Impact of Sodium N-[8-(2-Hydroxybenzoyl)amino]-caprylate on Intestinal Permeability for Notoginsenoside R1 and Salvianolic Acids in Caco-2 Cells Transport and Rat Pharmacokinetics
Abstract
1. Introduction
2. Results and Discussion
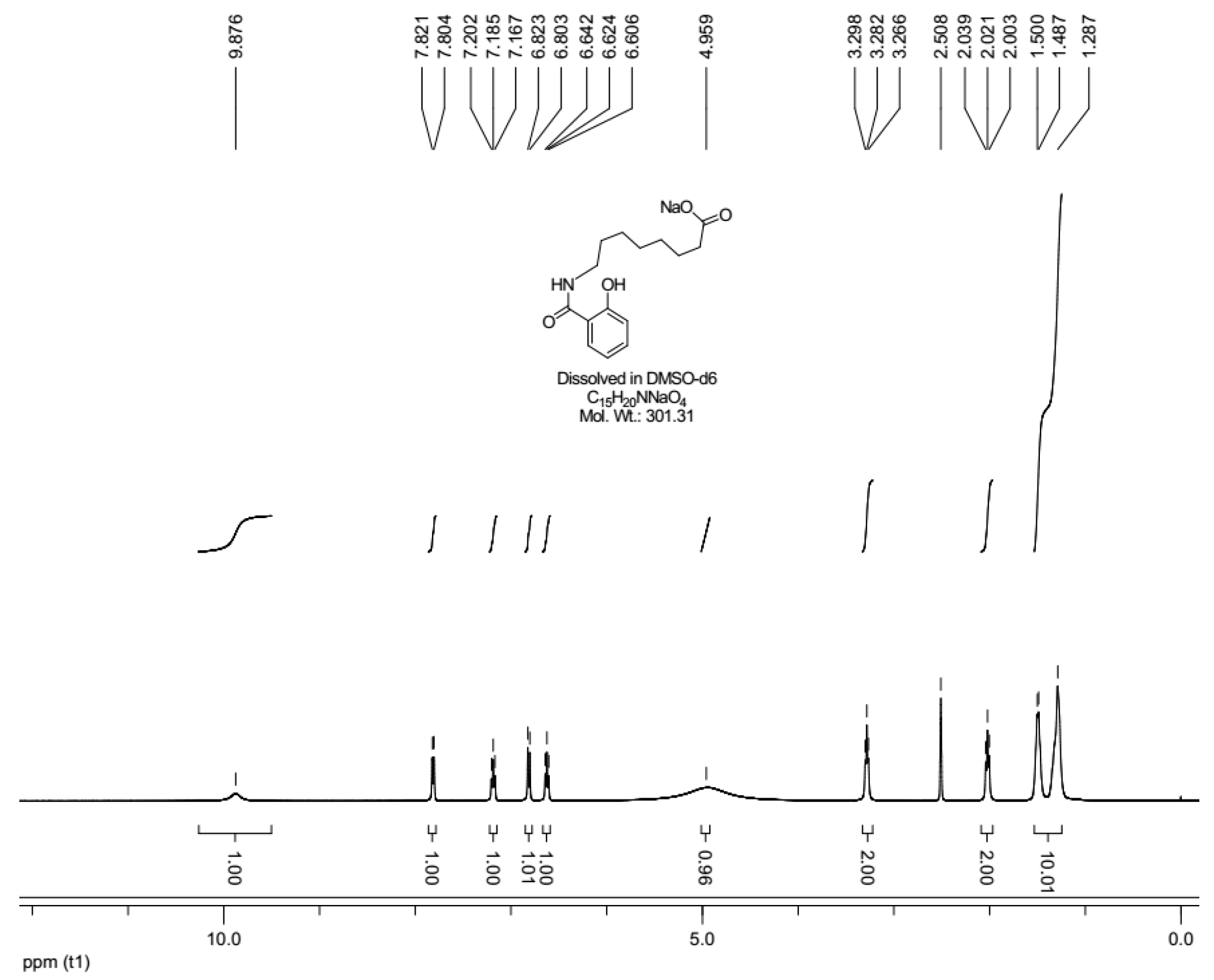
2.1. Sodium N-[8-(2-hydroxybenzoyl)amino]caprylate (SNAC) Characterization
2.2. Cytotoxicity Study (MTT Assay)
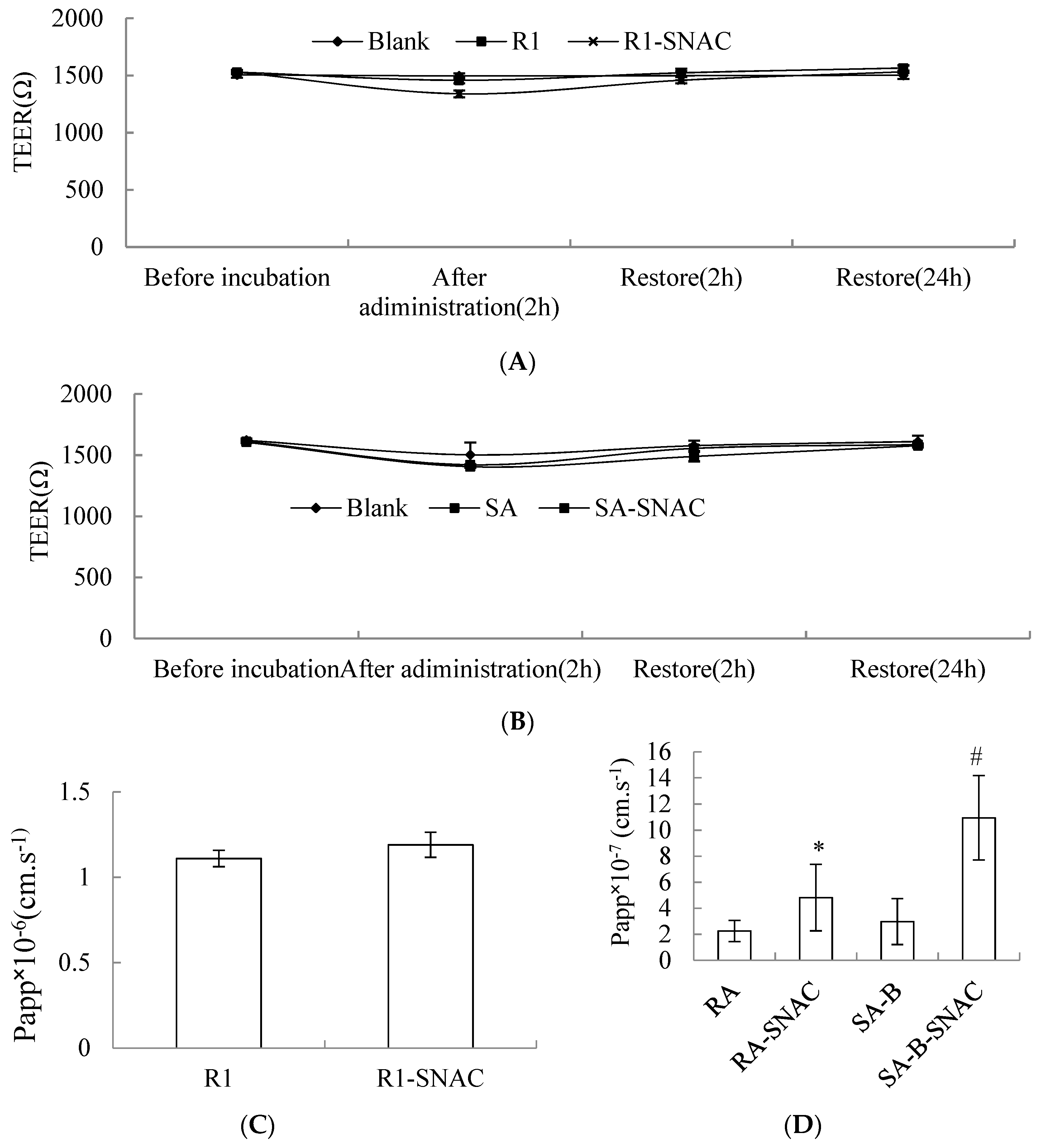
2.3. Caco-2 Cell Transport
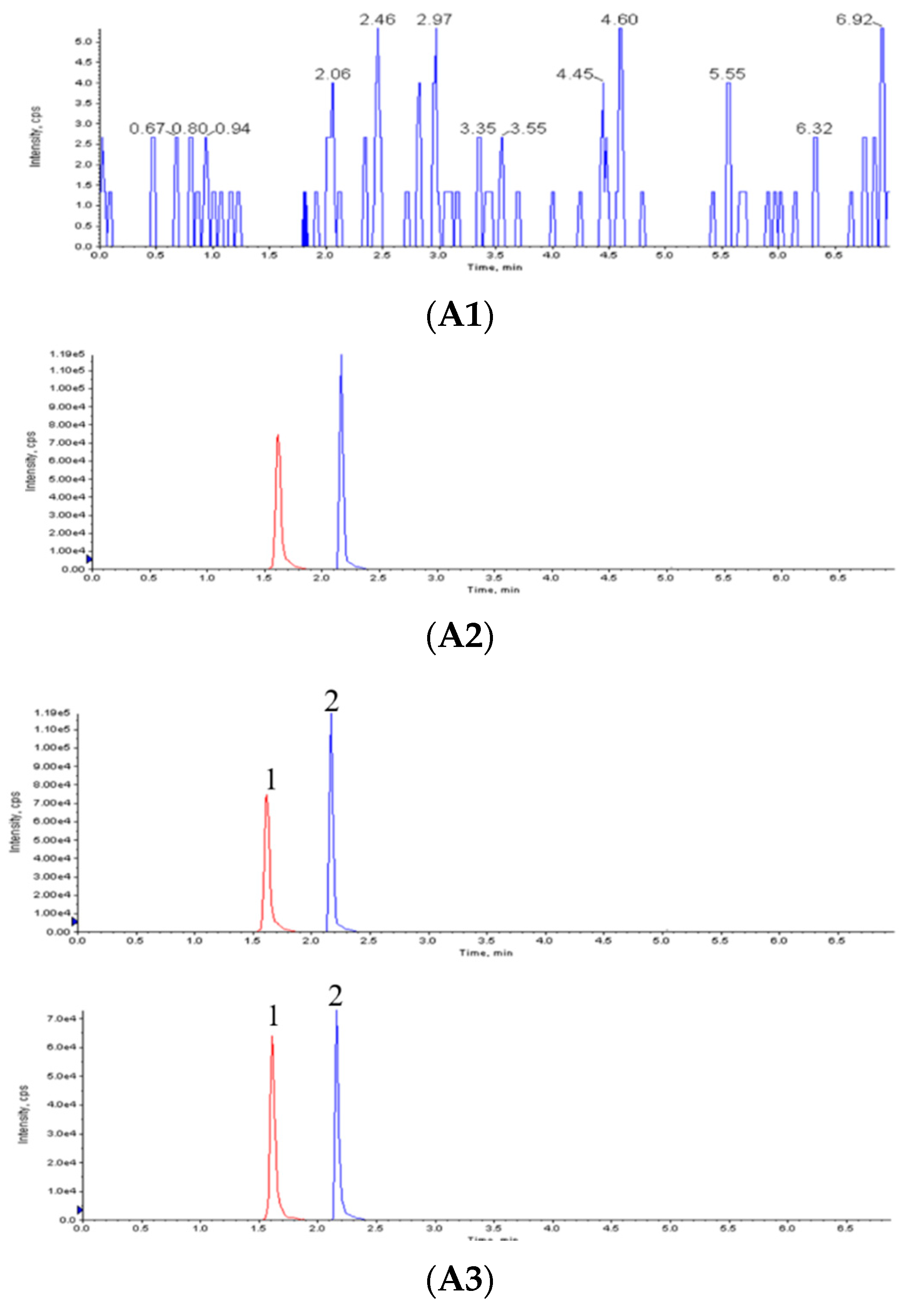
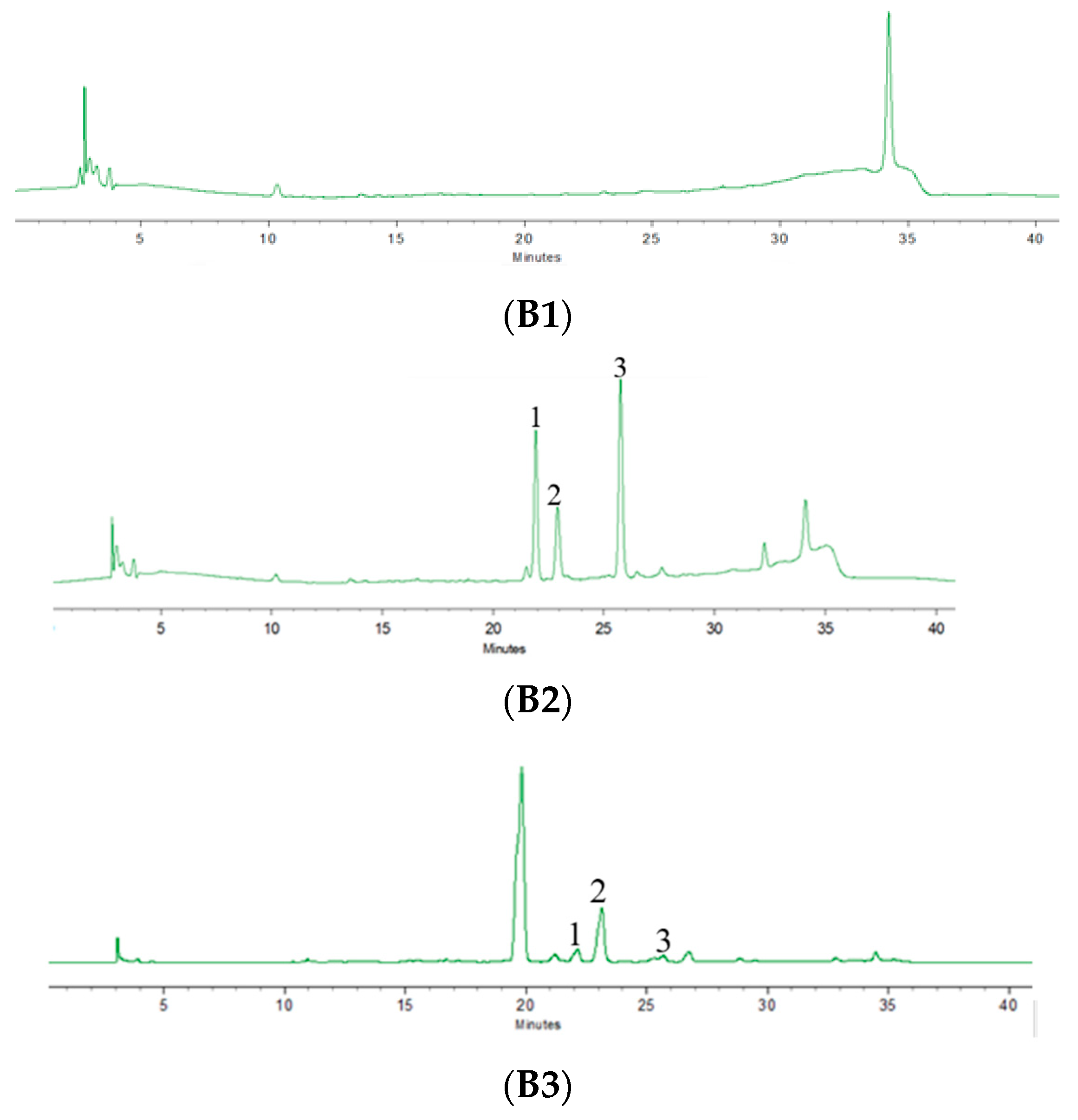
2.4. Chromatographic Condition
2.5. Validation of Analytical Methods
2.6. Pharmacokinetic Study
3. Materials and Methods
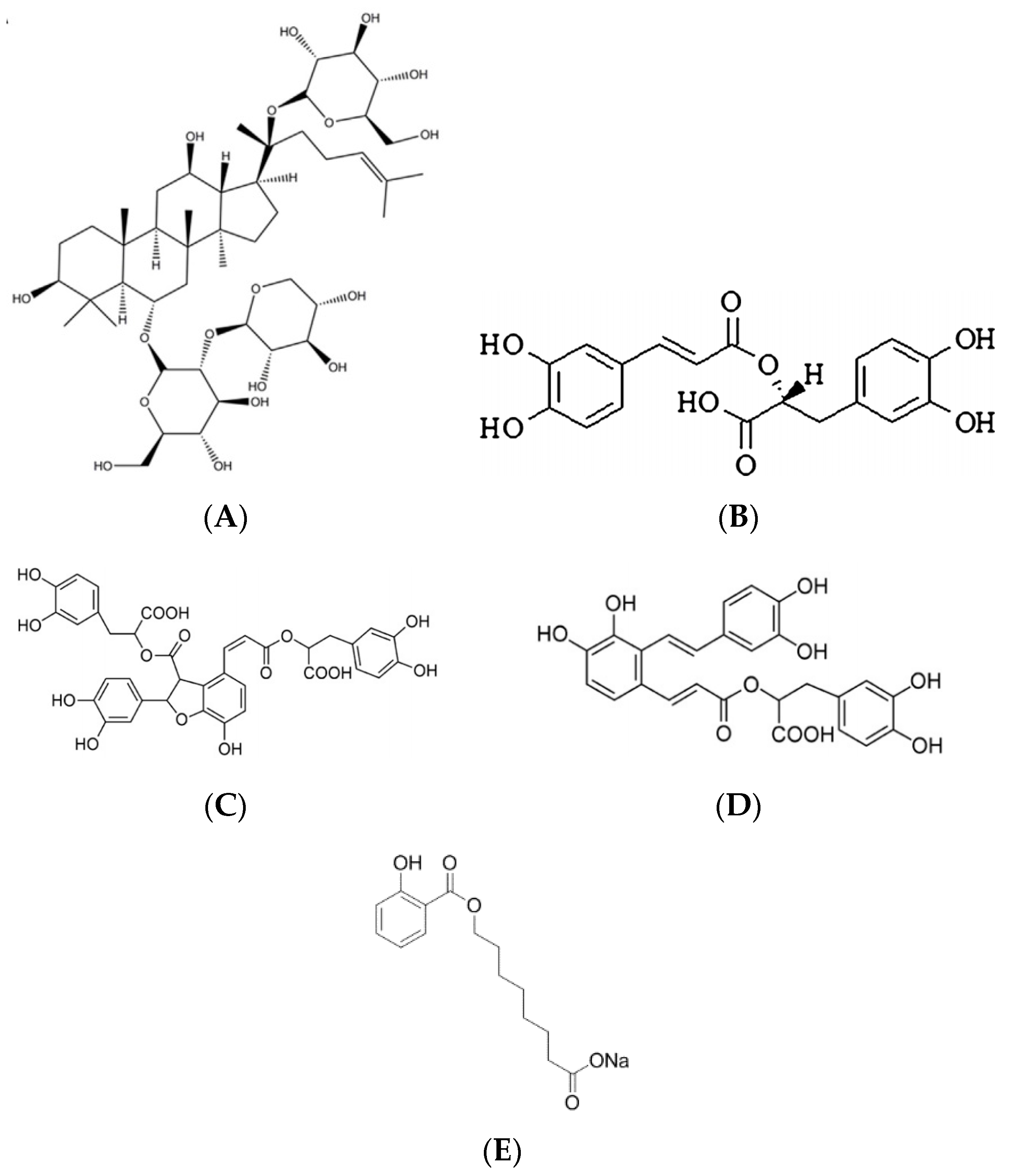
3.1. Chemicals, Reagents and Animals
3.2. SNAC Synthsis
3.3. 1H-NMR Characterization
3.4. Cytotoxicity Study (MTT Assay)
3.5. Caco-2 Monolayer Transport
3.6. Chromatographic System and Conditions
