New Photodegradation Products of the Fungicide Fluopyram: Structural Elucidation and Mechanism Identification
Abstract
1. Introduction
2. Results and Discussion
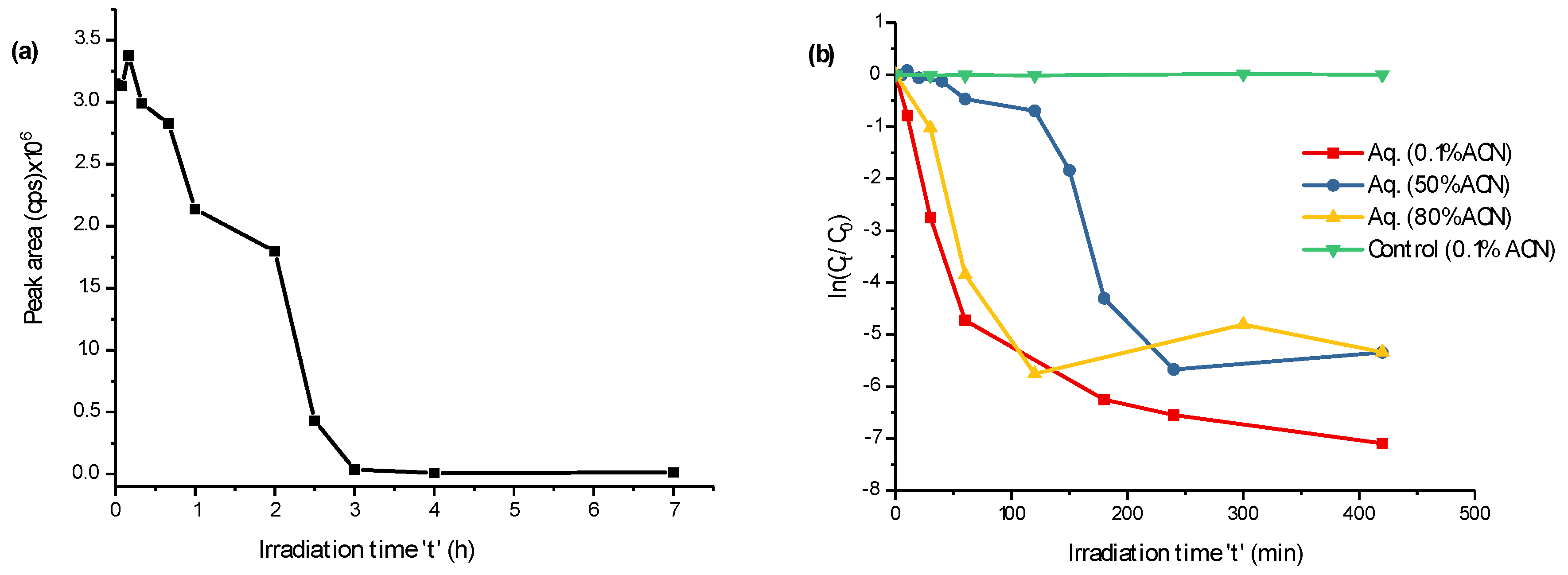
2.1. Photodegradation Properties of FLP and Its PPs
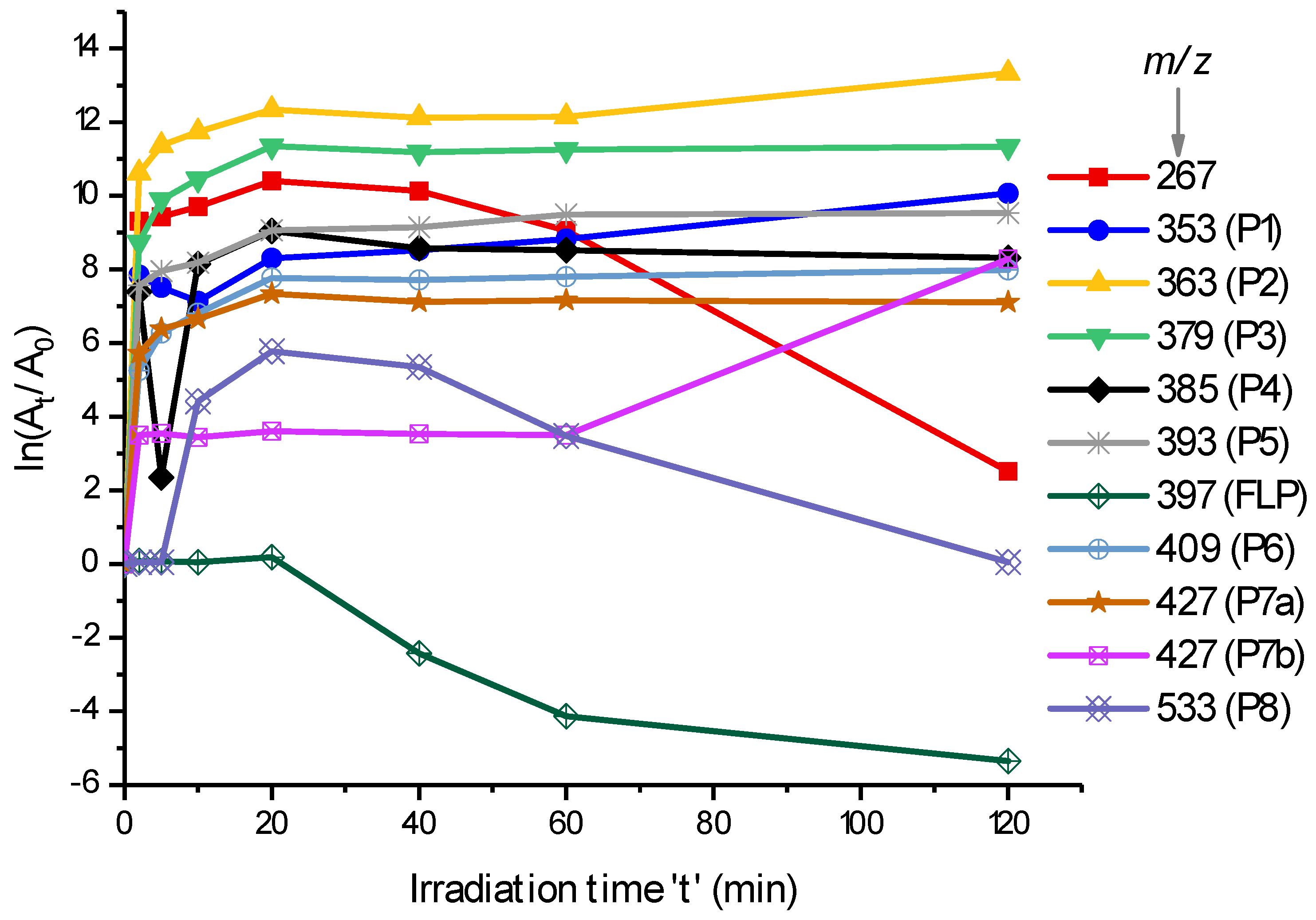
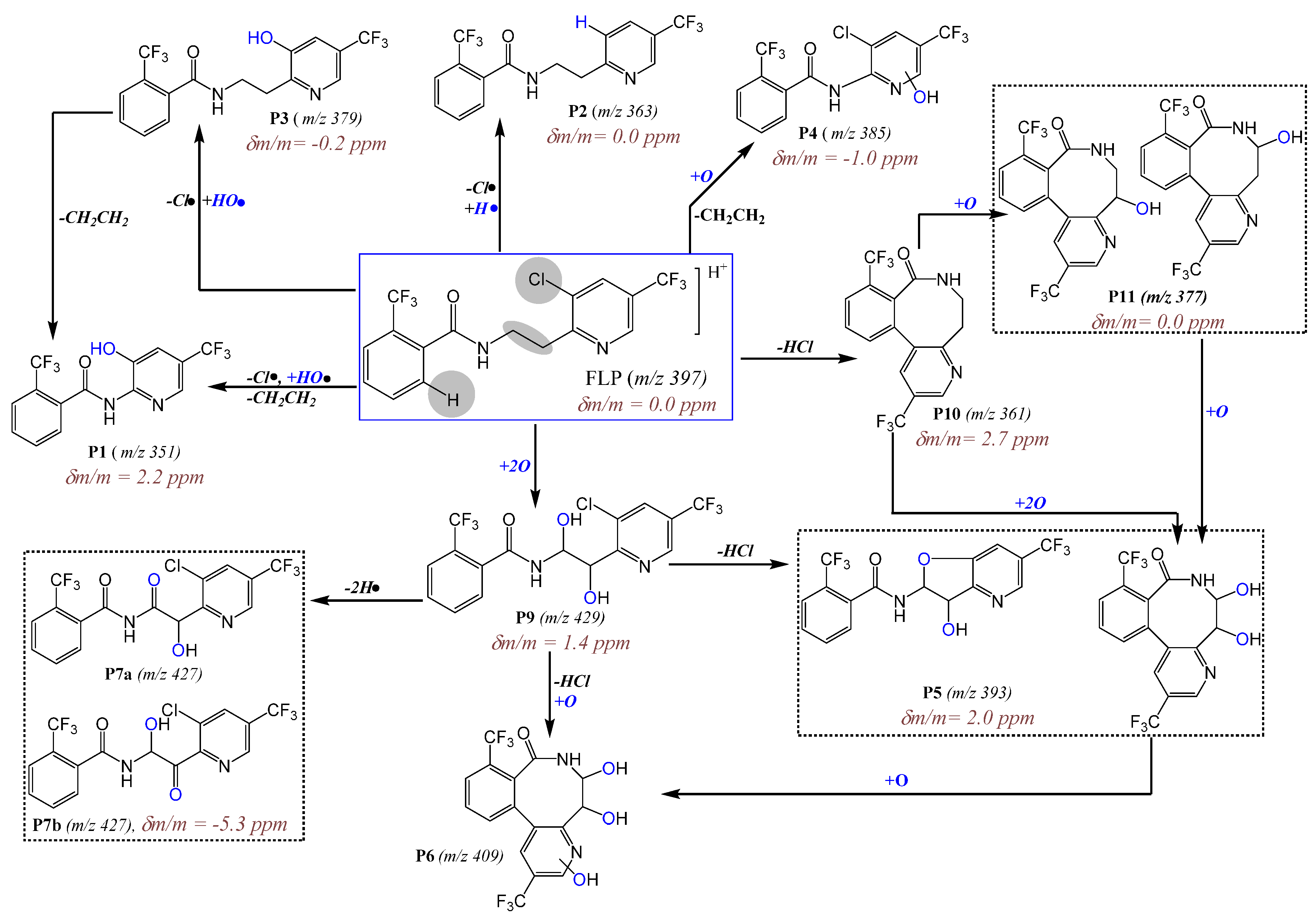
2.2. Identification of Dechlorinated PPs
2.3. LC-MS/MS Analysis of Hydroxyl FLP PPs
2.4. LC-MS/MS Analysis of Hydroxyl Lactam FLP PPs
3. Materials and Methods
3.1. Chemicals
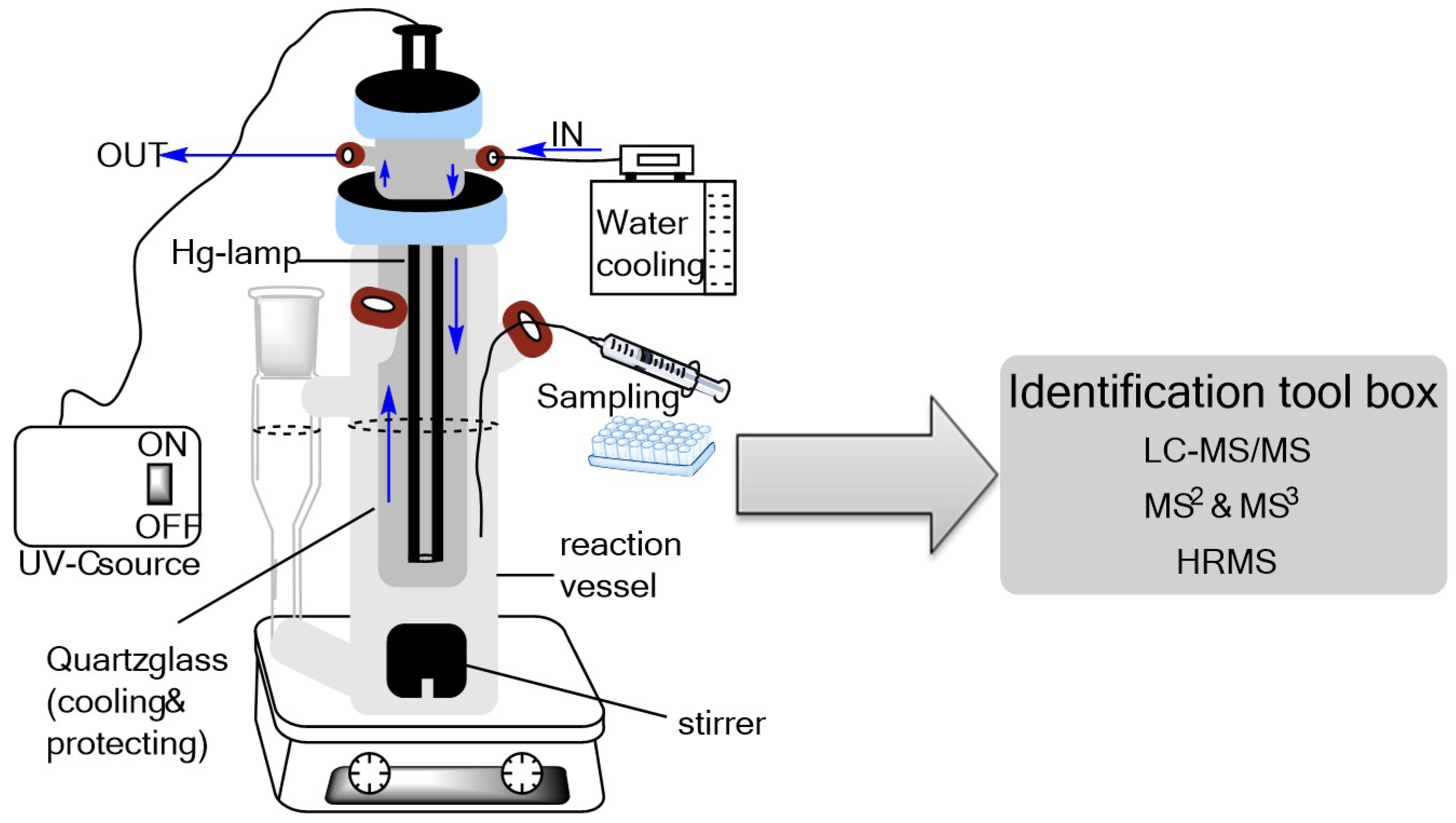
3.2. Photodegradation
3.3. LC-MS/MS Analysis of PPs
3.4. Confirmation by HRMS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liao, C.; Kim, U.J.; Kannan, K. A review of environmental occurrence, fate, exposure, and toxicity of benzothiazoles. Environ. Sci. Technol. 2018, 52, 5007–5026. [Google Scholar] [CrossRef] [PubMed]
