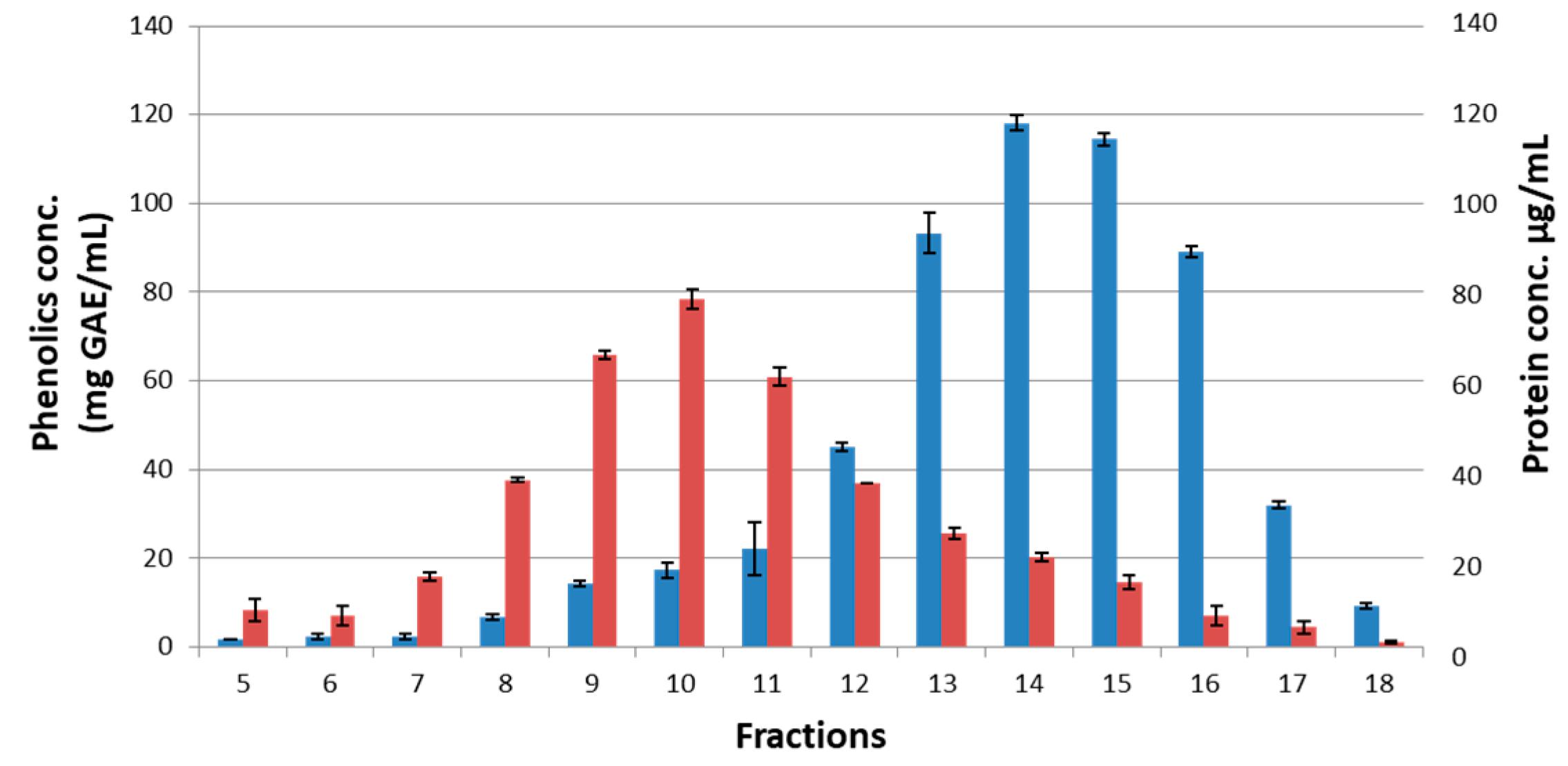
2.3. Partitioning of Ubiquinones between Free and Protein-Bound Fractions
2.4. Extraction of Ubiquinones from Polyphenol-Protein Complexes
3. Materials and Methods
3.1. Honeys
3.2. Sample Preparation
3.3. Extraction Procedures
3.4. Solid Phase Extraction (SPE)
3.5. LC-ESI-MS Analysis
3.6. Ultra-Performance Liquid Chromatography (UPLC)
3.7. The Exact Mass Measurement
3.8. Size-Exclusion Chromatography (SEC)
3.9. Determination of Protein Concentration
3.10. Determination of Polyphenol Concentration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paine, H.S.; Gertler, S.; Lothrop, R.E. The colloidal constituents of honey. Industr. Engng. Chem. 1934, 26, 73–81. [Google Scholar] [CrossRef]
- Brudzynski, K.; Maldonado-Alvarez, L. Polyphenol-protein complexes and their consequences for the redox activity, structure and function of honey. A current view and new hypothesis—A review. Pol. J. Food Nutr. Sci. 2015, 65, 71–80. [Google Scholar] [CrossRef]
- Brudzynski, K.; Miotto, D.; Kim, L.; Sjaarda, C.; Maldonado-Alvarez, L.; Fukś, H. Active macromolecules of honey form colloidal particles essential for honey antibacterial activity and hydrogen peroxide production. Sci. Rep. 2017, 7, 7637. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A.J.; Baxter, N.J.; Lokman Khan, M.; Moir, A.J.G.; Haslam, E.; Davies, A.P.; Williamson, M.P. Polyphenol/peptide binding and precipitation. J. Agric. Food Chem. 2002, 50, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Siebert, K.J.; Troukhanova, N.V.; Lynn, P.Y. Nature of polyphenols-protein interactions. J. Agric. Food Chem. 1996, 44, 80–85. [Google Scholar] [CrossRef]
