Phenolic Profiles, Antioxidant Capacities, and Inhibitory Effects on Digestive Enzymes of Different Kiwifruits
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of Different Kiwifruits
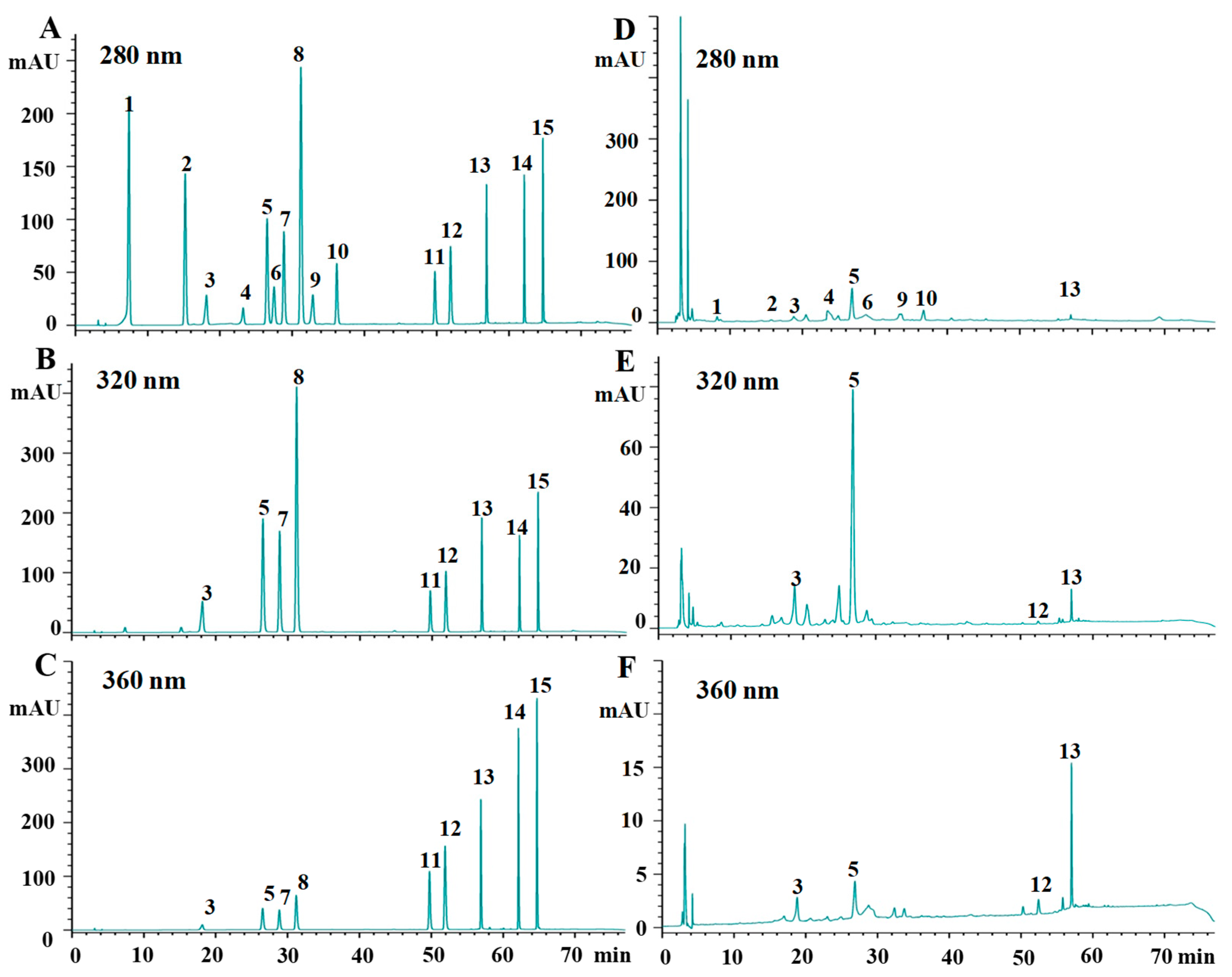
2.2. Phenolic Profiles of Different Kiwifruits
2.3. Antioxidant Capacities of Different Kiwifruits
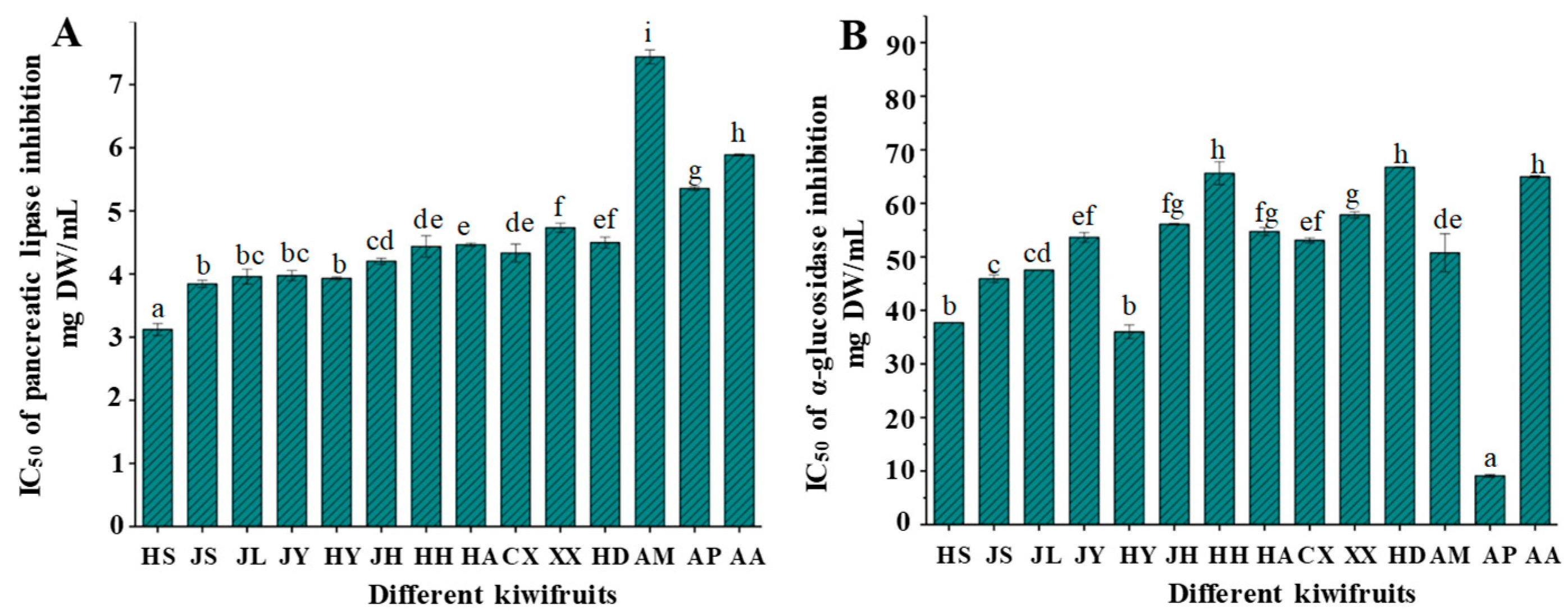
2.4. Inhibitory Effects on Digestive Enzymes of Different Kiwifruits
3. Materials and Methods
3.1. Samples and Chemicals
3.2. Determination of Dry Matter, Soluble Solids Content (SSC), and Ascorbic Acid
3.3. Extraction of Phenolic Compounds
3.4. Determination of Total Phenolic Content
3.5. HPLC Analysis of Individual Phenolic Compounds
3.6. Determination of Antioxidant Capacities of Kiwifruit Extract
3.6.1. ABTS Radical Cation Scavenging Capacity
3.6.2. DPPH Radical Scavenging Capacity
3.6.3. Ferric-Reducing Antioxidant Power (FRAP)
3.7. Pancreatic Lipase and α-Glucosidase Inhibition of Kiwifruit Extract
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Du, G.; Li, M.; Ma, F.; Liang, D. Antioxidant capacity and the relationship with polyphenol and Vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Zuo, L.L.; Wang, Z.Y.; Fan, Z.L.; Tian, S.Q.; Liu, J.R. Evaluation of antioxidant and antiproliferative properties of three Actinidia (Actinidia kolomikta, Actinidia arguta, Actinidia chinensis) extracts in vitro. Int. J. Mol. Sci. 2012, 13, 5506–5518. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.; Kucharska, A.Z.; Sokol-Letowska, A.; Fecka, I. Comparison of polyphenol content and antioxidant capacity of strawberry fruit from 90 cultivars of Fragaria × ananassa Duch. Food Chem. 2019, 270, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Boudaoud, H.; Debbache, N.; Berboucha, M.; Atmani, D.; Chaher, N.; Atmani, D. Flavonoids in Human Health: From Structure to Biological Activity. Curr. Nutr. Food Sci. 2009, 5, 225–237. [Google Scholar]
