Bioactive Natural Spirolactone-Type 6,7-seco-ent-Kaurane Diterpenoids and Synthetic Derivatives
Abstract
1. Introduction
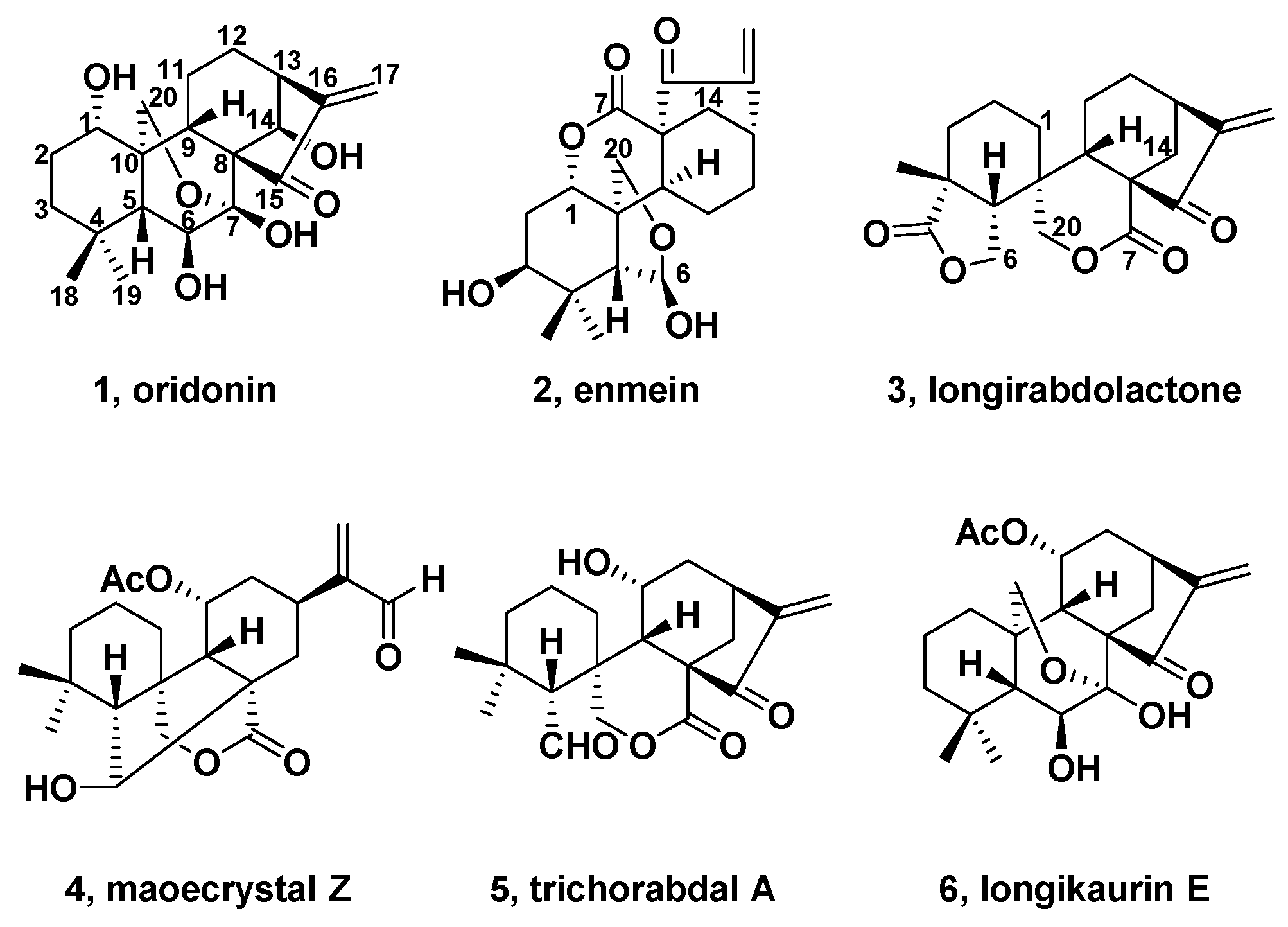
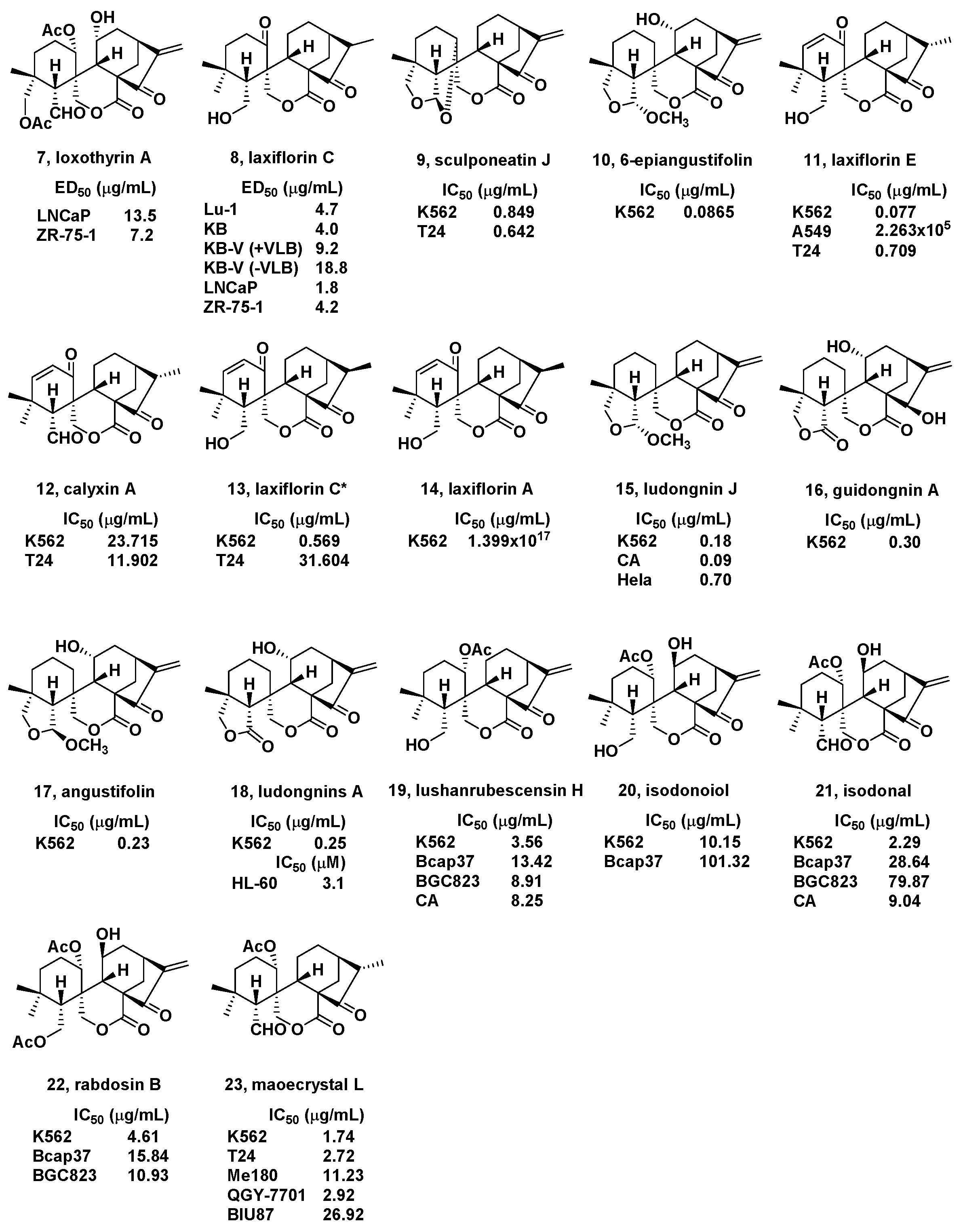
