Non-Linear Enthalpy-Entropy Correlation for Nitrogen Adsorption in Zeolites
Abstract
:1. Introduction
2. Outline of the VTIR Method
3. Experimental Protocol
4. Results and Discussion
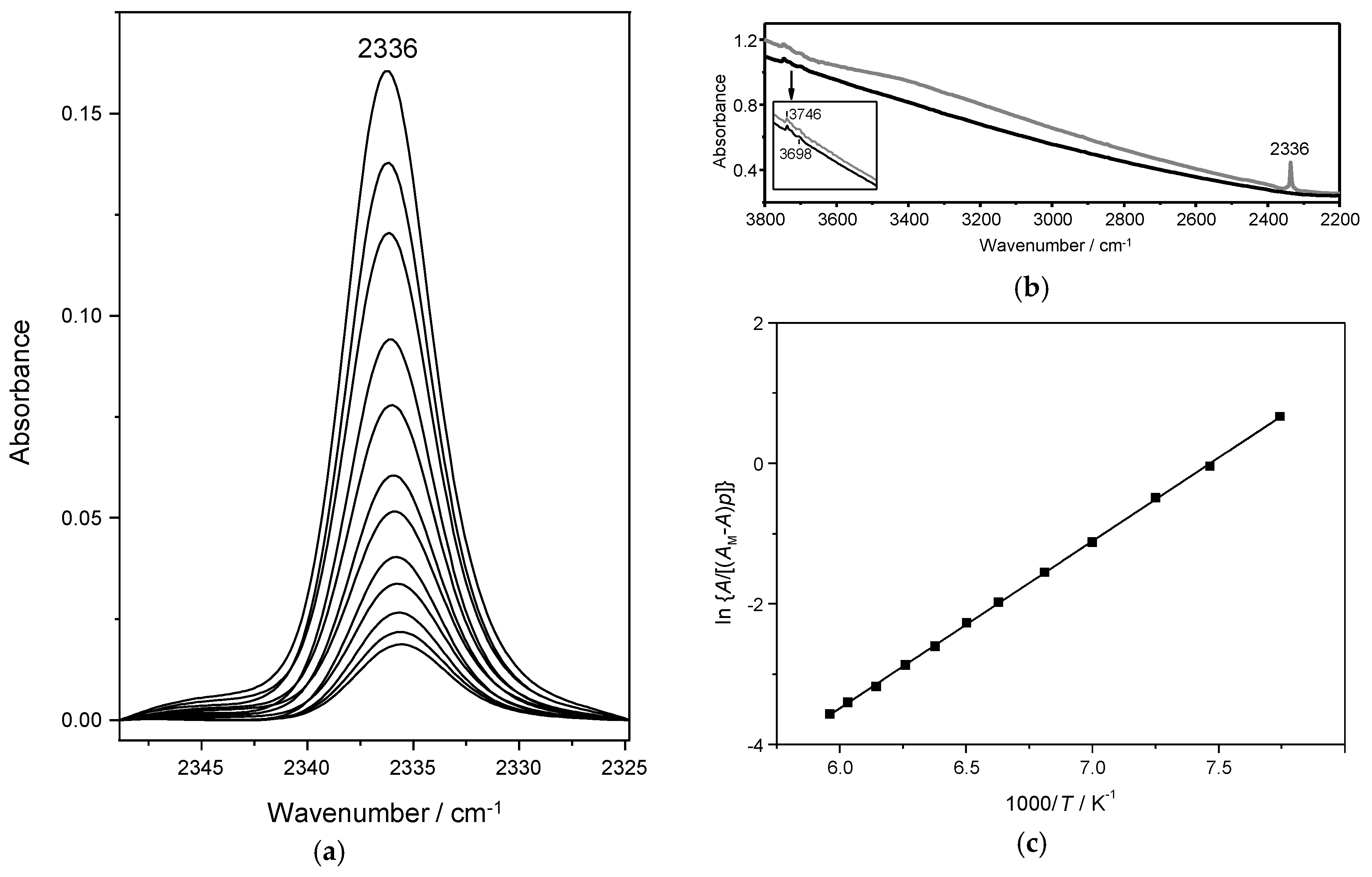
4.1. Dinitrogen Adsorption in Na-Y
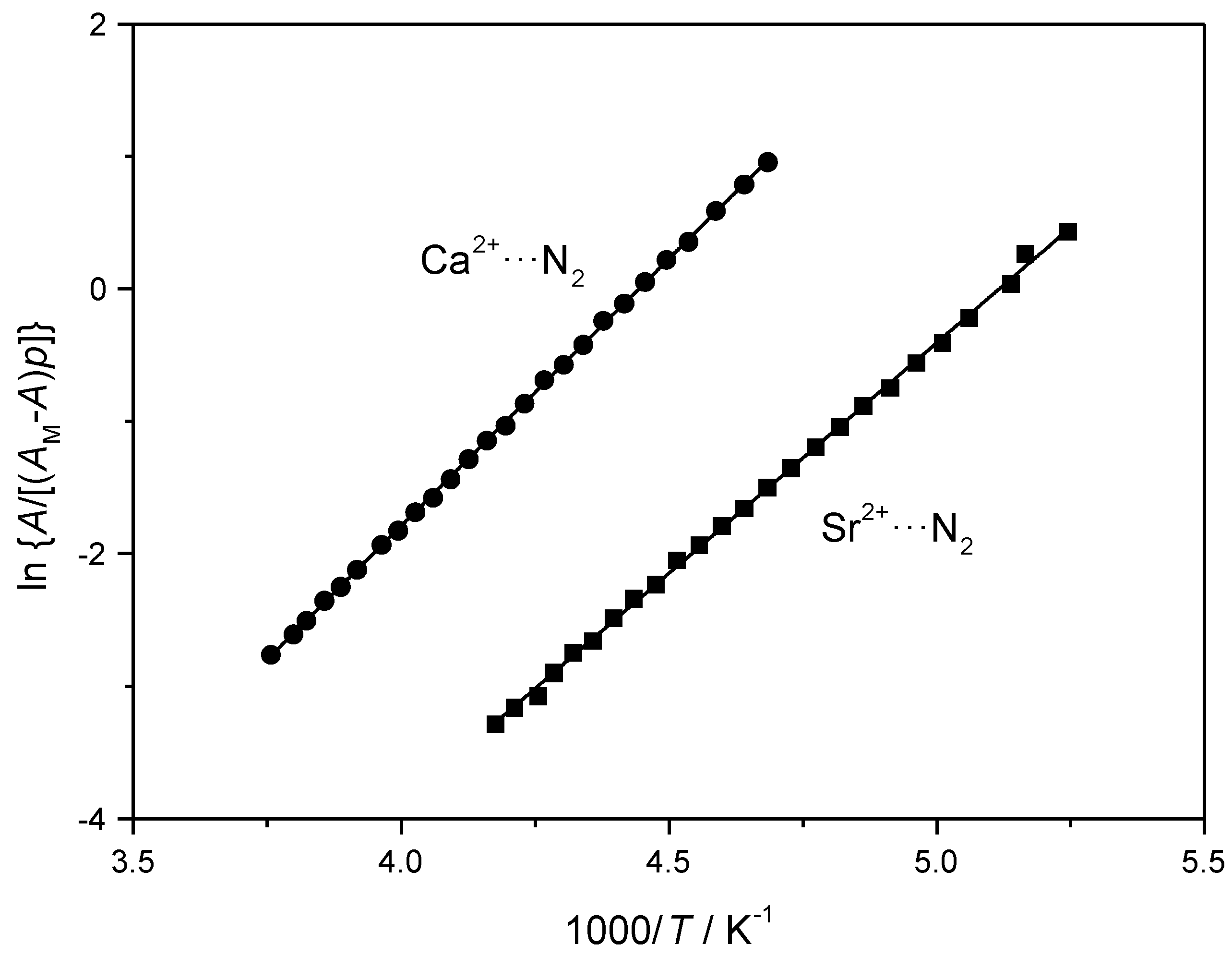
4.2. Dinitrogen Adsorption in Ca-Y and Sr-Y
Author Contributions
Funding
Conflicts of Interest
References
- Nandi, S.P.; Walker, P.L., Jr. Separation of Oxygen and Nitrogen Using 5A Zeolite and Carbon Molecular Sieves. Sep. Sci. 1976, 11, 441–453. [Google Scholar] [CrossRef]
- Mofarahi, M.; Towfighi, J.; Fathi, L. Oxygen separation from air by four-bed pressure swing adsorption. Ind. Eng. Chem. Res. 2009, 48, 5439–5444. [Google Scholar] [CrossRef]
- Hamed, H.H. Oxygen separation from air using zeolite type 5A. Int. J. Sci. Eng. Res. 2015, 6, 597–602. [Google Scholar]
- Rufford, T.E.; Smart, S.; Watson, G.C.Y.; Graham, B.F.; Boxall, J.; Diniz da Costa, J.C.; May, E.F. The removal of CO2 and N2 from natural gas: A review of conventional and emerging process technologies. J. Petrol. Sci. Technol. 2012, 94, 123–154. [Google Scholar] [CrossRef]
- Shah, M.S.; Tsapatsis, M.; Siepman, J.I. Identifying optimal zeolitic sorbents for sweetening of highly sour natural gas. Angew. Chem. Int. Ed. 2016, 55, 5938–5942. [Google Scholar] [CrossRef] [PubMed]
- Bae, T.H.; Hudson, M.R.; Mason, J.A.; Queen, W.L.; Dutton, J.J.; Sumida, K.; Micklash, K.J.; Kaye, S.S.; Browns, C.M.; Long, J.R. Evaluation of cations exchanged zeolite adsorbents for post-combustion carbon dioxide capture. Energy Environ. Sci. 2013, 6, 128–138. [Google Scholar] [CrossRef]