- Ariza, M.T.; Reboredo-Rodriguez, P.; Cervantes, L.; Soria, C.; Martinez-Ferri, E.; Gonzalez-Barreiro, C.; Cancho-Grande, B.; Battino, M.; Simal-Gandara, J. Bioaccessibility and potential bioavailability of phenolic compounds from achenes as a new target for strawberry breeding programs. Food Chem. 2018, 248, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.C.; Joshipura, K.J.; Jiang, R.; Hu, F.B.; Hunter, D.; Smithwarner, S.A.; Colditz, G.A.; Rosner, B.; Spiegelman, D.; Willett, W.C. Fruit and Vegetable Intake and Risk of Major Chronic Disease. J. Natl. Cancer Inst. 2004, 96, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- D’Evoli, L.; Moscatello, S.; Lucarini, M.; Aguzzi, A.; Gabrielli, P.; Proietti, S.; Battistelli, A.; Famiani, F.; Böhm, V.; Lombardi-Boccia, G. Nutritional traits and antioxidant capacity of kiwifruit (Actinidia deliciosa Planch., cv. Hayward) grown in Italy. J. Food Compos. Anal. 2015, 37, 25–29. [Google Scholar] [CrossRef]
- Podsedek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziolkiewicz, M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Liu, Q.; Tsao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, I. Fruits of the Actinidia Genus. Adv. Food Nutr. Res. 2007, 52, 293–324. [Google Scholar] [PubMed]
- Latocha, P.; Jankowski, P.; Radzanowska, J. Genotypic difference in postharvest characteristics of hardy kiwifruit (Actinidia arguta and its hybrids), as a new commercial crop Part I. Sensory profiling and physicochemical differences. Food Res. Int. 2011, 44, 1936–1945. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Oliveras, M.J.; Quesada, J.; Rufián-Henares, J.A.; Pastoriza, S. Relationship between composition and bioactivity of persimmon and kiwifruit. Food Res. Int. 2018, 105, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Wojdylo, A.; Nowicka, P.; Oszmianski, J.; Golis, T. Phytochemical compounds and biological effects of Actinidia fruits. J. Funct. Food 2017, 30, 194–202. [Google Scholar] [CrossRef]
- Ciacci, C.; Russo, I.; Bucci, C.; Iovino, P.; Pellegrini, L.; Giangrieco, I.; Tamburrini, M.; Ciardiello, M.A. The kiwi fruit peptide kissper displays anti-inflammatory and anti-oxidant effects in in-vitro and ex-vivo human intestinal models. Clin. Exp. Immunol. 2014, 175, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.V.; Quek, S.Y.; Stevenson, R.J.; Winz, R.A. Kiwifruit flavour: A review. Trends Food Sci. Technol. 2012, 24, 82–91. [Google Scholar] [CrossRef]
