Insecticidal Activities of Chloramphenicol Derivatives Isolated from a Marine Alga-Derived Endophytic Fungus, Acremonium vitellinum, against the Cotton Bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae)
Abstract
:1. Introduction
2. Results and Discussion
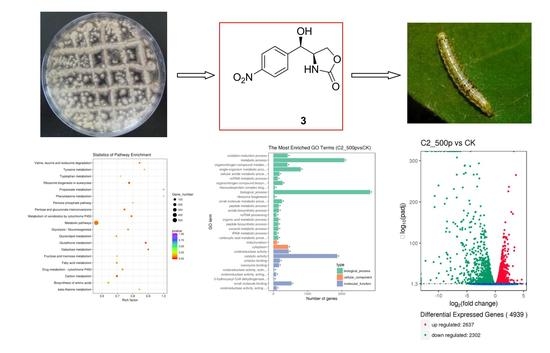
2.1. Elucidation of the Structure of Isolated Compounds 1–3
2.2. Insecticidal Activities of the Isolated Compounds
2.3. Effects of Compound 2 on the Activities of GST, CAT, AChE and T-AOC in H. armigera
2.4. Molecular Mechanism of Action of Compound 2
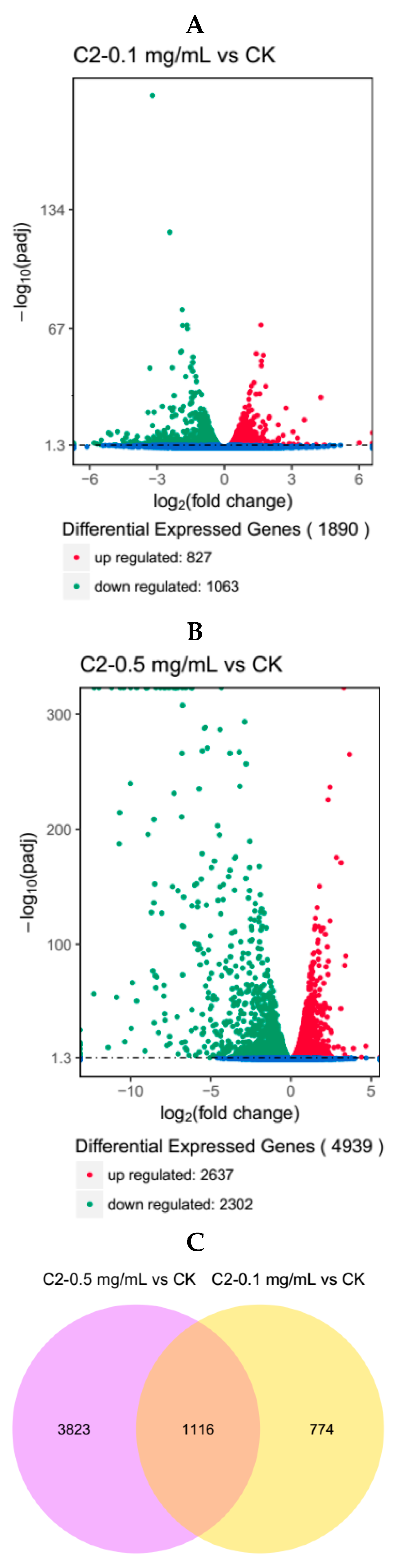
2.4.1. Analysis of Differentially Expressed Genes
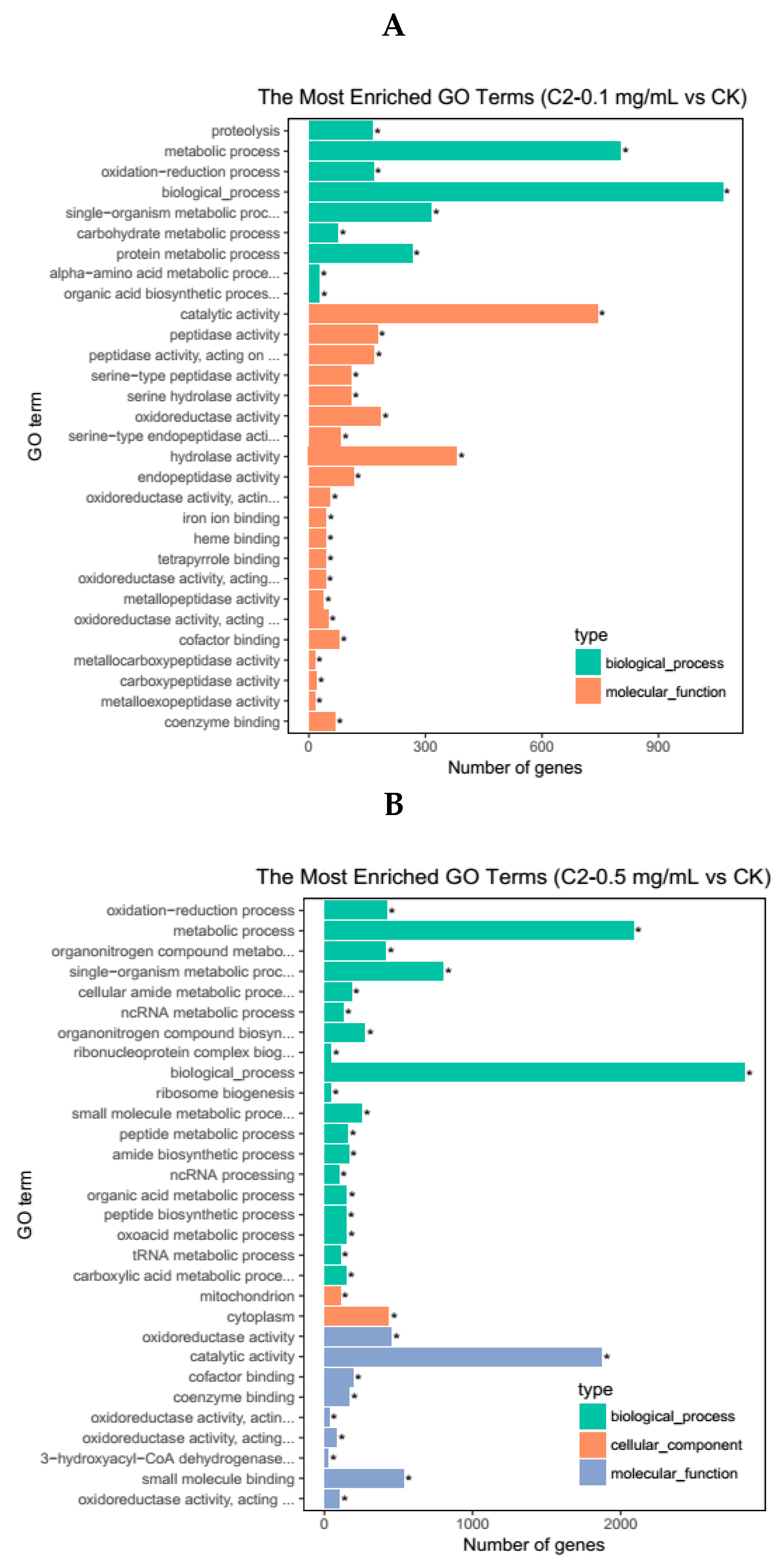
2.4.2. GO Enrichment Analysis of DEGs
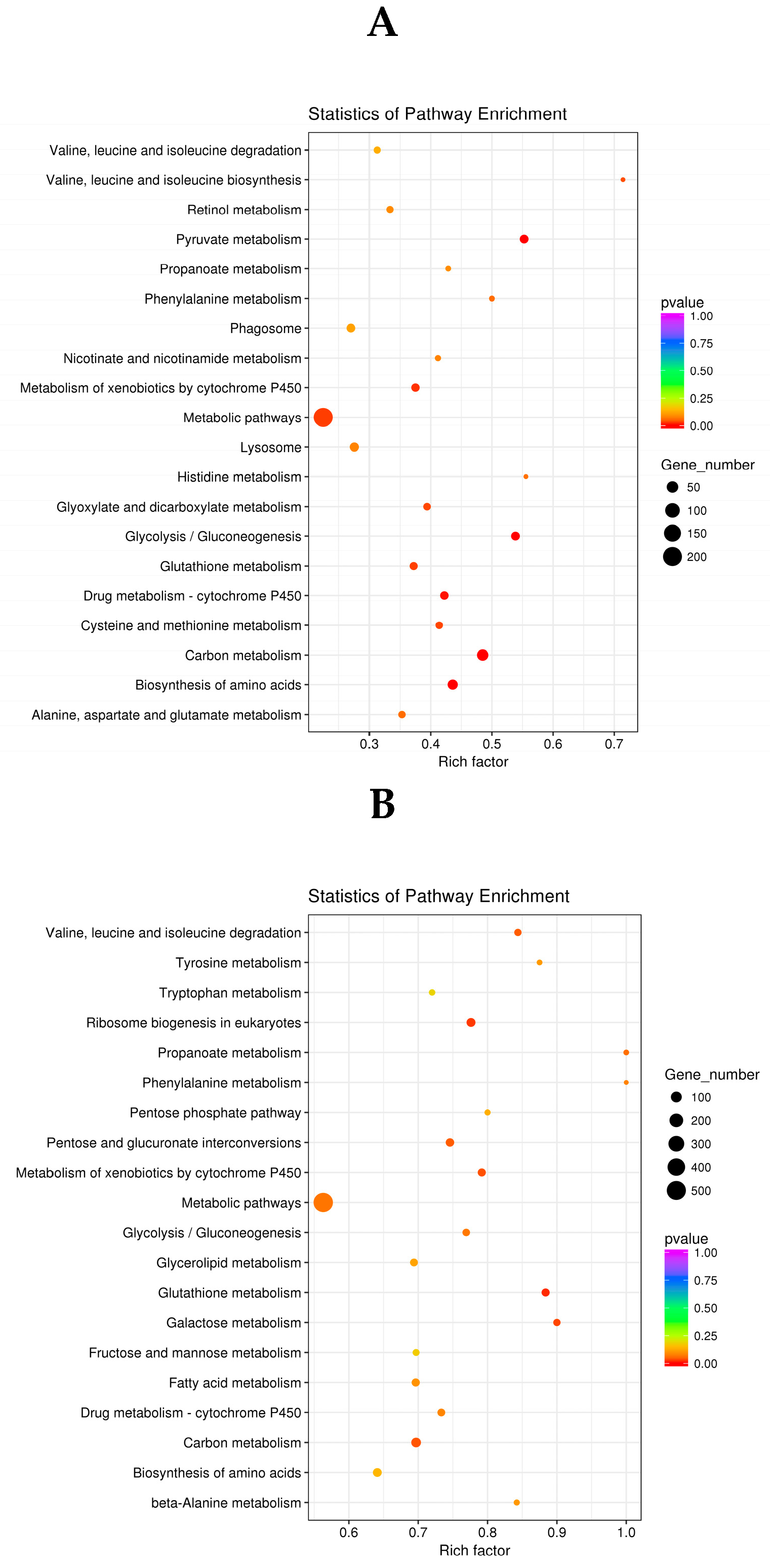
2.4.3. KEGG Analysis of DEGs
2.4.4. Differentially Expressed Genes Associated with the Insecticide Target
2.4.5. Differentially Expressed Genes Associated with Digestive, Detoxification, and Protective Enzymes
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction and Isolation
3.4. Insecticidal Assay
3.5. Enzyme Assays
3.5.1. Enzyme Preparation
3.5.2. Activity Assays
3.5.3. Statistical Analysis
3.6. Transcriptomic Analysis
3.6.1. Insect Treatments
3.6.2. Library Construction
3.6.3. Read Mapping to the Reference Genome
3.6.4. Bioinformatic Analysis of RNA-seq Data
3.6.5. GO and KEGG Pathway Enrichment Analysis
3.6.6. Availability of Supporting Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jaglan, M.S.; Khokhar, K.S.; Malik, M.S.; Singh, R. Evaluation of neem (Azadirachta indica A. Juss) extracts against American bollworm, Helicoverpa armigera (Hubner). J. Agric. Food Chem. 1997, 45, 3262–3268. [Google Scholar] [CrossRef]