- Thang, H.V.; Grajciar, L.; Nachtigall, P.; Bludsky, O.; Arean, C.O.; Frydova, E.; Bulanek, R. Adsorption of CO2 in FAU zeolites: Effect of zeolite composition. Catal. Today 2014, 227, 50–56. [Google Scholar] [CrossRef]
- Pham, T.D.; Hudson, M.R.; Brown, C.M.; Lobo, R.F. On the structure-property relationships of cation-exchanged ZK-5 zeolites for CO2 adsorption. ChemSusChem. 2017, 10, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Alvarez, R.; Calero, S. Highly selective zeolite topologies for flue gas separation. Chem. Eur. J. 2016, 52, 18705–18708. [Google Scholar] [CrossRef] [PubMed]
- Pulido, A.; Delgado, M.R.; Bludsky, O.; Nachtigall, P.; Arean, C.O. Combined DFT/CC and IR spectroscopic studies on carbon dioxide adsorption on the zeolite H-FER. Energy Environ. Sci. 2009, 2, 1187–1195. [Google Scholar] [CrossRef]
- Ackley, M.W.; Rege, S.V.; Saxena, H. Application of natural zeolites in the purification and separation of gases. Microporous Mesoporous Mater. 2003, 61, 25–42. [Google Scholar] [CrossRef]
- Armandi, M.; Bonelli, B.; Bottero, I.; Arean, C.O.; Garrone, E. Synthesis and characterization of ordered porous carbons with potential applications as hydrogen storage media. Microporous Mesoporous Mater. 2007, 103, 150–157. [Google Scholar] [CrossRef]
- Arean, C.O.; Groppo, E.; Liu, W.; Velasco, L.F.; Parra, J.B. Carbon dioxide and nitrogen adsorption on porous copolymers of divinylbenzene and acrylic acid. Adsorption 2013, 19, 367–372. [Google Scholar] [CrossRef]
- Boots, H.M.J.; Boks, P.K. Theory of enthalpy-entropy compensation. J. Phys. Chem. 1989, 93, 8240–8243. [Google Scholar] [CrossRef]
- Pan, A.; Biswas, T.; Rakshit, A.K.; Moulik, S.P. Enthalpy-entropy compensation (EEC) effect: A revisit. J. Phys. Chem. B 2015, 119, 15876–15884. [Google Scholar] [CrossRef] [PubMed]