- Podbielska, M.; Szpyrka, E.; Piechowicz, B.; Zwolak, A.; Sadlo, S. Behavior of fluopyram and tebuconazole and some selected pesticides in ripe apples and consumer exposure assessment in the applied crop protection framework. Environ. Monit. Assess. 2017, 189, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Scherr, K.E.; Bielska, L.; Kosubova, P.; Dinisova, P.; Hvezdova, M.; Simek, Z.; Hofman, J. Occurrence of chlorotriazine herbicides and their transformation products in arable soils. Environ. Pollut. 2017, 222, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.M.; Lester, Y.; Spangler, E.K.; von Gunten, U.; Linden, K.G. Uv/H2O2 advanced oxidation for abatement of organophosphorous pesticides and the effects on various toxicity screening assays. Chemosphere 2017, 182, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Marie, L.; Sylvain, P.; Benoit, G.; Maurice, M.; Gwenael, I. Degradation and transport of the chiral herbicide s-metolachlor at the catchment scale: Combining observation scales and analytical approaches. Environ. Sci. Technol. 2017, 51, 13231–13240. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Liang, Y.; Liu, D.; Liu, C.; Liu, H.; Wang, P.; Zhou, Z. Organochlorine pesticide acetofenate and its hydrolytic metabolite in rabbits: Enantioselective metabolism and cytotoxicity. Pestic. Biochem. Physiol. 2018, 145, 76–83. [Google Scholar] [CrossRef] [PubMed]
- von Gunten, U. Oxidation processes in water treatment: Are we on track? Environ. Sci. Technol. 2018, 52, 5062–5075. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Huang, L. Stereoselective bioaccumulation, transformation, and toxicity of triadimefon in scenedesmus obliquus. Chirality 2017, 29, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Chiaia-Hernandez, A.C.; Keller, A.; Wachter, D.; Steinlin, C.; Camenzuli, L.; Hollender, J.; Krauss, M. Long-term persistence of pesticides and TPs in archived agricultural soil samples and comparison with pesticide application. Environ. Sci. Technol. 