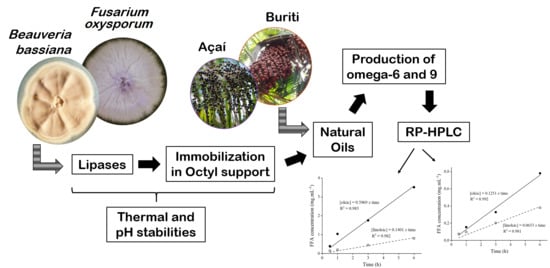
Production of Omegas-6 and 9 from the Hydrolysis of Açaí and Buriti Oils by Lipase Immobilized on a Hydrophobic Support
Abstract
1. Introduction
2. Results and Discussion
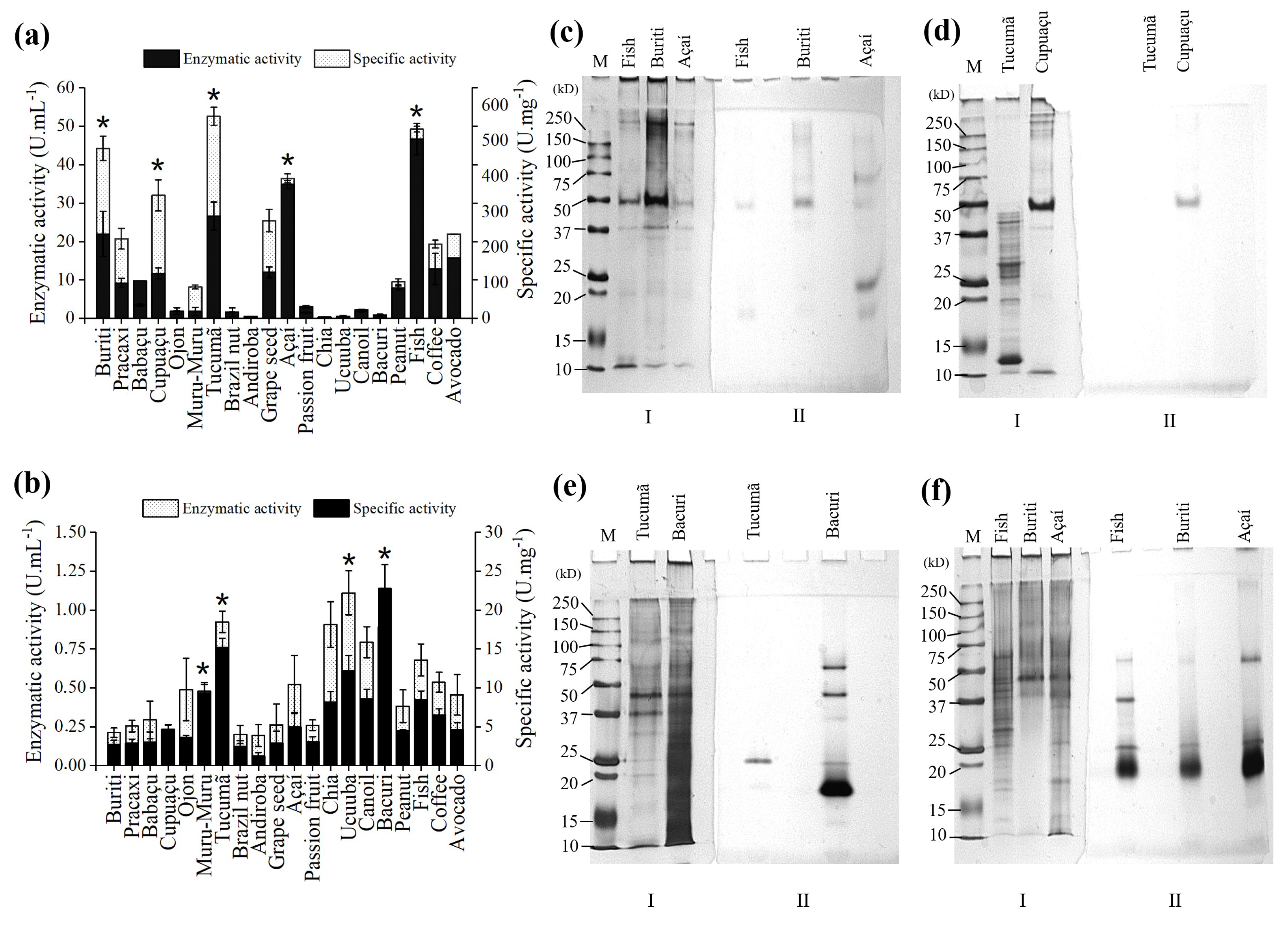
2.1. Selection of Carbon Source for Lipase Production
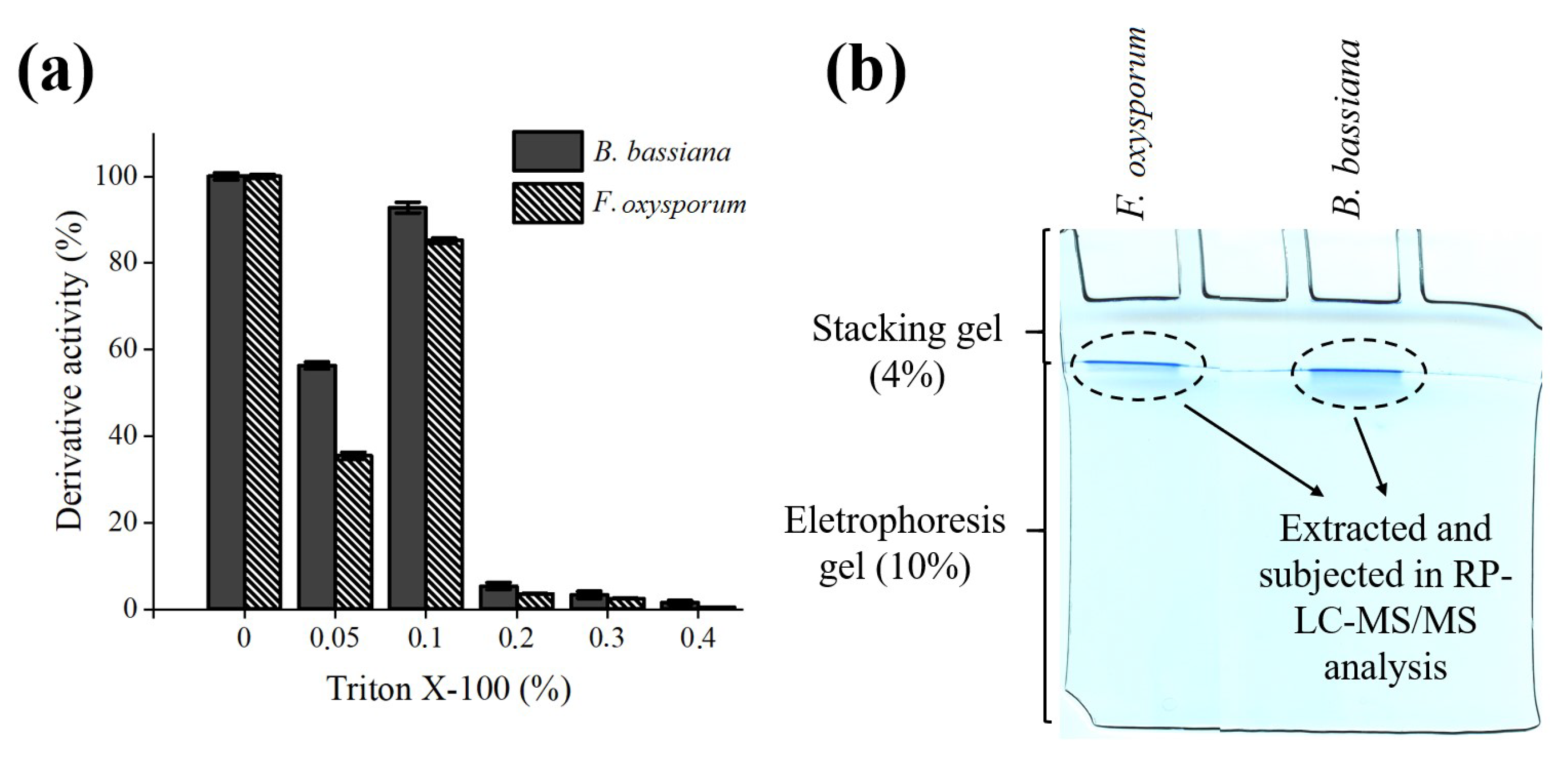
2.2. Immobilization of B. bassiana and F. oxysporum Lipase on Octyl-Sepharose
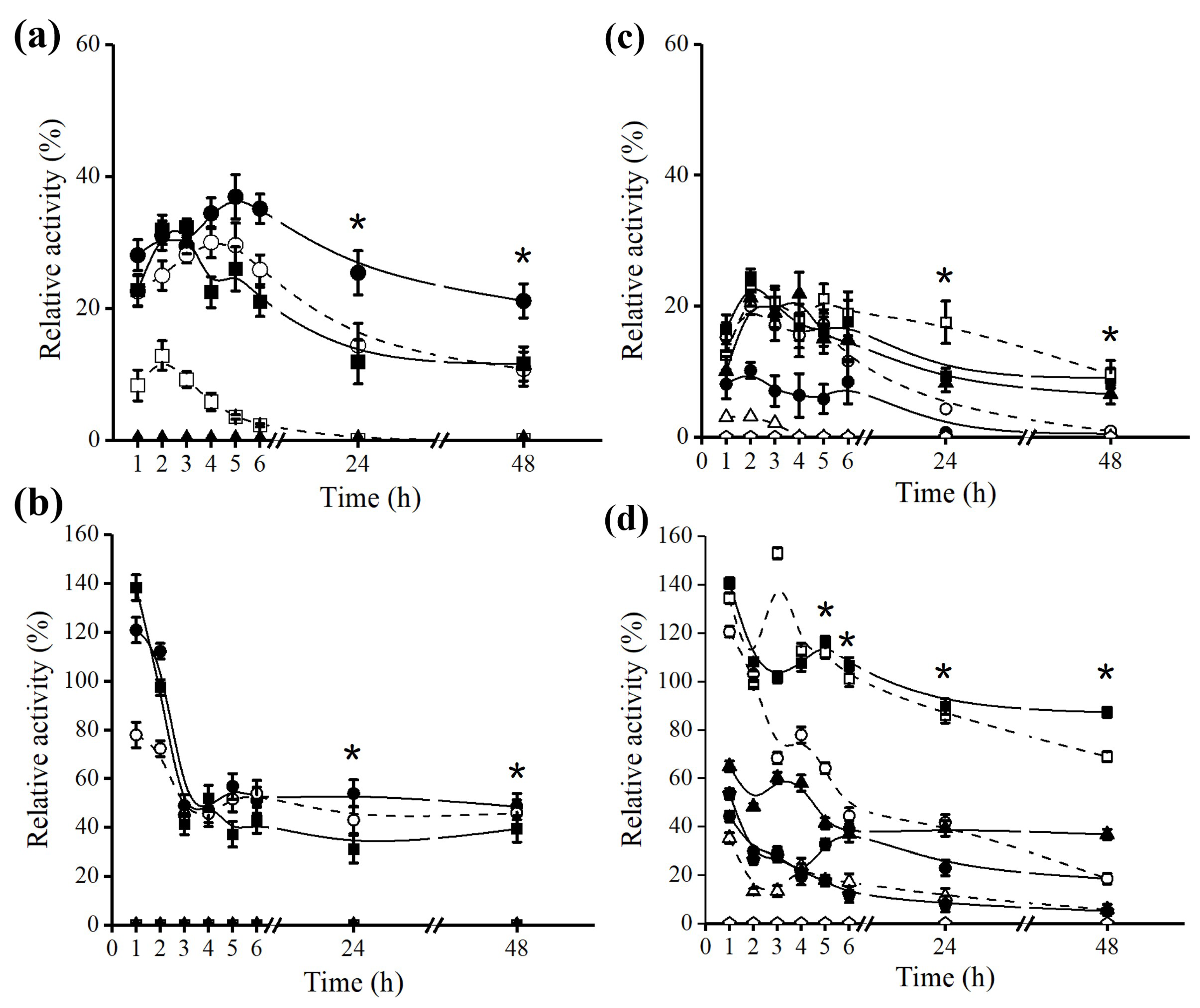
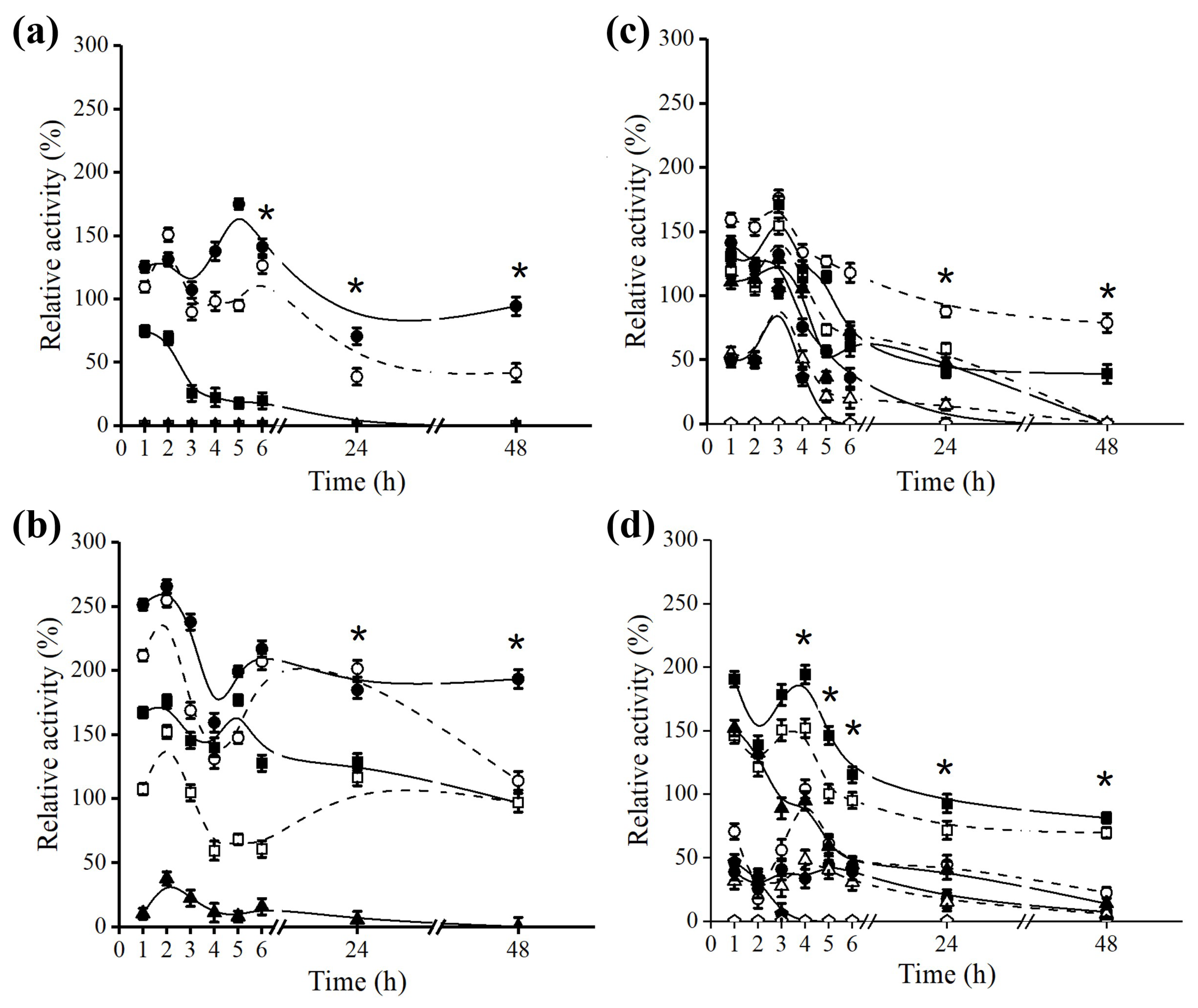