- Arean, C.O.; Bonelli, B.; Delgado, M.R.; Garrone, E. Hydrogen storage via physisorption: the combined role of adsorption enthalpy and entropy. Turk. J. Chem. 2009, 33, 599–606. [Google Scholar]
- Garrone, E.; Bonelli, B.; Arean, C.O. Enthalpy-entropy correlation for hydrogen adsorption on zeolites. Chem. Phys. Lett. 2008, 456, 68–70. [Google Scholar]
- Arean, C.O.; Manoilova, O.V.; Palomino, G.T.; Delgado, M.R.; Tsyganenko, A.A.; Bonelli, B.; Garrone, E. Variable-temperature infrared spectroscopy. An access to adsorption thermodynamics of weakly interacting systems. Phys. Chem. Chem. Phys. 2002, 4, 5713–5715. [Google Scholar] [CrossRef]
- Garrone, E.; Arean, C.O. Variable temperature infrared spectroscopy: A convenient tool for studying the thermodynamics of weak solid-gas interactions. Chem. Soc. Rev. 2005, 34, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Arean, C.O. Probing Brønsted acidity of protonic zeolites with variable-temperature infrared spectroscopy. Ukr. J. Phys. 2018, 63, 538–545. [Google Scholar] [CrossRef]
- Tsyganenko, A.A.; Storozhev, P.Y.; Arean, C.O. Infrared spectroscopic studies on the binding isomerism of adsorbed molecules. Kinet. Catal. 2004, 45, 530–540. [Google Scholar] [CrossRef]
- Garrone, E.; Delgado, M.R.; Bonelli, B.; Arean, C.O. Probing gas adsorption in zeolites by variable-temperature IR spectroscopy: An overview of current research. Molecules 2017, 22, 1557. [Google Scholar] [CrossRef] [PubMed]
- Chakarova, K.; Andonova, S.; Hadjiivanov, K. FTIR study of CO and N2 adsorption on [Ge]FAU zeolites in their Na- and H-forms. Microporous Mesoporous Mater. 2016, 220, 188–197. [Google Scholar] [CrossRef]
- Yamazaki, T.; Watanuki, I.; Ozawa, S.; Ogino, Y. Infrared spectra of nitrogen adsorbed on ion-exchanged ZSM-5 zeolite. Bull. Chem. Soc. Jpn. 1988, 61, 1039–1044. [Google Scholar] [CrossRef]