- Rahimi, V.; Hajizadeh, J.; Zibaee, A.; Sendi, J.J. Effect of Polygonum persicaria (Polygonales: Polygonaceae) extracted agglutinin on life table and antioxidant responses in Helicoverpa armigera (Lepidoptera: Noctuidae) larvae. J. Econ. Entomol. 2018, 111, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Arasu, M.V.; Al-Dhabi, N.A.; Saritha, V.; Duraipandiyan, V.; Muthukumar, C.; Kim, S.J. Antifeedant, larvicidal and growth inhibitory bioactivities of novel polyketide metabolite isolated from Streptomyces sp. AP-123 against Helicoverpa armigera and Spodoptera litura. BMC Microbiol. 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Xiao, J.J.; Zhou, L.J.; Yao, X.; Tang, F.; Hua, R.M.; Wu, X.W.; Cao, H.Q. Chemical composition, insecticidal and biochemical effects of Melaleuca alternifolia essential oil on the Helicoverpa armigera. J. Appl. Entomol. 2017, 141, 721–728. [Google Scholar] [CrossRef]
- Abbaszadeh, G.; Srivastava, C.; Walia, S. Insecticidal and antifeedant activities of clerodane diterpenoids isolated from the Indian bhant tree, Clerodendron infortunatum, against the cotton bollworm, Helicoverpa armigera. J. Insect. Sci. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Baskar, K.; Duraipandiyan, V.; Ignacimuthu, S. Bioefficacy of the triterpenoid friedelin against Helicoverpa armigera (Hub.) and Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Pest Manag. Sci. 2014, 70, 1877–1883. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Wang, B.G. Secondary metabolites from the marine algal-derived endophytic fungi: Chemical diversity and biological activity. Planta Med. 2016, 82, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.K.; Gai, C.J.; Cai, C.H.; Chen, L.L.; Liu, S.B.; Zeng, Y.B.; Yuan, J.Z.; Mei, W.L.; Dai, H.F. Metabolites with insecticidal activity from Aspergillus fumigatus JRJ111048 isolated from mangrove plant Acrostichum specioum endemic to Hainan Island. Mar. Drugs 2017, 15, 381. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Li, Y.Y.; Zhang, X.P.; Lai, D.W.; Zhou, L.G. Structural diversity and biological activities of the cyclodipeptides from fungi. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.; Bartz, Q.R.; Smith, R.M.; Josylyn, D.A.; Burkholder, P.R. Chloromycetin, a new antibiotic from a soil actinomycete. Science 1947, 106. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, H.; Li, C.; Xu, P. Stereoselective synthesis of (−)-chloramphenicol, (+)-thiamphenicol and (+)-sphinganine via chiral tricyclic iminolactone. Chin. J. Chem. 2013, 31, 149–153. [Google Scholar] [CrossRef]
- Cwik, A.; Fuchs, A.; Hell, Z.; Böjtös, I.; Halmai, D.; Bombicz, P. Modified Mg:Al hydrotalcite in the synthesis of oxazolidin-2-ones. Org. Biomol. Chem. 2005, 3, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Shirahata, K.; Hayashi, T.; Deguchi, T.; Suzuki, T.; Matsubara, I. The structures of corynecins; Chloramphenicol analogues produced by a n-paraffin-grown bacterium. Agric. Biol. Chem. 1972, 36, 2229–2232. [Google Scholar] [CrossRef]
- Abdallah, I.S.; Abouyousef, H.M.; Fouad, E.A.; Kandil, E.H. The role of detoxifying enzymes in the resistance of the cowpea aphid (Aphis craccivora Koch) to thiamethoxam. J. Plant Prot. Res. 2016, 56, 67–72. [Google Scholar] [CrossRef]
- Shi, H.; Pei, L.; Gu, S.; Zhu, S.; Wang, Y.; Zhang, Y.; Li, B. Glutathione S-transferase (GST) genes in the red flour beetle, Tribolium castaneum, and comparative analysis with five additional insects. Genomics 2012, 100, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Oxygen Is Toxic! Bioscience 1977, 27, 462–466. [Google Scholar] [CrossRef]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef]