- Haslam, E. Polyphenol-protein interactions. Biochem. J. 1974, 139, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Renard, C.M. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K.; Biesaga, M. Analysis of phenolic acids and flavonoids in honey. Trends Analyt Chem. 2009, 28, 893–902. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Jones, D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implications. Microbiol. Rev. 1981, 45, 316–354. [Google Scholar] [PubMed]
- Brudzynski, K.; Sjaarda, C.; Maldonado-Alvarez, L. A new look on polyphenol-protein complexation during honey storage: Is this a random or organized event with the help of dirigent-like proteins? PLoS ONE 2013, 8, 72897. [Google Scholar] [CrossRef] [PubMed]
- Paz, M.A.; Flückiger, R.; Boak, A.; Kagan, H.M.; Gallop, P.M. Specific detection of quinoproteins by redox-cycling staining. J. Biol. Chem. 1991, 266, 689–692. [Google Scholar] [PubMed]
- Trumpower, B.L. New concepts on the role of ubiquinones in the mitochondrial respiratory chain. J. Bioenerg. Biomembr. 1981, 13, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, B.; Kruk, J. Occurance, biosynthesis and function of isoprenoid quinones. Biochem. Biophys. Acta 2010, 1797, 1587–1605. [Google Scholar] [PubMed]
- Brudzynski, K.; Miotto, D. The recognition of high molecular weight melanoidins as the main components responsible for radical-scavenging capacity of unheated and heat-treated Canadian honeys. Food Chem. 2011, 125, 570–575. [Google Scholar] [CrossRef]