2.3. Effect of Octyl Immobilization on B. bassiana Lipase Stability
2.4. Effect of Octyl Immobilization on F. oxysporum Lipase Stability
2.5. Desorption and Identification of Lipases
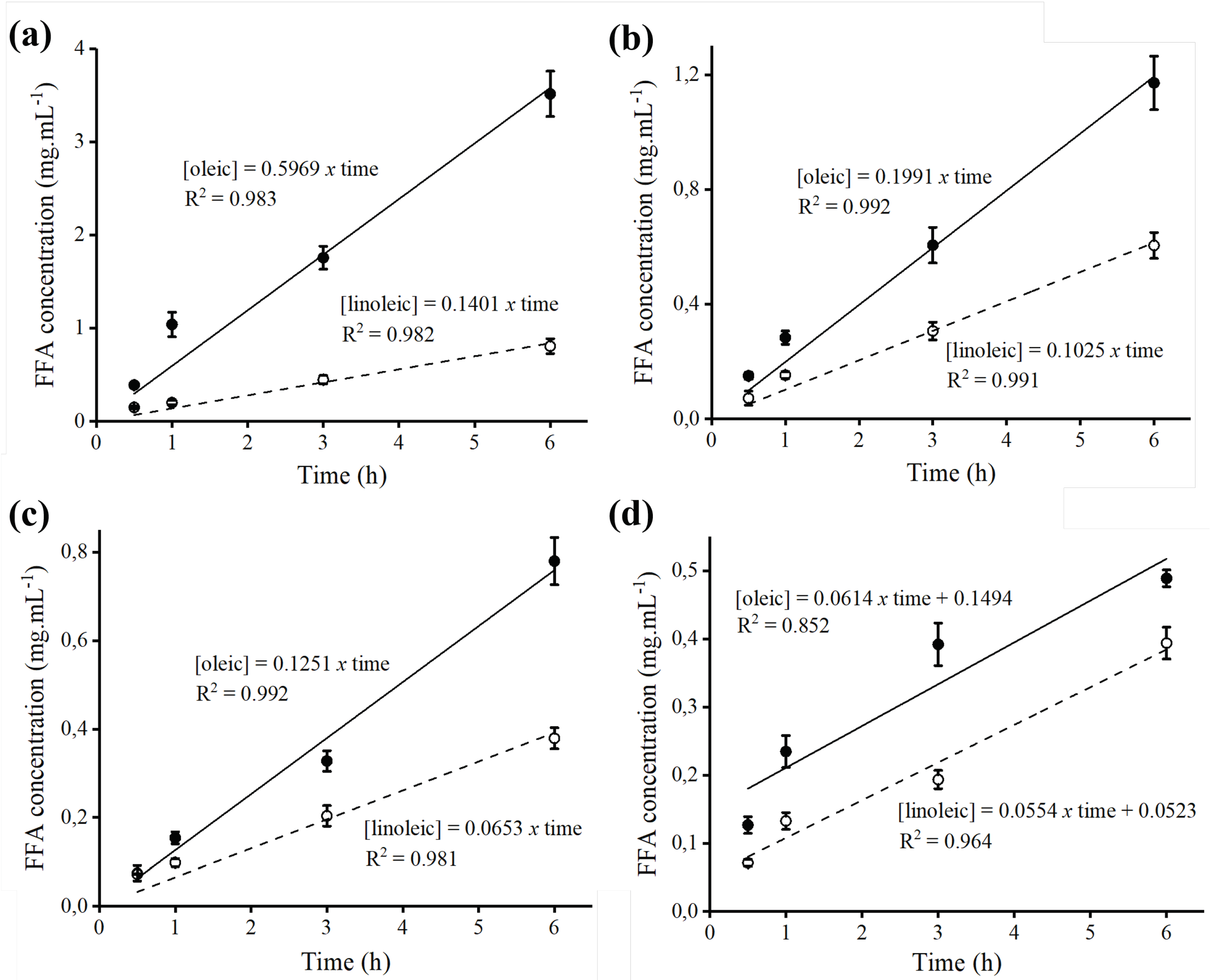
2.6. Hydrolysis of Açaí and Buriti Oils by Lipase Immobilized on Octyl-Sepharose
3. Materials and Methods
3.1. Microorganisms and Oils
3.2. Carbon Source Selection and Lipase Production
3.3. Enzyme Activity Assay
3.4. Protein Measurement
3.5. Protein Electrophoresis in Polyacrylamide Gel under Semi-Denaturing Conditions
3.6. Zymogram
3.7. Immobilization of Lipase on Octyl-Sepharose Support and Coating with Polyethyleneimine (PEI)
3.8. Thermal Inactivation and pH Stability of the Crude Extract and Derivative
3.9. Desorption of Lipase from Octyl-Sepharose Support
3.10. Reverse Phase-Liquid Chromatography RP-LC-MS/MS Analysis
3.11. Hydrolysis of Açaí and Buriti Oils
3.12. Analysis of Free Fatty Acids (Omega-6 and 9) by HPLC-UV
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zielińska, A.; Nowak, I. Fatty Acids in Vegetable Oils and Their Importance in Cosmetic Industry. Chemik 2014, 68, 103–110. [Google Scholar]
- Bhardwaj, K.; Verma, N.; Trivedi, R.K.; Bhardwaj, S.; Shukla, N. Significance of Ratio of Omega-3 and Omega-6 in Human Health with Special Reference to Flaxseed Oil. Int. J. Biol. Chem. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Omega-3, 6, and 9 and How They Add Up. Available online: https://www.uccs.edu/healthcircle/sites/healthcircle/files/inline-files/Omega-3_6_and_9_Fats.pdf (accessed on 5 October 2018).