- Meng, J.Y.; Zhang, C.Y.; Zhu, F.; Wang, X.P.; Lei, C.L. Ultraviolet light-induced oxidative stress: Effects on antioxidant response of Helicoverpa armigera adults. J. Insect Physiol. 2009, 55, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.X.; Liu, G.Y.; Wang, J.J. Toxicological and biochemical characterization of AChE in Liposcelis bostrychophila Badonnel (Psocoptera: Liposcelididae). Pestic. Biochem. Physiol. 2007, 88, 197–202. [Google Scholar] [CrossRef]
- Ding, J.N.; Zhang, H.H.; Chi, D.F. Effects of a pathogenic beauveria bassiana (hypocreales: Cordycipitaceae) strain on detoxifying and protective enzyme activities in xylotrechus rusticus (coleoptera: Cerambycidae) larvae. Fla. Entomol. 2002, 98, 1148–1156. [Google Scholar] [CrossRef]
- Mahmud, S.A.; Hirasawa, T.; Shimizu, H. Differential importance of trehalose accumulation in Saccharomyces cerevisiae in response to various environmental stresses. J. Biosci. Bioeng. 2010, 109, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Alam, M.S.; Athar, M. Cumene hydroperoxide debilitates macrophage physiology by inducing oxidative stress: Possible protection by α-tocopherol. Chem. Biol. Interact. 2009, 179, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Boily, M.; Sarrasin, B.; Deblois, C.; Aras, P.; Chagnon, M. Acetylcholinesterase in honey bees (Apis mellifera) exposed to neonicotinoids, atrazine and glyphosate: Laboratory and field experiments. Environ. Sci. Pollut. Res. Int. 2013, 20, 5603–5614. [Google Scholar] [CrossRef] [PubMed]
- Samsonrobert, O.; Labrie, G.; Mercier, P.L.; Chagnon, M.; Derome, N.; Fournier, V. Increased acetylcholinesterase expression in bumble bees during neonicotinoid-coated corn sowing. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11. [Google Scholar] [CrossRef] [PubMed]
- Chemendoza, A.; Penilla, R.P.; Américo Rodríguez, D. Insecticide resistance and glutathione S-transferases in mosquitoes: A review. Afr. J. Biotechnol. 2009, 8, 1386–1397. [Google Scholar]