- Maroz, A.; Anderson, R.F.; Smith, R.A.; Murphy, M.P. Reactivity of ubiquinone and ubiquinol with superoxide and the hydroperoxyl radical: Implications for in vivo antioxidant activity. Free Radic. Biol. Med. 2009, 46, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Marbois, B.N.; Clarke, C.F. The COQ7 Gene encodes a protein in Saccharomyces cerevisiae necessary for ubiquinone biosynthesis. J. Biol. Chem. 1996, 271, 2995–3004. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S. Harmonized Methods of the International Honey Commission; Swiss Bee Research Centre, FAM: Liebefeld, Switzerland, 2002. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
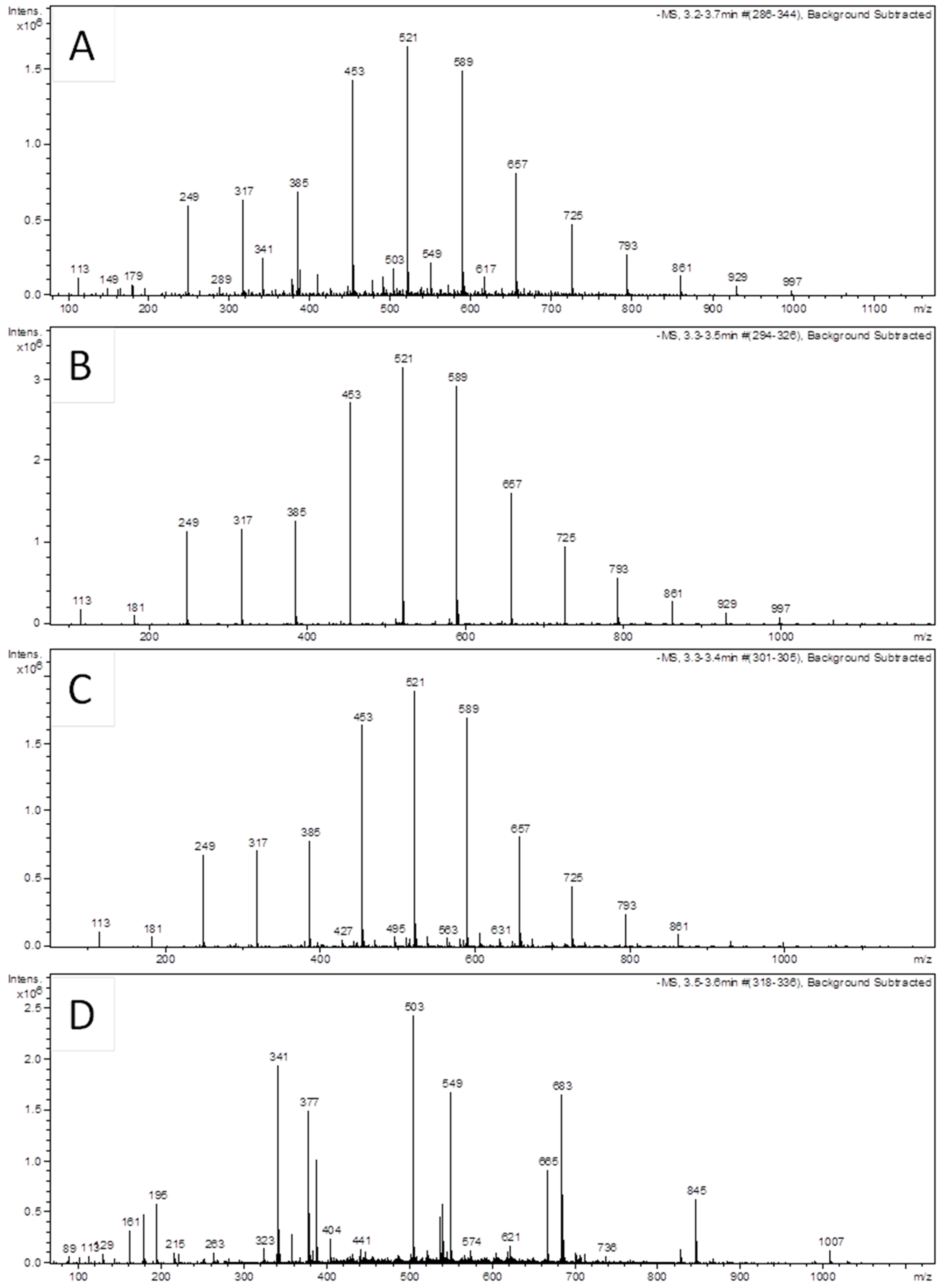
| Compound | Mol. Mass | Detected Mass [M − H]− (m/z) | Theoretical Mass (Da) | Formula | Accurate Mass [M + H]+ (m/z) | ||
|---|---|---|---|---|---|---|---|
| SN1 | SN2 | SN3 | |||||
| UQ-12 | 999.603 | 997 | 997 | - | 998.809 | C69H106O4 | |
| UQ-11 | 931.484 | 929 | 929 | - | 930.747 | C64H98O4 | |
| UQ-10 | 863.34 | 861 | 861 | 861 | 862.6839 | C59H90O4 | |
| UQ-9 | 794.6 | 793 | 793 | 793 | 794.621 | C54H82O4 | 794.5476 |
| UQ-8 | 726.6 | 725 | 725 | 725 | 727.56599 | C49H75O4 | |
| UQ-7 | 658.5 | 657 | 657 | 657 | 658.496 | C44H66O4 | 659.5829 |
| UQ-6 | 590.899 | 589 | 589 | 589 | 590.432022 | C39H58O4 | 591.4408 |
| UQ-5 | 522.758 | 521 | 521 | 521 | 522.370911 | C34H50O4 | 523.3782 |
| UQ-4 | 454.308 | 453 | 453 | 453 | 454.308 | C29H42O4 | |
| UQ-3 | 386.532 | 385 | 385 | 385 | 386.246 | C24H34O4 | 386.246 |
| UQ-2 | 318.413 | 317 | 317 | 317 | 318.183 | C19H26O4 | |
| UQ-1 | 250.29 | 249 | 249 | 249 | 250.121 | C14H18O4 | |
| UQ-0 | 182.17 | - | 181 | 181 | 182.05790 | C9H10O4 | |
| UQ Type | Detected [M − H]−, m/z | Diagnostic Fragment | ||||||
|---|---|---|---|---|---|---|---|---|