3.6.1. Chromatographic System and Conditions of Notoginsenoside R1
3.6.2. Chromatographic System and Conditions of SAs
3.7. Preparation of Calibration Standard and Quality Control Samples
3.7.1. Notoginsenoside R1
3.7.2. Salvianolic Acids
3.8. Preparation of Blood Samples
3.8.1. Notoginsenoside R1
3.8.2. Salvianolic Acids
3.9. Method Validation
3.9.1. Specificity
3.9.2. Recovery Rate
3.9.3. Precision
3.9.4. Lower Limit of Quantitation (LOQ)
3.10. Administration of Drugs Containing SNAC
3.11. Data Processing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alama, T.; Katayama, H.; Hirai, S.; Ono, S.; Kajiyama, A.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Enhanced oral delivery of alendronate by sucrose fatty acids esters in rats and their absorption-enhancing mechanisms. Int. J. Pharm. 2016, 515, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, M.; Canney, F.; Pacciana, C.; Colombo, G.; Young, P.M.; Traini, D. The use of fatty acids as absorption enhancer for pulmonary drug delivery. Int. J. Pharm. 2018, 541, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, T.A.S.; Rosa, M.; Guterres, S.S.; Pohlmann, A.R.; Coulter, I.; Brayden, D.J. Investigation of Coco-glucoside as a novel intestinal permeation enhancer in rat models. Eur. J. Pharm. Biopharm. 2014, 88, 856–865. [Google Scholar] [CrossRef] [PubMed]
- McCartney, F.; Gleeson, J.P.; Brayden, D.J. Safety concerns over the use of intestinal permeation enhancers: A mini-review. Tissue Barriers 2016, 4, e1176822. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Mrsny, R.J.; Brayden, D.J. Intestinal permeation enhancers for oral peptide delivery. Adv. Drug Deliv. Rev. 2016, 106, 277–319. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Brayden, D.J. Overcoming poor permeability: translating permeation enhancers for oral peptide delivery. Drug Discov. Today Technol. 2012, 9, e113–e119. [Google Scholar] [CrossRef] [PubMed]