- Wondji, C.S.; Irving, H.; Morgan, J.; Lobo, N.F.; Collins, F.H.; Hunt, R.H.; Coetzee, M.; Hemingway, J.; Ranson, H. Two duplicated P450 genes are associated with pyrethroid resistance in anopheles funestus, a major malaria vector. Genome Res. 2009, 19, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Ortelli, F.; Rossiter, L.C.; Vontas, J.; Ranson, H.; Hemingway, J. Heterologous expression of four glutathione transferase genes genetically linked to a major insecticide-resistance locus from the malaria vector anopheles gambiae. Biochem. J. 2003, 373, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Bolt, H.M.; Hengstler, J.G. Most cited articles: Metal toxicity, oxidative stress control and induction as well as inhibition of cytochrome P450 enzymes. Arch. Toxicol. 2010, 84, 903–905. [Google Scholar] [CrossRef]
- Meister, A. Glutamate, glutamine, glutathione, and related compounds. Methods Enzymol. 1985, 113, 1–723. [Google Scholar]
- Kerr, D.I.; Ong, J. GABA agonists and antagonists. Med. Res. Rev. 1992, 12, 593–636. [Google Scholar] [CrossRef] [PubMed]
- He, W.; You, M.; Vasseur, L.; Yang, G.; Xie, M.; Cui, K.; Bai, J.; Liu, C.; Li, X.; Xu, X.; et al. Developmental and insecticide-resistant insights from the de novo assembled transcriptome of the diamondback moth, Plutella xylostella. Genomics 2012, 99, 169–177. [Google Scholar]
- Esposti, M.D. Inhibitors of NADH–ubiquinone reductase: An overview. Biochim. Biophys. Acta 1998, 1364, 222–235. [Google Scholar] [CrossRef]
- Cutler, G.C.; Scott-Dupree, C.D.; Tolman, J.H.; Harris, C.R. Toxicity of the insect growth regulator novaluron to the non-target predatory bug Podisus maculivebtris (Heteroptera; Pentatomidae). Biol. Control 2006, 38, 196–204. [Google Scholar] [CrossRef]
- NY/T 1154.10-2008, Agricultural Industry Standard of the People’s Republic of China Guideline for Laboratory Bioassay of Pesticides Part 10: Diet Incorporation Method; Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2008.