- Ma, T.; Sun, X.; Zhao, J.; You, Y.; Lei, Y.; Gao, G.; Zhan, J. Nutrient compositions and antioxidant capacity of kiwifruit (Actinidia) and their relationship with flesh color and commercial value. Food Chem. 2017, 218, 294–304. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Nihal de Silva, H.; Requejo-Tapia, C.; Roger Harker, F. Evaluation of softening characteristics of fruit from 14 species of Actinidia. Postharvest Biol. Technol. 2005, 35, 143–151. [Google Scholar] [CrossRef]
- Burdon, J.; Lallu, N.; Pidakala, P.; Barnett, A. Soluble solids accumulation and postharvest performance of ‘Hayward’ kiwifruit. Postharvest Biol. Technol. 2013, 80, 1–8. [Google Scholar] [CrossRef]
- Ercisli, S.; Orhan, E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Krupa, T.; Latocha, P.; Liwińska, A. Changes of physicochemical quality, phenolics and vitamin C content in hardy kiwifruit (Actinidia arguta and its hybrid) during storage. Sci. Hortic. 2011, 130, 410–417. [Google Scholar] [CrossRef]
- Lim, Y.J.; Oh, C.S.; Park, Y.D.; Eom, S.H.; Kim, D.O.; Kim, U.J.; Cho, Y.S. Physiological components of kiwifruits with in vitro antioxidant and acetylcholinesterase inhibitory activities. Food Sci. Biotechnol. 2014, 23, 943–949. [Google Scholar] [CrossRef]
- Park, Y.S.; Namiesnik, J.; Vearasilp, K.; Leontowicz, H.; Leontowicz, M.; Barasch, D.; Nemirovski, A.; Trakhtenberg, S.; Gorinstein, S. Bioactive compounds and the antioxidant capacity in new kiwi fruit cultivars. Food Chem. 2014, 165, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Leontowicz, H.; Leontowicz, M.; Latocha, P.; Jesion, I.; Park, Y.S.; Katrich, E.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Bioactivity and nutritional properties of hardy kiwi fruit Actinidia arguta in comparison with Actinidia deliciosa ‘Hayward’ and Actinidia eriantha ‘Bidan’. Food Chem. 2016, 196, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yuan, Y.; Dou, P.; Yue, T. Multivariate statistical analysis of the polyphenolic constituents in kiwifruit juices to trace fruit varieties and geographical origins. Food Chem. 2017, 232, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Beppu, K.; Kataoka, I. Varietal differences in phenolic content and astringency in skin and flesh of hardy kiwifruit resources in Japan. Sci. Hortic. 2009, 120, 551–554. [Google Scholar] [CrossRef]