- Kapitza, C.; Zijlstra, E.; Heinemann, L.; Castelli, M.C.; Riley, G.; Heise, T. Oral insulin: a comparison with subcutaneous regular human insulin in patients with type 2 diabetes. Diabetes Care 2010, 33, 1288–1290. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, R.C.; Daley, D.N. Oral calcitonin. Int. J. Womens Health 2012, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Pineo, G.; Hull, R. Oral delivery of heparin: SNAC and related formulations. Best Pract. Res. Clin. Haematol. 2004, 17, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Sauerberg, P.; Bjerregaard, S.; Nielsen, F.S. Solid Compositions Comprising a GLP-1 Agonist and a Salt of N-(8-(2-Hydroxybenzoyl)amino) Caprylic Acid. U.S. Patent US20130345134, 16 September 2013. [Google Scholar]
- Holst, J.J.; Madsbad, S. Erratum to semaglutide seems to be more effective the other GLP-1Ras. Ann. Transl. Med. 2018, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Brayden, D.J.; Maher, S.; Bahar, B.; Walsh, E. Sodium caprate-induced increases in intestinal permeability and epithelial damage are prevented by misoprostol. Eur. J. Pharm. Biopharm. 2015, 94, 194–206. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.; Schmidt, J.; Castelli, M.C.; Bittner, B. Study on the impact of SNAC (sodium N-[8-(2-hydroxybenzoyl)amino]caprylate) on the bioavailability of ibandronate (IBN) in postmenopausal women. J. Drug Deliv. Sci. TEC 2011, 21, 521–525. [Google Scholar] [CrossRef]
- Nordisk A/S, N. Effect of Food on the Pharmacokinetics of Oral Semaglutide in Healthy Subjects. Available online: https://clinicaltrials.gov/ct2/show/NCT02172313 (accessed on 21 October 2014).