- Lao, S.H.; Huang, X.H.; Huang, H.J.; Liu, C.W.; Zhang, C.X.; Bao, Y.Y. Genomic and transcriptomic insights into the cytochrome P450 monooxygenase gene repertoire in the rice pest brown planthopper, Nilaparvata lugens. Genomics 2015, 106, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Caboni, P.; Ntalli, N.G.; Aissani, N.; Cavoski, I.; Angioni, A. Nematicidal activity of (E,E)-2,4-decadienal and (E)-2-decenal from Ailanthus altissima against Meloidogyne javanica. J. Agric. Food Chem. 2012, 60, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Guo, X.; Zhao, P.; Ruan, M.; Yu, X.; Zou, L.; Yang, Y.; Li, X.; Deng, D.; Xiao, J. Molecular diversity analysis, drought related marker-traits association mapping and discovery of excellent alleles for 100-day old plants by EST-SSRs in cassava germplasms (Manihot esculenta Cranz). PLoS ONE 2017, 12, e0177456. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Labade, C.P.; Jadhav, A.R.; Ahire, M.; Zinjarde, S.S.; Tamhane, V.A. Role of induced glutathione-S-transferase from Helicoverpa armigera (Lepidoptera: Noctuidae) HaGST-8 in detoxification of pesticides. Ecotoxicol. Environ. Saf. 2018, 147, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, D.; Li, Y.; Wang, X.; Wang, F. Enantioselective bioaccumulation and toxicity of the neonicotinoid insecticide dinotefuran in earthworms (Eisenia fetida). J. Agric. Food Chem. 2018, 66, 4531–4540. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–3 are available from the authors. |

| Compound 1 | Compound 2 | Compound 3 | ||||
|---|---|---|---|---|---|---|
| No. | δH (mult, J in Hz) | δC, type | δH (mult, J in Hz) | δC, type | δH (mult, J in Hz) | δC, type |
| 1 | 151.7, C | 149.4, C | 152.5, C | |||
| 2 | 7.58, d (8.6) | 127.8, CH | 7.66, d (8.6) | 128.4, CH | 7.58, d (8.3) | 127.8, CH |
| 3 | 8.14, d (8.6) | 123.3, CH | 8.21, d (8.6) | 123.6, CH | 8.16, d (8.3) | 123.2, CH |
| 4 | 146.9, C | 147.3, C | 146.7, C | |||
| 5 | 8.14, d (8.6) | 127.8, CH | 8.21, d (8.6) | 128.4, CH | 8.16, d (8.3) | 127.8, CH |
| 6 | 7.58, d (8.6) | 123.3, CH | 7.66, d (8.6) | 123.6, CH | 7.58, d (8.3) | 123.2, CH |
| 7 | 5.05, br s | 69.4, CH | 4.72, t (4.0) | 72.7, CH | 5.00, br s | 69.8, CH |
| 8 | 3.92, dt (8.3, 4.2) | 57.3, CH | 4.02, m | 57.3, CH | 3.95, m | 56.4, CH |
| 9 | 3.58, dt (10.3, 7.2) 3.36, m | 60.7, CH2 | 4.20, t (8.8) 4.11, dd (8.8, 4.6) | 65.6, CH2 | 3.53, dd (14.5, 10.0) 3.28, dt (10.0, 5.1) | 60.9, CH2 |
| 10 | 163.8, C | 159.1, C | 169.5, C | |||
| 11 | 6.46, s | 66.9, CH | - | - | 1.69, s | 22.8, CH3 |
