An Intelligent Nanoscale Insulin Delivery System
Abstract
:1. Introduction
2. Results
2.1. Identification of Nanoparticles
2.2. Characterization of Nanoparticles
2.3. Glucose Response Study
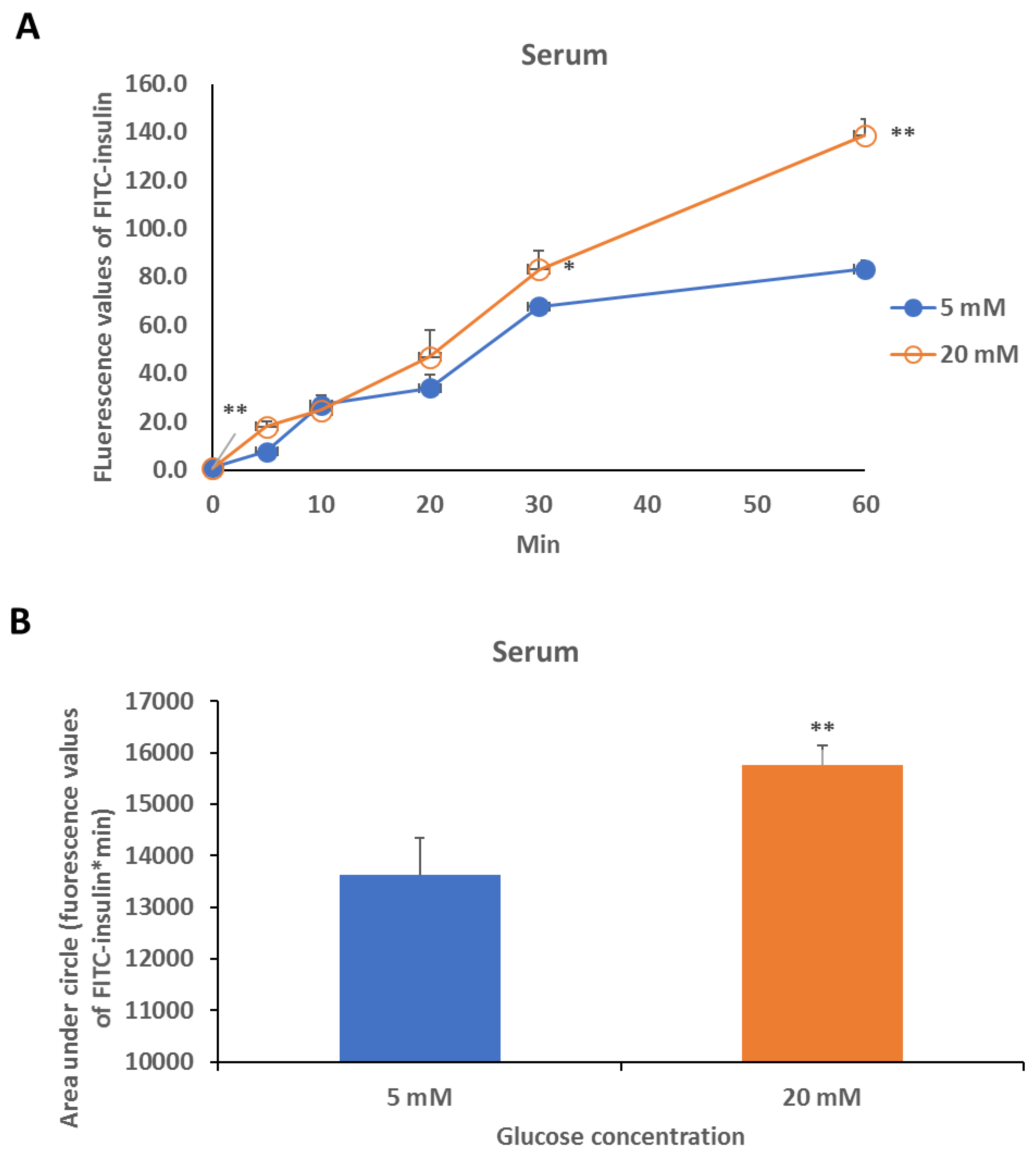
2.4. Safety and Effect Evaluation
2.5. Biocompatibility Tests
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Polymer
4.2.1. Synthesis of PAM-PBLA-NH2
4.2.2. Synthesis of PAM-PBLA-PEG
4.2.3. Synthesis of PAM-PAsp-b-PEG
4.2.4. Synthesis of PAM-PAspPBA-b-PEG
4.3. Preparation of FITC-Insulin Loaded Micelles
4.4. Characterization of Micelles
4.4.1. 1H-NMR
4.4.2. TEM Measurements
4.4.3. DLS measurements
4.4.4. Insulin Release Study
4.4.5. Safety and Effect Evaluation in Animals
4.5. Cytotoxicity and Inflammatory Factor Assays
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.E. Optimizing the treatment of type 2 diabetes using current and future insulin technologies. Medsurg Nurs. 2008, 17, 383–390. [Google Scholar] [PubMed]
- Kusunoki, Y.; Katsuno, T.; Nakae, R.; Watanabe, K.; Akagami, T.; Ochi, F.; Tokuda, M.; Murai, K.; Miuchi, M.; Miyagawa, J.; et al. Evaluation of blood glucose fluctuation in Japanese patients with type 1 diabetes mellitus by self-monitoring of blood glucose and continuous glucose monitoring. Diabetes Res. Clin. Pract. 2015, 108, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.H.; Sapir, T. Methods for insulin delivery and glucose monitoring in diabetes: Summary of a comparative effectiveness review. J. Manag Care Pharm. 2012, 18, 1–17. [Google Scholar] [CrossRef]
- Guo, X.; Wang, W. Challenges and recent advances in the subcutaneous delivery of insulin. Expert Opin. Drug Deliv. 2017, 14, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ma, R.; Ren, J.; Li, Z.; Zhang, H.; Zhang, Z.; An, Y.; Shi, L. A glucose-responsive complex polymeric micelle enabling repeated on–off release and insulin protection. Soft Matter 2013, 9, 1636–1644. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, S. Responsive materials for self-regulated insulin delivery. Macromol. Biosci. 2013, 13, 1464–1477. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xiao, C.; Wang, L.; Gai, G.; Ding, J. Glucose-sensitive polymer nanoparticles for self-regulated drug delivery. Chem. Commun. 2016, 52, 7633–7652. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ma, R.; Liu, G.; Li, Y.; Liu, X.; An, Y.; Shi, L. Glucose-responsive micelles from self-assembly of poly(ethylene glycol)-b-poly(acrylic acid-co-acrylamidophenylboronic acid) and the controlled release of insulin. Langmuir 2009, 25, 12522–12528. [Google Scholar] [CrossRef] [PubMed]