- Xiao, J.; Zhu, T.; Yin, Y.Z.; Sun, B. Notoginsenoside R1, a unique constituent of Panax notoginseng, blinds proinflammatory monocytes to protect against cardiac hypertrophy in ApoE-/- mice. Eur. J. Pharm. 2018, 833, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yan, L.; Li, X.; Zhang, Z.; Sun, Y.; Wang, J. Notoginsenoside R1 suppresses wear particle-induced osteolysis and RANKL mediated osteoclastogenesis in vivo and in vitro. Int. Immunopharmacol. 2017, 47, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Du, S.; Li, H.; Li, Z.; Xin, W.; Zhang, W. Notoginsenoside R1 inhibits oxidized low-density lipoprotein induced inflammatory cytokines production in human endothelial EA.hy926 cells. Eur. J. Pharmacol. 2016, 770, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Meng, X.; Wang, J.; Sun, J.; Ren, X.; Qin, M.; Sun, J.; Sun, G.; Sun, X. Notoginsenoside R1 attenuates amyloid-β-induced damage in neurons by inhibiting reactive oxygen species and modulating MAPK activation. Int. Immunopharmacol. 2014, 22, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Hsieh, S.; Hsieh, L.C.; Kuo, Y.H.; Hsieh, S.L. Regulation mechanism of Notoginsenoside R1 on human colorectal cancer metastasis. Eur. J. Cancer 2016, 61, S48. [Google Scholar] [CrossRef]
- Feng, L.; Jiang, X.H. Absorption profiles of sanchinoside R1 and ginsenoside Rg1 in the rat intestine. Eur. J. Drug Metab. Pharmacokinet. 2005, 30, 261–268. [Google Scholar]
- Ruan, J.Q.; Leong, W.I.; Yan, R.; Wang, Y.T. Characterization of metabolism and in vitro permeability study of notoginsenoside R1 from Radix notoginseng. J. Agric. Food Chem. 2010, 58, 5770–5776. [Google Scholar] [CrossRef] [PubMed]
- Trifana, A.; Opitz, S.E.W.; Josuran, R.; Grubelnikd, A.; Esslinger, N.; Peter, S.; Bräm, S.; Meier, N.; Wolfram, E. Is comfrey root more than toxic pyrrolizidine alkaloids? Salvianolic acids among antioxidant polyphenols in comfrey (Symphytum officinale L.) roots. Food Chem. Toxicol. 2018, 112, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; An, C.; Jia, L.; Li, Y.; Wang, M. Combination Therapy of Salvianolic Acid and Fluoxetine Improves the Cognitive Function of Rats with Chronic Stress-induced Depression. World Neurosurg. 2016, 86, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, W.; Pang, X.; Li, L.; Du, G. EETs mediate cardioprotection of salvianolic acids through MAPK signaling pathway. Acta Pharm. Sin. B 2013, 3, 25–31. [Google Scholar] [CrossRef]
- Feng, S.; Geng, J.; Sun, R.; Huang, J.; Wang, G. Protective Effect of Salvianolic Acid A on Brain Endothelial Cells after Treatment with Deprivation and Reperfusion of Oxygen-glucose. Chin. Herb. Med. 2017, 9, 335–343. [Google Scholar] [CrossRef]