- Asif, M. Health Effects of Omega-3,6,9 Fatty Acids: Perilla frutescens is a Good Example of Plant Oils. Orient. Pharm. Exp. Med. 2011, 11, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. J. Nutr. Metab. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Huang, Y.-S. Gamma Linolenic Acid: An Antiinflammatory Omega-6 Fatty Acid. Curr. Pharm. Biotechnol. 2006, 7, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E. Do Omega-3/6 Fatty Acids Have a Therapeutic Role in Children and Young People with ADHD? J. Lipids 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, G.; Sahin, H.; Inanc, N.; Uyanik, F.; Ok, E. Impact of Omega-3 and Omega-9 Fatty Acids Enriched Total Parenteral Nutrition on Blood Chemistry and Inflammatory Markers in Septic Patients. Pakistan J. Med. Sci. 2014, 30, 299–304. [Google Scholar] [CrossRef]
- Johnson, M.; Bradford, C. Omega-3, Omega-6 and Omega-9 Fatty Acids: Implications for Cardiovascular and Other Diseases. J. Glycomics Lipidomics 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Kien, C.L.; Bunn, J.Y.; Tompkins, C.L.; Dumas, J.A.; Crain, K.I.; Ebenstein, D.B.; Koves, T.R.; Muoio, D.M. Substituting Dietary Monounsaturated Fat for Saturated Fat is Associated with Increased Daily Physical Activity and Resting Energy Expenditure and with Changes in Mood. Am. J. Clin. Nutr. 2013, 97, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Ko, H.J.; Jeon, S.J.; Lee, S.; Lee, H.E.; Kim, H.N.; Woo, E.R.; Ryu, J.H. The Memory-Enhancing Effect of Erucic Acid on Scopolamine-Induced Cognitive Impairment in Mice. Pharmacol. Biochem. Behav. 2016, 142, 85–90. [Google Scholar] [CrossRef] [PubMed]
- De Rosso, V.V.; Mercadante, A.Z. Identification and Quantification of Carotenoids, by HPLC-PDA-MS/MS, from Amazonian Fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef] [PubMed]
- Rufino, M.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive Compounds and Antioxidant Capacities of 18 Non-Traditional Tropical Fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Pereira Lima, R.; Souza da Luz, P.T.; Braga, M.; dos Santos Batista, P.R.; Ferreira da Costa, C.E.; Zamian, J.R.; Santos do Nascimento, L.A.; da Rocha Filho, G.N. Murumuru (Astrocaryum murumuru Mart.) Butter and Oils of Buriti (Mauritia flexuosa Mart.) and Pracaxi (Pentaclethra macroloba (Willd.) Kuntze) Can be Used for Biodiesel Production: Physico-chemical Properties and Thermal and Kinetic Studies. Ind. Crops Prod. 2017, 97, 536–544. [Google Scholar] [CrossRef]
- Albuquerque, M.L.S.; Guedes, I.; Alcantara, P.; Moreira, S.G.C.; Barbosa Neto, N.M.; Correa, D.S.; Zilio, S.C. Characterization of Buriti (Mauritia flexuosa L.) Oil by Absorption and Emission Spectroscopies. J. Braz. Chem. Soc. 2005, 16, 1113–1117. [Google Scholar] [CrossRef]
- Nascimento, R.J.S.; Couri, S.; Antoniassi, R.; Freitas, S.P. Composição em Ácidos Graxos do Óleo da Polpa de Açaí Extraído com Enzimas e com Hexano. Rev. Bras. Frutic. 2008, 30, 498–502. [Google Scholar] [CrossRef]
- Darnet, S.H.; Silva, L.H.M.; Rodrigues, A.M.C.; Lins, R.T. Nutritional Composition, Fatty Acid and Tocopherol Contents of Buriti (Mauritia flexuosa) and Patawa (Oenocarpus bataua) Fruit Pulp from the Amazon Region. Ciência e Tecnol. Aliment. 2011, 31, 488–491. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Pizarro, C.; López-Vela, D.; Betancor, L.; Carrascosa, A.V.; Pessela, B.; Guisan, J.M. Hydrolysis of Fish Oil by Lipases Immobilized Inside Porous Supports. J. Am. Oil Chem. Soc. 2011, 88, 819–826. [Google Scholar] [CrossRef]
- Shimada, Y.; Sugihara, A.; Tominaga, Y. Enzymatic Purification of Polyunsaturated Fatty Acids. J. Biosci. Bioeng. 2001, 91, 529–538. [Google Scholar] [CrossRef]
- Singh, A.K.; Mukhopadhyay, M. Overview of Fungal Lipase: A Review. Appl. Biochem. Biotechnol. 2012, 166, 486–520. [Google Scholar] [CrossRef] [PubMed]
- Peirce, S.; Tacias-Pascacio, V.; Russo, M.; Marzocchella, A.; Virgen-Ortíz, J.; Fernandez-Lafuente, R. Stabilization of Candida antarctica Lipase B (CALB) Immobilized on Octyl Agarose by Treatment with Polyethyleneimine (PEI). Molecules 2016, 21, 751. [Google Scholar] [CrossRef] [PubMed]