2017, 51, 10642–10651. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M. Fate of pesticides in the environment and its bioremediation. Eng. Life Sci. 2005, 5, 497–526. [Google Scholar] [CrossRef]
- Mekonnen, T.F.; Byrne, L.; Panne, U.; Koch, M. Investigation of chlorpyrifos and its transformation products in fruits and spices by combining electrochemistry and liquid chromatography coupled to tandem mass spectrometry. Food Anal. Methods 2018, 11, 2657–2665. [Google Scholar] [CrossRef]
- The Rapid Alert System for Food and Feed (RASFF). Available online: https://ec.europa.eu/food/safety/rasff/for_consumers_en (accessed on 1 October 2018).
- Mel’nikova, T.V.; Polyakova, L.P.; Oudalova, A.A. Assessment of Organochlorine Hydrocarbons Transformation in Contaminated Agricultural Products and Foodstuffs under Gamma-radiation. In Proceedings of the 1st International Symposium on Physics, Engineering and Technologies for Bio-Medicine, Moscow, Russia, 18–23 October 2016. [Google Scholar]
- Mekonnen, T.F.; Panne, U.; Koch, M. Prediction of biotransformation products of the fungicide fluopyram by electrochemistry coupled online to liquid chromatography-mass spectrometry and comparison with in vitro microsomal assays. Anal. Bioanal. Chem. 2018, 410, 2607–2617. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Hu, J. Photodegradation of the novel fungicide fluopyram in aqueous solution: Kinetics, transformation products, and toxicity evolvement. Environ. Sci. Pollut. Res. Int. 2016, 23, 19096–19106. [Google Scholar] [CrossRef] [PubMed]
- Australian Pesticides and Veterinary Medicines Authority. Available online: https://apvma.gov.au/sites/.../publication/14166-prs-fluopyram.pdf (accessed on 25 July 2017).