- Dawes, H.M.; Keene, J.B. Phenolic Composition of Kiwifruit Juice. J. Agric. Food Chem. 1999, 47, 2398–2403. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Suhaj, M.; Cvikrová, M.; Martincová, O.; Weisz, M.; Gorinstein, S. Comparison of the contents of bioactive compounds and the level of antioxidant activity in different kiwifruit cultivars. J. Food Compos. Anal. 2011, 24, 963–970. [Google Scholar] [CrossRef]
- Juárez-Trujillo, N.; Monribot-Villanueva, J.L.; Alvarado-Olivarez, M.; Luna-Solano, G.; Guerrero-Analco, J.A.; Jiménez-Fernández, M. Phenolic profile and antioxidative properties of pulp and seeds of Randia monantha Benth. Ind. Crop. Prod. 2018, 124, 53–58. [Google Scholar] [CrossRef]
- Moreno, D.A.; Ilic, N.; Poulev, A.; Brasaemle, D.L.; Fried, S.K.; Raskin, I. Inhibitory effects of grape seed extract on lipases. Nutrition 2003, 19, 876–879. [Google Scholar] [CrossRef]
- McDougall, G.J.; Kulkarni, N.N.; Stewart, D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009, 115, 193–199. [Google Scholar] [CrossRef]
- Sakulnarmrat, K.; Konczak, I. Composition of native Australian herbs polyphenolic-rich fractions and in vitro inhibitory activities against key enzymes relevant to metabolic syndrome. Food Chem. 2012, 134, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Sergent, T.; Vanderstraeten, J.; Winand, J.; Beguin, P.; Schneider, Y.J. Phenolic compounds and plant extracts as potential natural anti-obesity substances. Food Chem. 2012, 135, 68–73. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Samoticha, J. Evaluation of phytochemicals, antioxidant capacity, and antidiabetic activity of novel smoothies from selected Prunus fruits. J. Funct. Food. 2016, 25, 397–407. [Google Scholar] [CrossRef]
- Ishikawa, A.; Yamashita, H.; Hiemori, M.; Inagaki, E.; Kimoto, M.; Okamoto, M.; Tsuji, H.; Memon, A.N.; Mohammadi, A.; Natori, Y. Characterization of inhibitors of postprandial hyperglycemia from the leaves of Nerium indicum. J. Nutr. Sci. Vitaminol. 2007, 53, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.N.; Liu, H.F.; Zheng, J.B.; Fan, M.T.; Cao, W. Distribution of phenolic acids in different tissues of jujube and their antioxidant activity. J. Agric. Food Chem. 2011, 59, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Guo, H.; Gong, J.D.B.; Lu, M.; Lu, M.Y.; Wang, L.; Zhang, Q.; Qin, W.; Wu, D.T. Phenolic profiles, β-glucan contents, and antioxidant capacities of colored Qingke (Tibetan hulless barley) cultivars. J. Cereal Sci. 2018, 81, 69–75. [Google Scholar] [CrossRef]