- Hasan, F.; Shah, A.A.; Hameed, A. Industrial Applications of Microbial Lipases. Enzyme Microb. Technol. 2006, 39, 235–251. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Andualema, B.; Gessesse, A. Microbial Lipases and Their Industrial Applications: Review. Biotechnology 2012, 11, 100–118. [Google Scholar] [CrossRef]
- Palomo, J.M.; Muñoz, G.; Fernández-Lorente, G.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Interfacial Adsorption of Lipases on Very Hydrophobic Support (Octadecyl–Sepabeads): Immobilization, Hyperactivation and Stabilization of the Open Form of Lipases. J. Mol. Catal. B Enzym. 2002, 19–20, 279–286. [Google Scholar] [CrossRef]
- Manoel, E.A.; dos Santos, J.C.S.; Freire, D.M.G.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of Lipases on Hydrophobic Supports Involves the Open Form Of the Enzyme. Enzyme Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.G.; Facchini, F.D.A.; Filó, L.E.C.; Polizeli, A.M.; Vici, A.C.; Jorge, J.A.; Lorente, G.F.; Pessela, B.C.; Guisan, J.M.; Polizeli, M.L.T.M. Immobilized Lipase From Hypocrea pseudokoningii on Hydrophobic and Ionic Supports: Determination of Thermal and Organic Solvent Stabilities for Applications in the Oleochemical Industry. Process Biochem. 2015, 50, 561–570. [Google Scholar] [CrossRef]
- Palomo, J.M.; Fernandez-Lorente, G.; Mateo, C.; Ortiz, C.; Fernandez-Lafuente, R.; Guisan, J.M. Modulation Of The Enantioselectivity of Lipases Via Controlled Immobilization and Medium Engineering: Hydrolytic Resolution of Mandelic Acid Esters. Enzyme Microb. Technol. 2002, 31, 775–783. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Betancor, L.; Carrascosa, A.V.; Palomo, J.M.; Guisan, J.M. Modulation of the Selectivity of Immobilized Lipases by Chemical and Physical Modifications: Release of Omega-3 Fatty Acids from Fish Oil. J. Am. Oil Chem. Soc. 2012, 89, 97–102. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of Enzyme Activity, Stability and Selectivity Via Immobilization Techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Rueda, N.; Dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Improved Performance of Lipases Immobilized on Heterofunctional Octyl-Glyoxyl Agarose Beads. RSC Adv. 2015, 5, 11212–11222. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Hirata, D.B.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Relevance of Substrates and Products on the Desorption of Lipases Physically Adsorbed on Hydrophobic Supports. Enzyme Microb. Technol. 2017, 96, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, N.; Bernal, C.; Wilson, L.; Cabrera, Z. Selectivity of R-α-monobenzoate Glycerol Synthesis Catalyzed by Candida antarctica Lipase B Immobilized on Heterofunctional Supports. Process Biochem. 2015, 50, 1870–1877. [Google Scholar] [CrossRef]
- Albuquerque, T.L.D.; Rueda, N.; dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Binay, B.; Özdemir, E.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Easy Stabilization of Interfacially Activated Lipases Using Heterofunctional Divinyl Sulfone Activated-Octyl Agarose Beads. Modulation of The Immobilized Enzymes by Altering Their Nanoenvironment. Process Biochem. 2016, 51, 865–874. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, C.S.; Rodriguez, M.D.; Albuquerque, T.L.; Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Reversible Immobilization of Lipases on Octyl-Glutamic Agarose Beads: A Mixed Adsorption that Reinforces Enzyme Immobilization. J. Mol. Catal. B Enzym. 2016, 128, 10–18. [Google Scholar] [CrossRef]
- Rueda, N.; Albuquerque, T.; Bartolome-Cabrero, R.; Fernandez-Lopez, L.; Torres, R.; Ortiz, C.; dos Santos, J.; Barbosa, O.; Fernandez-Lafuente, R. Reversible Immobilization of Lipases on Heterofunctional Octyl-Amino Agarose Beads Prevents Enzyme Desorption. Molecules 2016, 21, 646. [Google Scholar] [CrossRef] [PubMed]
- Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A Very Useful Ionic Polymer in the Design of Immobilized Enzyme Biocatalysts. J. Mater. Chem. B 2017, 5, 7461–7490. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; López-Gallego, F.; Vázquez-Duhalt, R.; Mateos-Díaz, J.C.; Guisán, J.M.; Favela-Torres, E. Carrier-Free Immobilization of Lipase from Candida rugosa with Polyethyleneimines by Carboxyl-Activated Cross-Linking. Biomacromolecules 2014, 15, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, L.; Virgen-OrtÍz, J.J.; Pedrero, S.G.; Lopez-Carrobles, N.; Gorines, B.C.; Otero, C.; Fernandez-Lafuente, R. Optimization of the Coating Of Octyl-CALB with Ionic Polymers to Improve Stability and Decrease Enzyme Leakage. Biocatal. Biotransformation 2018, 36, 47–56. [Google Scholar] [CrossRef]