| H208 F15 | H208 F16 | H220 F16 | H220 F17 | H177 H15 | H177 F16 | H229 F15 | m/z | |
| UQ-3 | 384.9 | 384.9 | 384.9 | 384.9 | 385 | 385 | 384.9 | 197.1 |
| UQ-5 | 520.8 | 520.8 | - | - | 521 | 521 | 520.9 | 197.1 |
| UQ-7 | 656.8 | 656.8 | 656.8 | 656.8 | 657 | 657 | 656.8 | 197.1 |
| UQ-11 | 928.8 | - | - | - | 929 | 861 | 928.9 | 197.1 |
| Honey | Color | Source | Water Activity | Refractive Index | Moisture (%) | Brix | Specific Gravity |
|---|---|---|---|---|---|---|---|
| H208 | Medium | Buckwheat/golden rod | 0.580 | 1.4900 | 18.6 | 79.8 | 1.4129 |
| H177 | Dark | buckwheat | 0.572 | 1.4964 | 16.2 | 82.3 | 1.4295 |
| H220 | Dark | buckwheat | 0.601 | 1.4988 | 15.8 | 83.4 | 1.4370 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brudzynski, K.; Maldonado-Alvarez, L. Identification of Ubiquinones in Honey: A New View on Their Potential Contribution to Honey’s Antioxidant State. Molecules 2018, 23, 3067. https://doi.org/10.3390/molecules23123067
Brudzynski K, Maldonado-Alvarez L. Identification of Ubiquinones in Honey: A New View on Their Potential Contribution to Honey’s Antioxidant State. Molecules. 2018; 23(12):3067. https://doi.org/10.3390/molecules23123067
Chicago/Turabian StyleBrudzynski, Katrina, and Liset Maldonado-Alvarez. 2018. "Identification of Ubiquinones in Honey: A New View on Their Potential Contribution to Honey’s Antioxidant State" Molecules 23, no. 12: 3067. https://doi.org/10.3390/molecules23123067
APA StyleBrudzynski, K., & Maldonado-Alvarez, L. (2018). Identification of Ubiquinones in Honey: A New View on Their Potential Contribution to Honey’s Antioxidant State. Molecules, 23(12), 3067. https://doi.org/10.3390/molecules23123067