- Dhapare, S.; Li, H.; Sakagami, M. Salvianolic acid B as an anti-emphysema agent II: In vivo reversal activities in two rat models of emphysema. Pulm. Pharmacol. Ther. 2018, 53, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Ye, S.; Cai, C.; Chen, J.; Zeng, C. Salvianolic acid a attenuates limb ischemia/reperfusion injury in skeletal muscle of rats. Biomed. Pharmacother. 2018, 97, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Cui, L.; Chen, R.; Li, P. Salvianolic Acids for Injection (SAFI) promotes functional recovery and neurogenesis via sonic hedgehog pathway after stroke in mice. Neurochem. Int. 2017, 110, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chow, M.S.S.; Zuo, Z. Effect of sodium caprate on the oral absorptions of danshensu and salvianolic acid B. Int. J. Pharm. 2009, 379, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, K.; Karr, N.; Mitragotri, S. Safe and effective permeation enhancers for oral drug delivery. Pharm. Res. 2008, 25, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, K.; Mitragotri, S. Mechanistic analysis of chemical permeation enhancers for oral drug delivery. Pharm. Res. 2008, 25, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, P.; Badarinath, A.V.; Naveen, N.; Prasad, K.; Ravi Shankar Reddy, B.; Hyndhavi, M. Rationalized description on study of intestinal barrier, drug permeability and permeation enhancers. J. Glob. Trends Pharm. Sci. 2011, 2, 431–449. [Google Scholar]
- Antunes, F.; Andrade, F.; Ferreira, D.; Nielson, H.M.; Sarmento, B. Models to predict intestinal absorption of therapeutic peptides and proteins. Curr. Drug. Metab. 2013, 14, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Al Kawas, S.; Rahim, Z.H.; Ferguson, D.B. Potential uses of human salivary protein and peptide analysis in the diagnosis of disease. Arch. Oral Biol. 2012, 57, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aungst, B.J. Absorption enhancers: applications and advances. AAPS J. 2011, 14, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Uehara, S.; Sawangrat, K.; Morishita, M.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Improvement of intestinal absorption of curcumin by cyclodextrins and the mechanisms underlying absorption enhancement. Int. J. Pharm. 2018, 535, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Zhu, C. Pharmacokinetics and correlation between in vitro release and in vivo absorption of bio-adhesive pellets of panax notoginseng saponins. Chin. J. Nat. Med. 2017, 15, 142–151. [Google Scholar] [CrossRef]