- Zaak, H.; Fernandez-Lopez, L.; Otero, C.; Sassi, M.; Fernandez-Lafuente, R. Improved Stability of Immobilized Lipases Via Modification with Polyethylenimine and Glutaraldehyde. Enzyme Microb. Technol. 2017, 106, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.M.; Rocha-Martin, J.; Mateo, C.; Cava, F.; Berenguer, J.; Fernandez-Lafuente, R.; Guisan, J.M. Coating of Soluble and Immobilized Enzymes with Ionic Polymers: Full Stabilization of the Quaternary Structure of Multimeric Enzymes. Biomacromolecules 2009, 10, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.M.; Breccia, J.D.; Hatti-Kaul, R. Stabilizing Effect of Chemical Additives Against Oxidation of Lactate Dehydrogenase. Biotechnol. Appl. Biochem. 2000, 32, 145. [Google Scholar] [CrossRef] [PubMed]
- Breccia, J.D.; Andersson, M.M.; Hatti-Kaul, R. The Role of Poly(Ethyleneimine) in Stabilization Against Metal-Catalyzed Oxidation of Proteins: A Case Study with Lactate Dehydrogenase. Biochim. Biophys. Acta Gen. Subj. 2002, 1570, 165–173. [Google Scholar] [CrossRef]
- Cabrera, Z.; Gutarra, M.L.E.; Guisan, J.M.; Palomo, J.M. Highly Enantioselective Biocatalysts by Coating Immobilized Lipases with Polyethyleneimine. Catal. Commun. 2010, 11, 964–967. [Google Scholar] [CrossRef]
- Ranjan Moharana, T.; Byreddy, A.R.; Puri, M.; Barrow, C.; Rao, N.M. Selective Enrichment of Omega-3 Fatty Acids in Oils by Phospholipase A1. PLoS One 2016, 11, e0151370. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lorente, G.; Betancor, L.; Carrascosa, A.V.; Guisán, J.M. Release of Omega-3 Fatty Acids by the Hydrolysis of Fish Oil Catalyzed by Lipases Immobilized on Hydrophobic Supports. J. Am. Oil Chem. Soc. 2011, 88, 1173–1178. [Google Scholar] [CrossRef]
- Moreno, M.L.; Escobar, J.; Izquierdo-Álvarez, A.; Gil, A.; Pérez, S.; Pereda, J.; Zapico, I.; Vento, M.; Sabater, L.; Marina, A.; Martínez-Ruiz, A.; Sastre, J. Disulfide stress: A Novel Type of Oxidative Stress in Acute Pancreatitis. Free Radic. Biol. Med. 2014, 70, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.C.; Filho, A.A.M.; Chagas, E.A.; Takahashi, J.A.; Montero, I.F.; Holanda, L.C.; Ribeiro, P.R.E.; Santos, G.F.; Melo, A.C.G.R. Chemical Characterization of Oils and Fats from Amazonian Fruits by 1H-NMR. 2017. [Google Scholar] [CrossRef]
- Hiane, P.A.; Bogo, D.; Ramos, M.I.L.; Ramos Filho, M.M. Carotenóides Pró-Vitamínicos A e Composição em Ácidos Graxos do Fruto e da Farinha do Bacuri (Scheelea phalerata Mart.). Ciência e Tecnol. Aliment. 2003, 23, 206–209. [Google Scholar] [CrossRef]
- Lakshmi, B.S.; Kangueane, P.; Abraham, B.; Pennathur, G. Effect of Vegetable Oils in the Secretion of Lipase from Candida rugosa (DSM 2031). Lett. Appl. Microbiol. 1999, 29, 66–70. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Palomo, J.M.; Fuentes, M.; Mateo, C.; Guisán, J.M.; Fernández-Lafuente, R. Self-assembly of Pseudomonas fluorescens Lipase into Bimolecular Aggregates Dramatically Affects Functional Properties. Biotechnol. Bioeng. 2003, 82, 232–237. [Google Scholar] [CrossRef] [PubMed]
- González-Navarro, H.; Bañó, M.C.; Abad, C. The Closed/open Model for Lipase Activation. Addressing Intermediate Active Forms of Fungal Enzymes by Trapping of Conformers in Water-Restricted Environments. Biochemistry 2001, 40, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Maiangwa, J.; Mohamad Ali, M.S.; Salleh, A.B.; Rahman, R.N.Z.R.A.; Normi, Y.M.; Mohd Shariff, F.; Leow, T.C. Lid Opening and Conformational Stability of T1 Lipase is Mediated by Increasing Chain Length Polar Solvents. Peer J. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An Overview of Technologies for Immobilization of Enzymes and Surface Analysis Techniques for Immobilized Enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, B.; Zhang, Z.; Lou, D.; Tan, J.; Zhu, L. The Effects of Macromolecular Crowding and Surface Charge on the Properties of an Immobilized Enzyme: Activity, Thermal Stability, Catalytic Efficiency and Reusability. RSC Adv. 2017, 7, 38028–38036. [Google Scholar] [CrossRef]