- Markowicz-Piasecka, M.; Luczak, E.; Chalubinski, M.; Broncel, M.; Mikiciuk-Olasik, E.; Sikora, J. Studies towards biocompatibility of PAMAM dendrimers—overall hemostasis potential and integrity of the human aortic endothelial barrier. Int. J. Pharm. 2014, 473, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Aljayyoussi, G.; Mansour, O.; Griffiths, P.; Gumbleton, M. Endocytic uptake, transport and macromolecular interactions of anionic PAMAM dendrimers within lung tissue. Pharm. Res. 2017, 34, 2517–2531. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Gao, H.; Zuo, Y.; Zeng, X.; Tao, W.; Tsai, H.I.; Mei, L. DACHPt-loaded unimolecular micelles based on hydrophilic dendritic block copolymers for enhanced therapy of lung cancer. ACS Appl. Mater. Interfaces 2017, 9, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.J.; Golden, S.H. Hypoglycemia in non-critically ill, hospitalized patients with diabetes: Evaluation, prevention, and management. Hosp. Pract. (1995) 2013, 41, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Adachi, H.; Oshima, S.; Kurabayashi, M. Glucose fluctuation and the resultant endothelial injury are correlated with pancreatic beta cell dysfunction in patients with coronary artery disease. Diabetes Res. Clin. Pract. 2017, 131, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Zhang, S.; Jiang, X.; Xie, W. Novel synergic antidiabetic effects of astragalus polysaccharides combined with crataegus flavonoids via improvement of islet function and liver metabolism. Mol. Med. Rep. 2016, 13, 4737–4744. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, W.; Zhong, J.; Lei, F.; Xu, N.; Zhang, Y.; Xie, W. Canagliflozin exerts anti-inflammatory effects by inhibiting intracellular glucose metabolism and promoting autophagy in immune cells. Biochem. Pharm. 2018, 152, 45–59. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: The sample of the compound PAM-PAspPBA-b-PEG is available from the authors. |







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Liao, L.; Zhang, X.; Lei, F.; Zhang, Y.; Liu, G.; Xie, W. An Intelligent Nanoscale Insulin Delivery System. Molecules 2018, 23, 2945. https://doi.org/10.3390/molecules23112945
Wang W, Liao L, Zhang X, Lei F, Zhang Y, Liu G, Xie W. An Intelligent Nanoscale Insulin Delivery System. Molecules. 2018; 23(11):2945. https://doi.org/10.3390/molecules23112945
Chicago/Turabian StyleWang, Wei, Ling Liao, Xiaobing Zhang, Fan Lei, Yaou Zhang, Gan Liu, and Weidong Xie. 2018. "An Intelligent Nanoscale Insulin Delivery System" Molecules 23, no. 11: 2945. https://doi.org/10.3390/molecules23112945
APA StyleWang, W., Liao, L., Zhang, X., Lei, F., Zhang, Y., Liu, G., & Xie, W. (2018). An Intelligent Nanoscale Insulin Delivery System. Molecules, 23(11), 2945. https://doi.org/10.3390/molecules23112945