- Smudde, G.H.; Slager, T.L.; Coe, C.G.; MacDougall, J.E.; Weigel, S.J. DRIFTS and Raman investigation of N2 and O2 adsorption on zeolites at ambient temperature. Appl. Spectrosc. 1995, 49, 1747–1755. [Google Scholar] [CrossRef]
- Zecchina, A.; Arean, C.O. Diatomic molecular probes for mid-IR studies of zeolites. Chem. Soc. Rev. 1996, 25, 187–197. [Google Scholar] [CrossRef]
- Bulanin, K.M.; Lobo, R.F.; Bulanin, M.O. Low-temperature adsorption of N2, O2, and D2 on LiX, NaX and NaLiX zeolites studied by FT-IR spectroscopy. J. Phys. Chem. B 2000, 104, 1269–1276. [Google Scholar] [CrossRef]
- Koubi, L.; Blain, M.; Cohen de Lara, E.; Leclercq, J.M. Vibrational frequency shifts of diatomic molecules in interaction with a Na+ cation by ab-initio calculations—comparison with experiment on H2 and N2 adsorbed in Na-A zeolite. Chem. Phys. Lett. 1994, 217, 544–550. [Google Scholar] [CrossRef]
- Cohen de Lara, E. Electric field effect on molecules: Relation between the orientation of the molecule with respect to the field and the vibrational frequency shift observed in IR spectra of molecules adsorbed in zeolites. Phys. Chem. Chem. Phys. 1999, 1, 501–505. [Google Scholar] [CrossRef]
- Arean, C.O. Dinitrogen and carbon monoxide hydrogen bonding in protonic zeolites: Studies from variable-temperature infrared spectroscopy. J. Mol. Struct. 2008, 880, 31–37. [Google Scholar] [CrossRef]
- Nachtigall, P.; Bludsky, O.; Grajciar, L.; Nachtigallova, D.; Delgado, M.R.; Arean, C.O. Computational and FTIR spectroscopic studies on carbon monoxide and dinitrogen adsorption on a high-silica H-FER zeolite. Phys. Chem. Chem. Phys. 2009, 11, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Arean, C.O.; Delgado, M.R.; Nachtigall, P.; Thang, H.V.; Rubes, M.; Bulanek, R.; Chlubna-Eliasova, P. Measuring the Brønsted acid strength of zeolites: does it correlate with the O-H frequency shift probed by a weak base? Phys. Chem. Chem. Phys. 2014, 21, 10129–10141. [Google Scholar] [CrossRef] [PubMed]
- Hercigonja, R.; Rac, V.; Rakic, V.; Auroux, A. Enthalpy-entropy compensation for n-hexane adsorption on HZSM-5 containing transition metal ions. J. Chem. Thermodyn. 2012, 48, 112–117. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


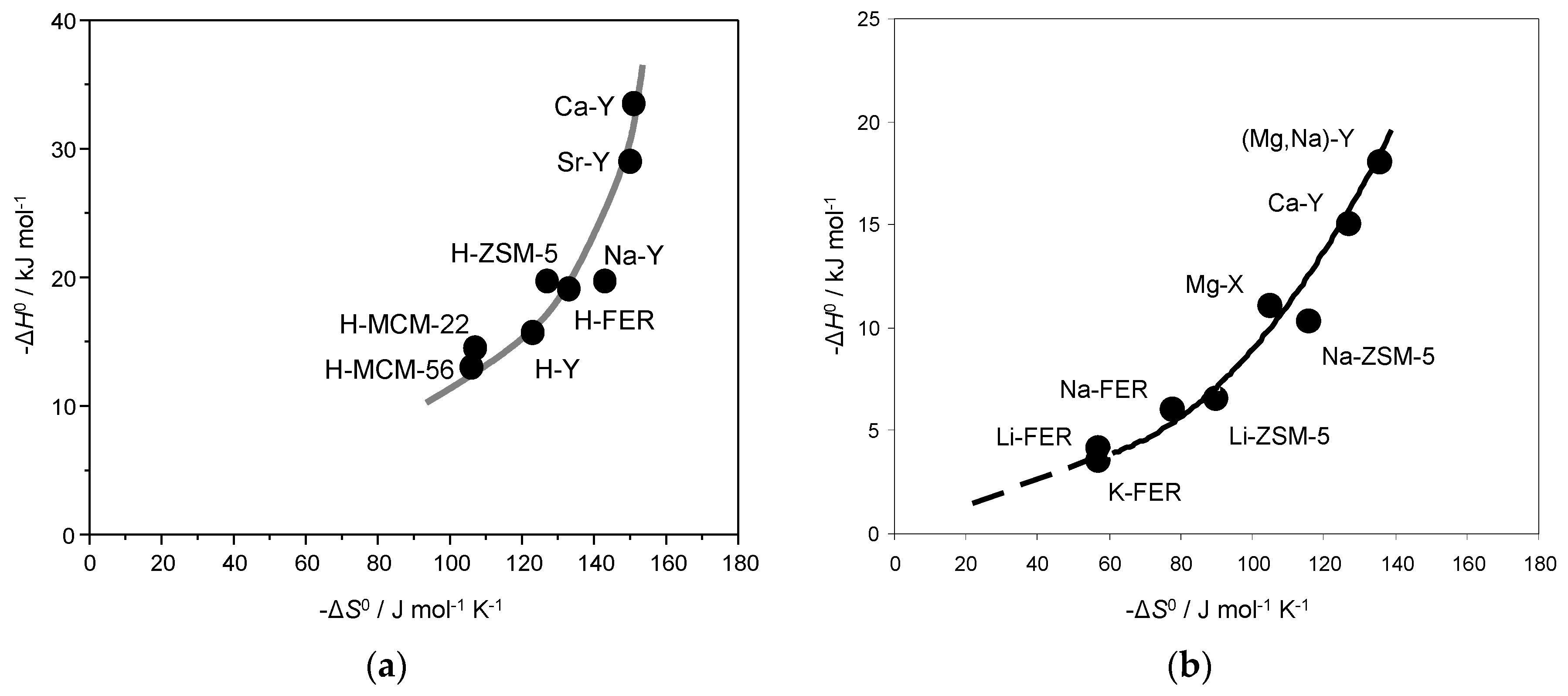
| Zeolite | −ΔH0 (kJ mol−1) | −ΔS0 b (J mol−1 K−1) | Ref |
|---|---|---|---|
| H-MCM-56 | 13 | 106 | [32] |
| H-MCM-22 | 14.5 | 107 | [32] |
| H-Y | 15.7 | 123 | [30] |
| H-FER | 19.1 | 133 | [30,31] |
| H-ZSM-5 | 19.7 | 127 | [30] |
| Na-Y | 19.7 | 143 | This work |
| Sr-Y | 29 | 150 | This work |
| Ca-Y | 33.5 | 151 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado, M.R.; Arean, C.O. Non-Linear Enthalpy-Entropy Correlation for Nitrogen Adsorption in Zeolites. Molecules 2018, 23, 2978. https://doi.org/10.3390/molecules23112978
Delgado MR, Arean CO. Non-Linear Enthalpy-Entropy Correlation for Nitrogen Adsorption in Zeolites. Molecules. 2018; 23(11):2978. https://doi.org/10.3390/molecules23112978
Chicago/Turabian StyleDelgado, Montserrat R., and Carlos O. Arean. 2018. "Non-Linear Enthalpy-Entropy Correlation for Nitrogen Adsorption in Zeolites" Molecules 23, no. 11: 2978. https://doi.org/10.3390/molecules23112978
APA StyleDelgado, M. R., & Arean, C. O. (2018). Non-Linear Enthalpy-Entropy Correlation for Nitrogen Adsorption in Zeolites. Molecules, 23(11), 2978. https://doi.org/10.3390/molecules23112978