- Padilha, M.E.S.; Augusto-Ruiz, W. Hidrólise Enzimática do Óleo de Pescado. Ciência e Tecnol. Aliment. 2007, 27, 285–290. [Google Scholar] [CrossRef]
- Ferreira-Dias, S.; Sandoval, G.; Plou, F.; Valero, F. The Potential Use of Lipases in the Production of Fatty Acid Derivatives for the Food and Nutraceutical Industries. Electron. J. Biotechnol. 2013, 16. [Google Scholar] [CrossRef]
- Pereira, M.G.; Vici, A.C.; Facchini, F.D.A.; Tristão, A.P.; Cursino-Santos, J.R.; Sanches, P.R.; Jorge, J.A.; Polizeli, M.L.T.M. Screening of Filamentous Fungi for Lipase Production: Hypocrea pseudokoningii A New Producer With a High Biotechnological Potential. Biocatal. Biotransform. 2014, 32, 74–83. [Google Scholar] [CrossRef]
- Khanna, P.; Sundari, S.S.; Kumar, N.J. Production, Isolation and Partial Purification of Xylanases from an Aspergillus sp. World J. Microbiol. Biotechnol. 1995, 11, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.R. Mycelial Amylase Activities of Thermophilic Species of Rhizomucor, Humicola and Papulaspora. Mycopathologia 1990, 112, 35–37. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins During the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.A.; Kim, H.S.; Hahm, D.H.; Song, J.K. Synthesis Activity-based Zymography for Detection of Lipases and Esterases. Biotechnol. Lett. 2011, 33, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Oakley, B.R.; Kirsch, D.R.; Morris, N.R. A Simplified Ultrasensitive Silver Stain for Detecting Proteins in Polyacrylamide Gels. Anal. Biochem. 1980, 105, 361–363. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
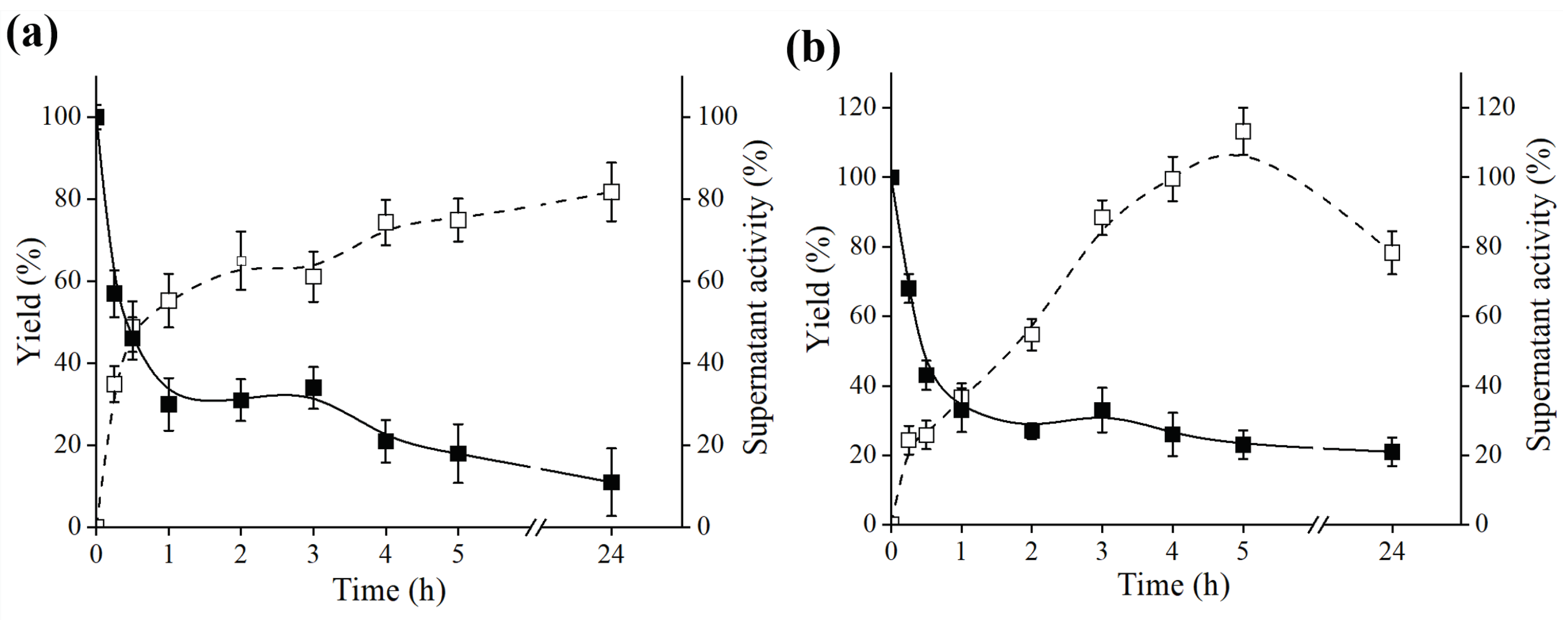
| Extract | Yield from Supernatant (%) | Relative Activity of Octyl Derivative (%) | Octyl Derivative Specific Activity (U·g−1) |
|---|---|---|---|
| B. bassiana | 81.78 ± 0.75 | 2350 ± 22.17 | 470 ± 13.46 |
| F.oxysporum | 78.27 ± 0.67 | 1100 ± 11.32 | 220 ± 18.32 |
| Lipase | Accession | Score | Coverage (%) | No. Peptides | MW (kDa) | Calc. pI |
|---|---|---|---|---|---|---|
| F. oxysporum lip 1 | A0A0C4DHT8 | 873.70 | 33.82 | 8 | 36.5 | 6.86 |
| F. oxysporum lip 2 | A0A0D2YK59 | 5.08 | 5.13 | 2 | 48.1 | 5.88 |
| B. bassiana lip | J4VUX9 | 825.96 | 21.23 | 10 | 52.9 | 6.57 |
| Parameter | Derivative | Açaí | Buriti | ||
|---|---|---|---|---|---|
| Oleic Acid | Linoleic Acid | Oleic Acid | Linoleic Acid | ||
| Productivity index (mg·h−1) | Bb-octyl | 6.68 ± 0.23 | 1.57 ± 0.09 | 2.22 ± 0.18 | 1.15 ± 0.02 |
| Fo-octyl | 1.41 ± 0.17 | 0.73 ± 0.12 | 1.06 ± 0.02 | 0.75 ± 0.01 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, M.M.; Gonçalves, E.C.S.; Salgado, J.C.S.; Rocha, M.d.S.; Almeida, P.Z.d.; Vici, A.C.; Infante, J.d.C.; Guisán, J.M.; Rocha-Martin, J.; Pessela, B.C.; et al. Production of Omegas-6 and 9 from the Hydrolysis of Açaí and Buriti Oils by Lipase Immobilized on a Hydrophobic Support. Molecules 2018, 23, 3015. https://doi.org/10.3390/molecules23113015
Pérez MM, Gonçalves ECS, Salgado JCS, Rocha MdS, Almeida PZd, Vici AC, Infante JdC, Guisán JM, Rocha-Martin J, Pessela BC, et al. Production of Omegas-6 and 9 from the Hydrolysis of Açaí and Buriti Oils by Lipase Immobilized on a Hydrophobic Support. Molecules. 2018; 23(11):3015. https://doi.org/10.3390/molecules23113015
Chicago/Turabian StylePérez, Malena Martínez, Enrico Cerioni Spiropulos Gonçalves, Jose Carlos Santos Salgado, Mariana de Souza Rocha, Paula Zaghetto de Almeida, Ana Claudia Vici, Juliana da Conceição Infante, Jose Manuel Guisán, Javier Rocha-Martin, Benevides Costa Pessela, and et al. 2018. "Production of Omegas-6 and 9 from the Hydrolysis of Açaí and Buriti Oils by Lipase Immobilized on a Hydrophobic Support" Molecules 23, no. 11: 3015. https://doi.org/10.3390/molecules23113015
APA StylePérez, M. M., Gonçalves, E. C. S., Salgado, J. C. S., Rocha, M. d. S., Almeida, P. Z. d., Vici, A. C., Infante, J. d. C., Guisán, J. M., Rocha-Martin, J., Pessela, B. C., & Polizeli, M. d. L. T. d. M. (2018). Production of Omegas-6 and 9 from the Hydrolysis of Açaí and Buriti Oils by Lipase Immobilized on a Hydrophobic Support. Molecules, 23(11), 3015. https://doi.org/10.3390/molecules23113015