- Almeida, D.; Pinto, D.; Santos, J.; Vinha, A.F.; Palmeira, J.; Ferreira, H.N.; Rodrigues, F.; Oliveira, M. Hardy kiwifruit leaves (Actinidia arguta): An extraordinary source of value-added compounds for food industry. Food Chem. 2018, 259, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Shen, Q.; Li, L.Q.; Huang, Y.Q.; Cheung, H.Y. Phytochemical profiles, antioxidant activities of functional herb Abrus cantoniensis and Abrus mollis. Food Chem. 2015, 177, 304–312. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of raw materials of different kiwifruits are available from the authors. |
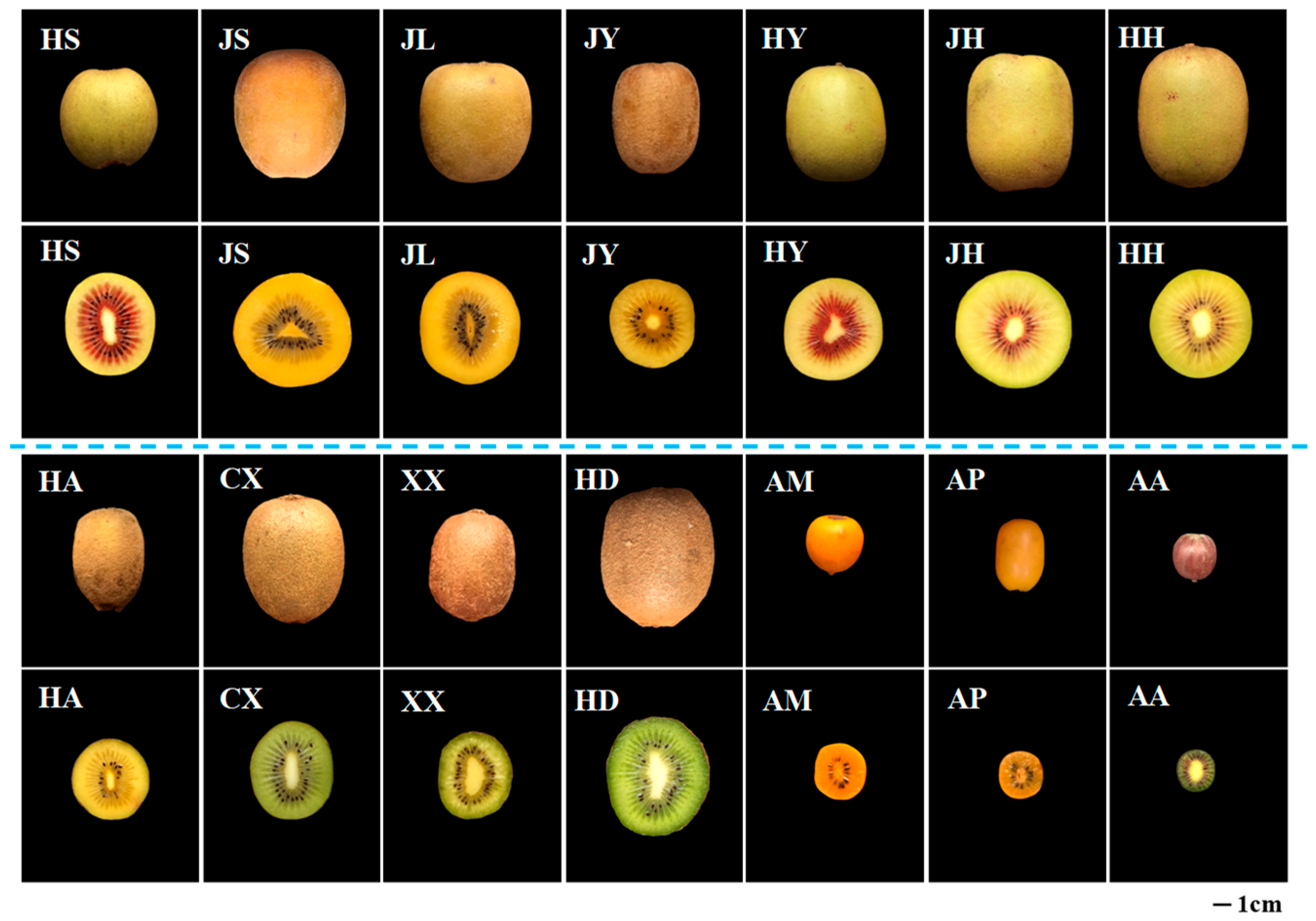
| Samples | Codes | Harvest Date | Regions | Flesh Color | Fruit Weight (g) | SSC (%) | Dry Matter (%) | Ascorbic Acid (mg/100 g FW) |
|---|---|---|---|---|---|---|---|---|
| A. chinensis | ||||||||
| Hongshi | HS | 28 September 2017 | Mianzhu, Sichuan, China | Green, and red (middle part) | 58.07 ± 2.17 g | 16.3 ± 0.1 b | 16.49 ± 0.18 e | 390.68 ± 4.24 a |
| Jinshi | JS | 15 October 2017 | Mianzhu, Sichuan, China | Yellow | 107.19 ± 1.48 c | 16.1 ± 0.1 b,c | 16.55 ± 0.05 e | 235.30 ± 8.27c |
| Jinlong | JL | 2 November 2017 | Xianyang, Shaanxi, China | Yellow | 118.25 ± 7.41 b | 16.1 ± 0.1 c | 15.88 ± 0.10 f,g | 335.06 ± 10.39 b |
| Jinyan | JY | 26 October 2017 | Chengdu, Sichuan, China | Yellow | 88.47 ± 5.30 e | 13.1 ± 0.1 i | 12.45 ± 0.01 j | 97.27 ± 3.59 g |
| Hongyang | HY | 28 September 2017 | Cangxi, Sichuan, China | Green, and red (middle part) | 95.57 ± 10.22 d | 17.6 ± 0.1 a | 18.33 ± 0.09 b | 107.56 ± 1.34 f |
| Jinhong | JH | 4 September 2017 | Dayi, Sichuan, China | Green, and red (middle part) | 133.74 ± 5.66 a | 16.1 ± 0.1 c | 16.63 ± 0.06 e | 53.42 ± 1.78 j,k |
| Honghua | HH | 8 September 2017 | Pujiang, Sichuan, China | Green, and red (middle part) | 107.80 ± 7.74 c | 14.3 ± 0.1 f | 15.75 ± 0.04 f,g | 61.45 ± 1.22 i |
| Hort16A | HA | 26 October 2017 | Chengdu, Sichuan, China | Yellow | 47.98 ± 4.49 h | 15.8 ± 0.3 d | 15.67 ± 0.02 g | 129.10 ± 3.24 e |
| A. deliciosa | ||||||||