- Wang, J.; Zhai, W.; Yu, J.; Wang, J.; Dai, J. Preparation and Quality Evaluation of Salvianolic Acids and Tanshinones Dry Powder Inhalation. J. Pharm. Sci. 2018, 107, 2451–2456. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, Y.; Zhu, C. Study on in Vitro Release of Salvianolic Acid Bioadhesive Floating Pellets and Pharmacokinetics in Rats. Chin. J. Inf. TCM 2018, 25, 77–81. [Google Scholar]
- Miller, J.M.; Dahan, A.; Gupta, D.; Varghese, S.; Amidon, G.L. Enabling the intestinal absorption of highly polar anti-viral agents: Ion-pair facilitated membrane permeation of zanamivir heptyl ester and guanidino oseltamivir. Mol. Pharmaceut. 2010, 7, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

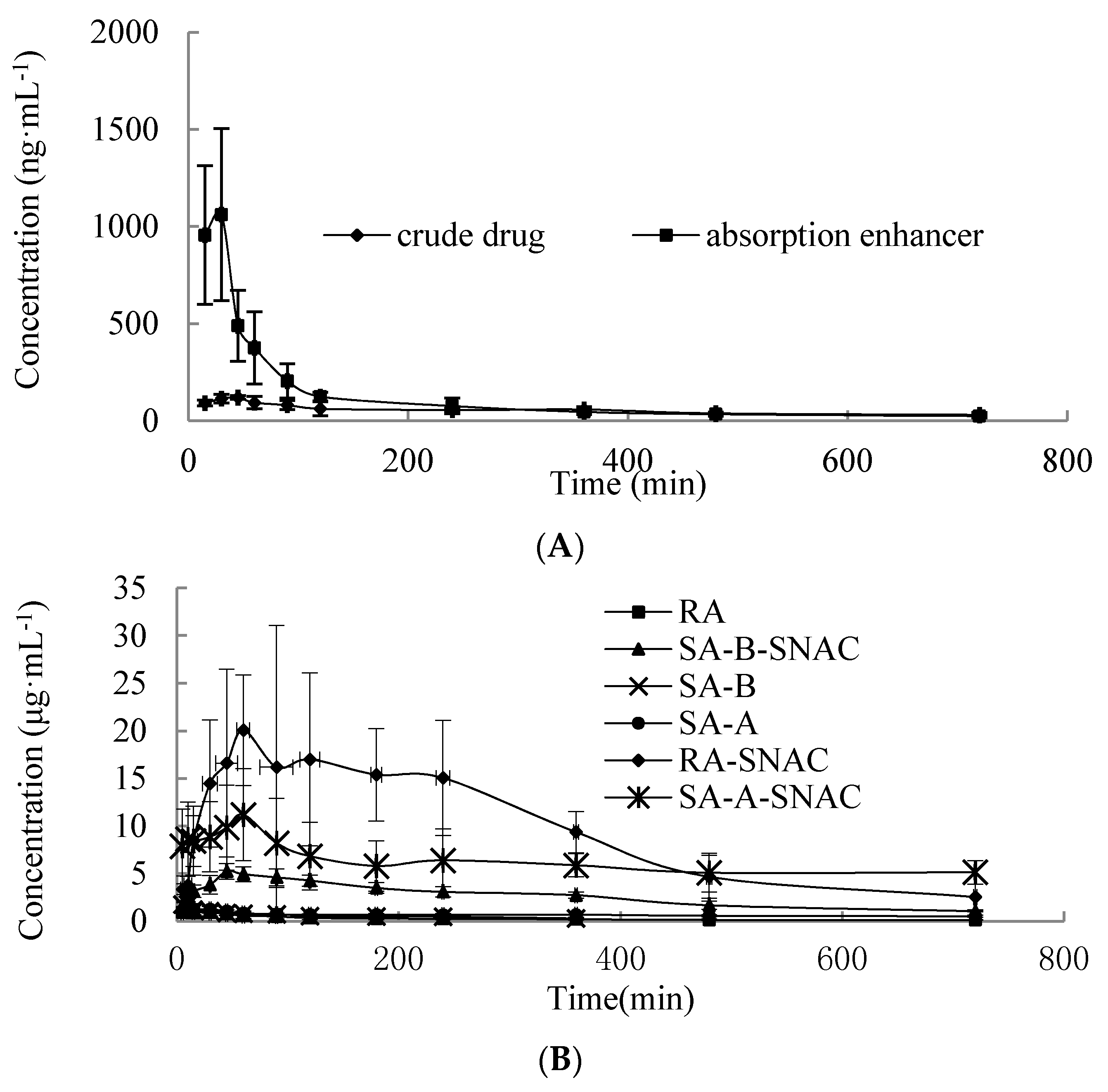
| Parameter | R1 | R1 containing SNAC |
|---|---|---|
| Cmax (ng·mL−1) | 119.40 ± 9.86 | 1061.40 ± 443.60 |
| Tmax (min) | 42.50 ± 11.29 | 20.00 ± 7.74 |
| AUC0→t/(ng/mL·min) | 37,991.01 ± 2746.71 | 84,930.61 ± 14,364.23 |
| Fr/% | 100 | 223.55 |
| Parameter | RA | RA Containing SNAC | SA-B | SA-B Containing SNAC | SA-A | SA-A Containing SNAC |
|---|---|---|---|---|---|---|
| Cmax (µg·mL−1) | 1.02 ± 0.51 | 20.06 ± 5.79 | 2.29 ± 0.77 | 5.62 ± 0.85 | 1.61 ± 0.44 | 11.19 ± 4.84 |
| Tmax (min) | 10 ± 5 | 63.75 ± 18.87 | 7.5 ± 2.74 | 57.5 ± 17.54 | 8.75 ± 2.5 | 67.5 ± 15 |
| AUC0→t/(µg/mL·min) | 193.79 ± 51.78 | 6835.35 ± 946.52 | 214.46 ± 37.86 | 1871.38 ± 424.47 | 478.54 ± 28.93 | 4416.15 ± 706.94 |
| Fr/% | 100 | 3527.21 | 100 | 872.30 | 100 | 922.83 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yang, D.; Zhu, C. Impact of Sodium N-[8-(2-Hydroxybenzoyl)amino]-caprylate on Intestinal Permeability for Notoginsenoside R1 and Salvianolic Acids in Caco-2 Cells Transport and Rat Pharmacokinetics. Molecules 2018, 23, 2990. https://doi.org/10.3390/molecules23112990
Li Y, Yang D, Zhu C. Impact of Sodium N-[8-(2-Hydroxybenzoyl)amino]-caprylate on Intestinal Permeability for Notoginsenoside R1 and Salvianolic Acids in Caco-2 Cells Transport and Rat Pharmacokinetics. Molecules. 2018; 23(11):2990. https://doi.org/10.3390/molecules23112990
Chicago/Turabian StyleLi, Ying, Dandan Yang, and Chunyan Zhu. 2018. "Impact of Sodium N-[8-(2-Hydroxybenzoyl)amino]-caprylate on Intestinal Permeability for Notoginsenoside R1 and Salvianolic Acids in Caco-2 Cells Transport and Rat Pharmacokinetics" Molecules 23, no. 11: 2990. https://doi.org/10.3390/molecules23112990
APA StyleLi, Y., Yang, D., & Zhu, C. (2018). Impact of Sodium N-[8-(2-Hydroxybenzoyl)amino]-caprylate on Intestinal Permeability for Notoginsenoside R1 and Salvianolic Acids in Caco-2 Cells Transport and Rat Pharmacokinetics. Molecules, 23(11), 2990. https://doi.org/10.3390/molecules23112990