| 7-OH | 6.03, d (4.5) | - | 6.06, d (4.0) | - | 5.81, d (4.6) | - |
| 8-NH | 8.32, d (9.1) | - | 7.74, s | - | 7.56, br s | - |
| 9-OH | 4.98, t (5.4) | - | - | - | 4.83, t (5.1) | - |
| GST (μmol/min/mg prot) | CAT (μmol/min/mg prot) | T-AOC (U/mg prot) | AChE (nmol/min/mg prot) | |
|---|---|---|---|---|
| blank control (CK) | 0.332 ± 0.022 a | 0.111 ± 0.003 a | 24.394 ± 0.827 c | 0.555 ± 0.018 c |
| 0.1 mg/mL | 0.312 ± 0.001 a | 0.065 ± 0.005 c | 38.813 ± 0.467 a | 0.887 ± 0.025 b |
| 0.5 mg/mL | 0.267 ± 0.013 b | 0.080 ± 0.003 b | 35.517 ± 0.744 b | 2.828 ± 0.081 a |
| C2-0.1 mg/mL vs. CK | C2-0.5 mg/mL vs. CK | |
|---|---|---|
| NADH dehydrogenase | 2 | 19 |
| Acetylcholinesterase | 4 | 4 |
| Acetylcholine | 2 | 2 |
| Gamma-aminobutyric acid | - | 1 |
| C2-0.1 mg/mL vs. CK | C2-0.5 mg/mL vs. CK | |
|---|---|---|
| Trypsin | 20 | 20 |
| Chymotrypsin | 4 | 4 |
| Lysosome | 0 | 2 |
| Catalase | 0 | 2 |
| Carboxylesterase | 2 | 4 |
| Glutathione S-transferase | 8 | 22 |
| Cytochrome P450 | 37 | 47 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Zhang, P.; Liu, T.; Wang, X.-F.; Li, Z.-X.; Li, W.; Wang, F.-L. Insecticidal Activities of Chloramphenicol Derivatives Isolated from a Marine Alga-Derived Endophytic Fungus, Acremonium vitellinum, against the Cotton Bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Molecules 2018, 23, 2995. https://doi.org/10.3390/molecules23112995
Chen D, Zhang P, Liu T, Wang X-F, Li Z-X, Li W, Wang F-L. Insecticidal Activities of Chloramphenicol Derivatives Isolated from a Marine Alga-Derived Endophytic Fungus, Acremonium vitellinum, against the Cotton Bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Molecules. 2018; 23(11):2995. https://doi.org/10.3390/molecules23112995
Chicago/Turabian StyleChen, Dan, Peng Zhang, Tong Liu, Xiu-Fang Wang, Zhao-Xia Li, Wei Li, and Feng-Long Wang. 2018. "Insecticidal Activities of Chloramphenicol Derivatives Isolated from a Marine Alga-Derived Endophytic Fungus, Acremonium vitellinum, against the Cotton Bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae)" Molecules 23, no. 11: 2995. https://doi.org/10.3390/molecules23112995
APA StyleChen, D., Zhang, P., Liu, T., Wang, X.-F., Li, Z.-X., Li, W., & Wang, F.-L. (2018). Insecticidal Activities of Chloramphenicol Derivatives Isolated from a Marine Alga-Derived Endophytic Fungus, Acremonium vitellinum, against the Cotton Bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Molecules, 23(11), 2995. https://doi.org/10.3390/molecules23112995