| Cuixing | CX | 27 October 2017 | Xianyang, Shaanxi, China | Green | 78.53 ± 3.16 f | 16.2 ± 0.1 b,c | 17.54 ± 0.05 c | 169.54 ± 2.88 d |
| Xuxiang | XX | 26 October 2017 | Chengdu, Sichuan, China | Green | 59.90 ± 3.53 g | 13.7± 0.1 f,h | 14.53 ± 0.09 h | 84.38 ± 1.44 h |
| Hayward | HD | 8 August 2017 | Xianyang, Shaanxi, China | Green | 113.77 ± 8.65 b | 13.6 ± 0.1 h | 13.48 ± 0.02 i | 59.32 ± 1.89 i,j |
| A. macrosperma | AM | 24 August 2017 | Pujiang, Sichuan, China | Orange | 12.79 ± 0.89 i | 13.9 ± 0.1 g,f | 15.97 ± 0.36 f | 61.06 ± 2.91 i |
| A. polygama | AP | 24 August 2017 | Pujiang, Sichuan, China | Orange | 14.31 ± 1.25 i | 14.0 ± 0.1 g | 17.08 ± 0.15 d | 85.94 ± 0.94 h |
| A. arguta | AA | 24 August 2017 | Pujiang, Sichuan, China | Green | 7.10 ± 0.37 j | 15.4 ± 0.1 e | 20.35 ± 0.31 a | 51.32 ± 0.42 k |
| Code | TPC (mg GAE/g DW) | ABTS (μmol Trolox/g DW) | DPPH (μmol Trolox/g DW) | FRAP (μmol Trolox/g DW) |
|---|---|---|---|---|
| HS | 16.52 ± 0.26 a | 160.36 ± 6.15 a | 87.38 ± 4.32 a | 149.97 ± 6.98 a |
| JS | 13.38 ± 0.20 b | 117.90 ± 2.09 b | 71.60 ± 4.04 b | 142.58 ± 3.72 b |
| JL | 11.02 ± 0.05 c | 90.78 ± 1.29 c | 60.14 ± 2.07 c | 120.04 ± 1.82 c |
| JY | 6.71 ± 0.12 e | 47.92 ± 1.64 g | 26.46 ± 0.66 e | 54.69 ± 1.48 d |
| HY | 6.22 ± 0.21 f | 62.93 ± 1.31 e | 22.97 ± 0.53 f | 52.73 ± 1.75 d |
| JH | 5.29 ± 0.10 h | 38.16 ± 1.03 i | 13.12 ± 0.07 h | 25.42 ± 0.77 f |
| HH | 4.70 ± 0.10 i | 42.68 ± 1.24 h | 13.55 ± 0.73 h | 29.03 ± 0.47 f |
| HA | 6.60 ± 0.14 e | 50.14 ± 0.24 g | 26.88 ± 0.09 e | 53.84 ± 2.06 d |
| CX | 6.06 ± 0.13 f | 55.71 ± 1.32 f | 28.10 ± 2.01 e | 54.11 ± 0.58 d |
| XX | 5.29 ± 0.10 h | 39.52 ± 0.56 h,i | 19.54 ± 0.71 g | 37.75 ± 1.67 e |
| HD | 3.75 ± 0.09 j | 32.95 ± 0.29 j | 15.67 ± 0.42 h | 26.43 ± 0.97 f |
| AM | 8.15 ± 0.19 d | 84.07 ± 0.61 d | 39.69 ± 1.85 d | 54.75 ± 0.37 d |
| AP | 5.57 ± 0.12 g | 64.71 ± 1.40 e | 21.47 ± 0.17 f,g | 38.18 ± 0.77 e |
| AA | 4.71 ± 0.18 i | 55.81 ± 0.71 f | 14.08 ± 0.20 h | 27.24 ± 0.41 f |
| Compounds | Regression Equation | Linear Range (μg/mL) | R2 | LOD (μg/mL) |
|---|---|---|---|---|
| gallic acid | Y = 54.107X − 8.928 | 0.50–10.00 | 0.9998 | 0.16 |
| protocatechuic acid | Y = 28.455X + 0.213 | 0.78–10.00 | 1.0000 | 0.26 |
| neochlorogenic acid | Y = 74.454X − 4.940 | 0.22–40.00 | 1.0000 | 0.07 |