- Verma, S.; Nasir Baig, R.B.; Nadagouda, M.N.; Varma, R.S. Aerobic oxidation of alcohols in visible light on Pd-grafted Ti cluster. Tetrahedron 2017, 73, 5577–5580. [Google Scholar] [CrossRef] [PubMed]
- Zabar, R.; Sarakha, M.; Lebedev, A.T.; Polyakova, O.V.; Trebse, P. Photochemical fate and photocatalysis of 3,5,6-trichloro-2-pyridinol, degradation product of chlorpyrifos. Chemosphere 2016, 144, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.J.; Wong, O.T.; Simlot, R.; Chang, J.-J.; Hall, I.H. Acute toxic and teratogenic effects of cyclic imides in rodents. Arch. Pharm. 1994, 327, 237–245. [Google Scholar] [CrossRef]
- Burrows, H.D.; Canle L., M.; Santaballa, J.A.; Steenken, S. Reaction pathways and mechanisms of photodegradation of pesticides. J. Photochem. Photobiol. B 2002, 67, 71–108. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. Mzmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Products | Molecular Formula | Retention Time (min) | Molecular Ion, [M + H]+ | Product Ions (Q3), [M + H]+ | Measured m/z, [M + H]+ | δm/m, ppm | Mechanism of Formation from FLP |
|---|---|---|---|---|---|---|---|
| P1 | C14H8F6N2O2 | 24.1 | 351.0563 | 335, 321, 291, 173, 164, 145, 115 | 351.0555 | 2.2 | −CH2=CH2, −Cl•, +HO• |
| P2 * | C16H12F6N2O | 26.5 | 363.0927 | 385, 190, 173, 145 | 363.0932 | 0.0 | −Cl•, +H• |
| P3 * | C16H12F6N2O2 | 25.9 | 379.0881 | 417, 361, 208, 190, 173, 152, 145 | 379.0880 | −0.2 | −Cl•, +HO• |
| P4 | C14H7ClF6N2O2 | 26.3 | 385.0173 | 387, 407/409, 198, 173, 162, 145 | 385.0169 | −1.0 | −CH2=CH2, +O |
| P5 | C16H10F6N2O3 | 24.8 a, 25.6 b | 393.0674 | 409, 427, 449, 375, 176, 188 | 393.0682 | 2.0 | −HCl, +2O |
| P6 | C16H10F6N2O4 | 24.4 | 409.0618 | 431, 391, 377, 359, 220, 202, 170, 150, 131 | 409.06899 | 3.8 | −HCl, +3O |
| P7 | C16H9ClF6N2O3 | 25.6 a, 27.0 b | 427.0279 | 429, 449, 409, 353, 302, 206, 185, 173, 145 | 427.0256 | −5.3 | +2O, −2H• |
| P8 U | - | 30.2 | 533 | 267, 279, 289, 555, 249 | - | - | - |
| P9 | C16H11ClF6N2O3 | 22.3 a, 24.1 b, 26.7 c, 28.5 b | 429.0435 | 431, 451, 411, 397, 240, 222, 190, 173, 145, 131, 115 | 429.0441 | 1.4 | +2O |
| P10 * | C16H10F6N2O | 31.8 | 361.0776 | 383, 343, 312, 271, 190, 173, 145 | 361.0786 | 2.7 | −HCl |
| P11 | C16H10F6N2O2 | 28.2 | 377.0719 | 359, 345, 331, 176, 188 | 377.0716 | 0.0 | −HCl, +O |
| FLP | C16H11ClF6N2O | 27.8/9 | 397.0535 | 419, 435, 208, 173, 145, 131, 115 | 397.0535 | 0.0 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekonnen, T.F.; Panne, U.; Koch, M. New Photodegradation Products of the Fungicide Fluopyram: Structural Elucidation and Mechanism Identification. Molecules 2018, 23, 2940. https://doi.org/10.3390/molecules23112940
Mekonnen TF, Panne U, Koch M. New Photodegradation Products of the Fungicide Fluopyram: Structural Elucidation and Mechanism Identification. Molecules. 2018; 23(11):2940. https://doi.org/10.3390/molecules23112940
Chicago/Turabian StyleMekonnen, Tessema F., Ulrich Panne, and Matthias Koch. 2018. "New Photodegradation Products of the Fungicide Fluopyram: Structural Elucidation and Mechanism Identification" Molecules 23, no. 11: 2940. https://doi.org/10.3390/molecules23112940
APA StyleMekonnen, T. F., Panne, U., & Koch, M. (2018). New Photodegradation Products of the Fungicide Fluopyram: Structural Elucidation and Mechanism Identification. Molecules, 23(11), 2940. https://doi.org/10.3390/molecules23112940