| procyanidin B1 | Y = 13.806X − 11.478 | 2.78–250.00 | 1.0000 | 0.92 |
| chlorogenic acid | Y = 71.270X − 3.391 | 0.10–40.00 | 1.0000 | 0.30 |
| (+)-catechin | Y = 16.756X − 9.765 | 2.00–40.00 | 0.9999 | 0.70 |
| cryptochlorogenic acid | Y = 59.701X + 0.326 | 0.19–1.50 | 1.0000 | 0.06 |
| caffeic acid | Y = 102.38X − 5.749 | 0.30–12.00 | 0.9999 | 0.10 |
| procyanidin B2 | Y = 14.637X − 2.1539 | 0.71–50.00 | 0.9999 | 0.23 |
| (−)-epicatechin | Y = 20.575X + 0.264 | 0.50–25.00 | 1.0000 | 0.16 |
| rutin | Y = 30.337X − 6.2411 | 1.00–10.00 | 0.9998 | 0.03 |
| quercetin-3-O-glucoside | Y = 39.956X − 3.1422 | 0.44–20.00 | 0.9998 | 0.12 |
| quercetin-3-rhamnoside | Y = 34.673X − 8.953 | 0.75–15.00 | 0.9993 | 0.25 |
| quercetin | Y = 67.113X − 22.838 | 0.80–8.00 | 0.9992 | 0.26 |
| kaempferol | Y = 94.982X − 16.021 | 0.33–10.00 | 0.9998 | 0.11 |
| Code | Flavan-3-ols (μg/g DW) | Phenolic Acids (μg/g DW) | Flavonols (μg/g DW) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PB1 | PB2 | EC | Ca | GA | PA | CHL | NCHL | CA | QRha | QGlu | Ru | |
| HS | 446.81 ± 1.51 a | 182.11 ± 1.16 a | 162.61 ± 0.99 a | 32.08 ± 0.35 c | 24.98 ± 0.19 d | 15.76 ± 0.18 a | 235.75 ± 5.44 a | 39.26 ± 1.41 c | n.d | 41.94 ± 1.44 a | 6.16 ± 0.05 c | n.d |
| JS | 201.69 ± 2.28 b | n.d | 60.70 ± 1.07 b | n.d | 53.76 ± 0.43 a | 6.55 ± 0.12 b | 78.32 ± 1.12 f | 107.06 ± 1.74 b | n.d | 4.59 ± 0.09 g | n.d | n.d |
| JL | 145.68 ± 3.12 f | 20.91 ± 0.53 h | 38.98 ± 0.94 e | n.d | 26.69 ± 0.45 c | n.d | 55.22 ± 0.48 h | 133.72 ± 3.98 a | n.d | n.d | n.d | n.d |
| JY | 195.99 ± 4.29 c | 125.38 ± 2.00 c | 43.41 ± 0.98 c | n.d | 49.13 ± 0.27 b | n.d | 121.41 ± 0.92 c | 6.29 ± 0.11 f | n.d | 4.73 ± 0.11 g | n.d | n.d |
| HY | 132.33 ± 1.33 g | 78.62 ± 1.18 e | 45.60 ± 0.97 c | n.d | 14.62 ± 0.16 g | n.d | 97.22 ± 1.60 e | 15.86 ± 1.04 e | n.d | 21.57 ± 1.08 c | n.d | n.d |
| JH | 188.54 ± 1.73 d | n.d | 22.97 ± 0.15 h | n.d | 21.25 ± 0.35 f | n.d | 184.15 ± 1.97 b | 8.27 ± 0.14 f | n.d | 9.16 ± 0.21 f | n.d | n.d |
| HH | 155.11 ± 2.32 e | n.d | n.d | n.d | 12.17 ± 0.25 h | n.d | 37.78 ± 1.19 i | 6.53 ± 0.17 f | n.d | 7.88 ± 0.04 f | n.d | n.d |
| HA | 203.68 ± 4.27 b | 114.95 ± 1.84 d | 60.56 ± 1.77 b | n.d | 22.68 ± 0.30 e | n.d | 103.65 ± 1.47 d | 17.78 ± 1.53 d,e | n.d | n.d | n.d | n.d |
| CX | 64.60 ± 1.33 k | 17.88 ± 0.66 h | 13.60 ± 0.30 i | n.d | 5.64 ± 0.13 k | n.d | 20.94 ± 0.61 k | n.d | n.d | 16.19 ± 0.53 e | n.d | n.d |
| XX | 118.58 ± 1.72 h | 146.28 ± 2.65 b | 27.96 ± 0.63 g | 14.71 ± 0.59 d | 9.12 ± 0.08 j | n.d | 75.25 ± 2.52 f | n.d | n.d | 32.25 ± 0.60 b | 5.10 ± 0.12 c | n.d |
| HD | 91.03 ± 1.44 j | 45.54 ± 0.87 g | n.d | n.d | 9.74 ± 0.13 i | n.d | 60.77 ± 0.49 g | n.d | n.d | 8.55 ± 0.17 f | n.d | n.d |
| AM | 107.98 ± 1.53 i | 112.74 ± 2.19 d | 31.48 ± 1.60 f | 28.72 ± 1.43 c | n.d | n.d | 27.12 ± 1.07 j | n.d | 40.97 ± 1.15 a | 8.69 ± 0.15 f | n.d | 17.58 ± 0.58 b |
| AP | n.d | 81.42 ± 0.98 e | 26.12 ± 0.63 g | 43.30 ± 2.47 b | 9.90 ± 0.09 i | 12.19 ± 0.24 c | 63.50 ± 0.89 g | n.d | 2.69 ± 0.03 c | 16.43 ± 0.41d,e | 108.60 ± 0.92 a | 48.23 ± 0.38 a |
| AA | 58.72 ± 0.74 l | 65.17 ± 0.45 f | n.d | 132.08 ± 1.16 a | n.d | n.d | 7.70 ± 0.04 l | 21.14 ± 1.11 d | 22.56 ± 0.90 b | 17.63 ± 0.59 d | 11.02 ± 0.20 b | 8.27 ± 0.08 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-Y.; Yuan, Q.; Yang, Y.-L.; Han, Q.-H.; He, J.-L.; Zhao, L.; Zhang, Q.; Liu, S.-X.; Lin, D.-R.; Wu, D.-T.; et al. Phenolic Profiles, Antioxidant Capacities, and Inhibitory Effects on Digestive Enzymes of Different Kiwifruits. Molecules 2018, 23, 2957. https://doi.org/10.3390/molecules23112957
Li H-Y, Yuan Q, Yang Y-L, Han Q-H, He J-L, Zhao L, Zhang Q, Liu S-X, Lin D-R, Wu D-T, et al. Phenolic Profiles, Antioxidant Capacities, and Inhibitory Effects on Digestive Enzymes of Different Kiwifruits. Molecules. 2018; 23(11):2957. https://doi.org/10.3390/molecules23112957
Chicago/Turabian StyleLi, Hong-Yi, Qin Yuan, Yu-Ling Yang, Qiao-Hong Han, Jing-Liu He, Li Zhao, Qing Zhang, Shu-Xiang Liu, De-Rong Lin, Ding-Tao Wu, and et al. 2018. "Phenolic Profiles, Antioxidant Capacities, and Inhibitory Effects on Digestive Enzymes of Different Kiwifruits" Molecules 23, no. 11: 2957. https://doi.org/10.3390/molecules23112957
APA StyleLi, H.-Y., Yuan, Q., Yang, Y.-L., Han, Q.-H., He, J.-L., Zhao, L., Zhang, Q., Liu, S.-X., Lin, D.-R., Wu, D.-T., & Qin, W. (2018). Phenolic Profiles, Antioxidant Capacities, and Inhibitory Effects on Digestive Enzymes of Different Kiwifruits. Molecules, 23(11), 2957. https://doi.org/10.3390/molecules23112957