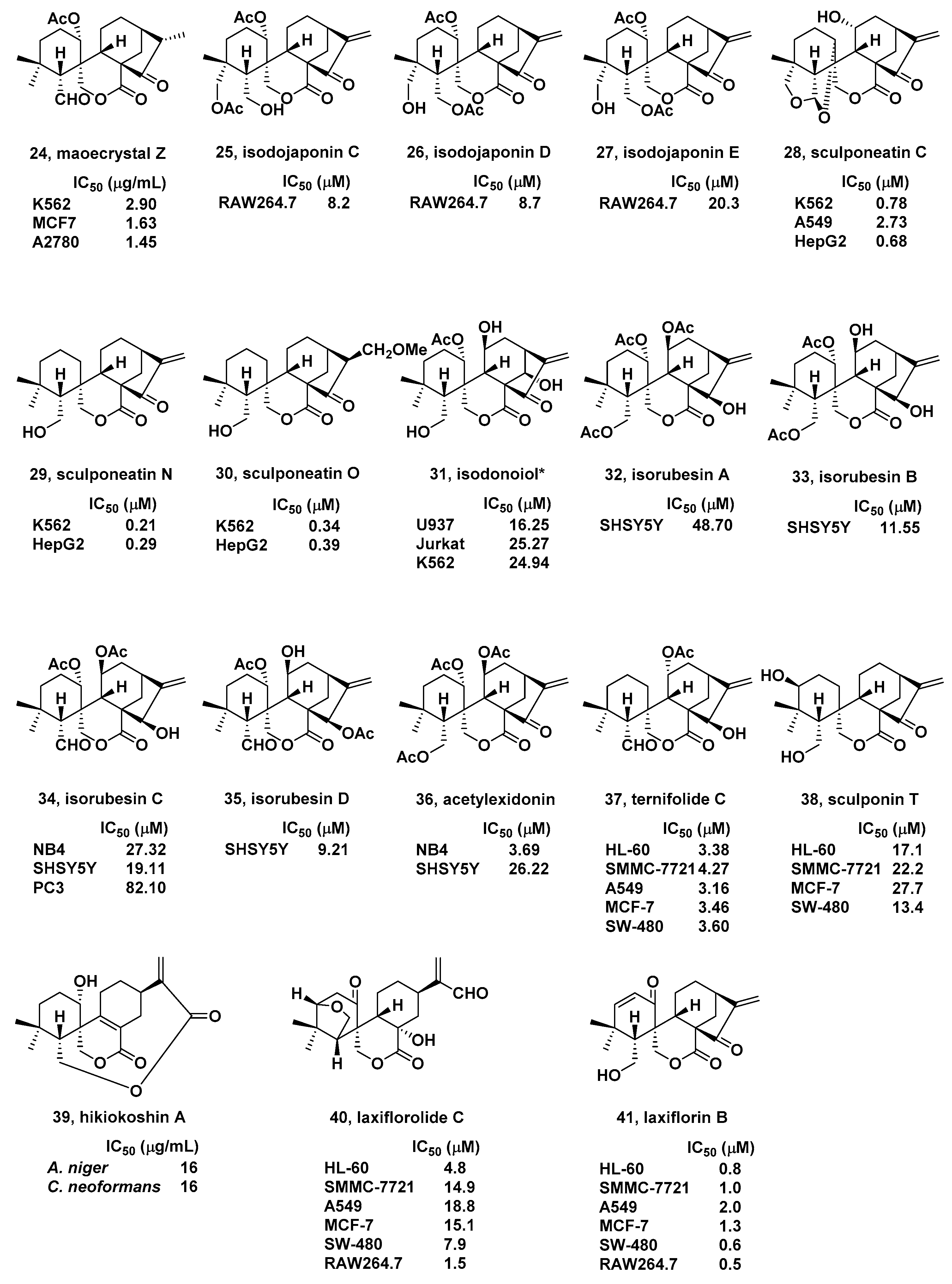
2. Natural Bioactive Spirolactone-Type Diterpenoids
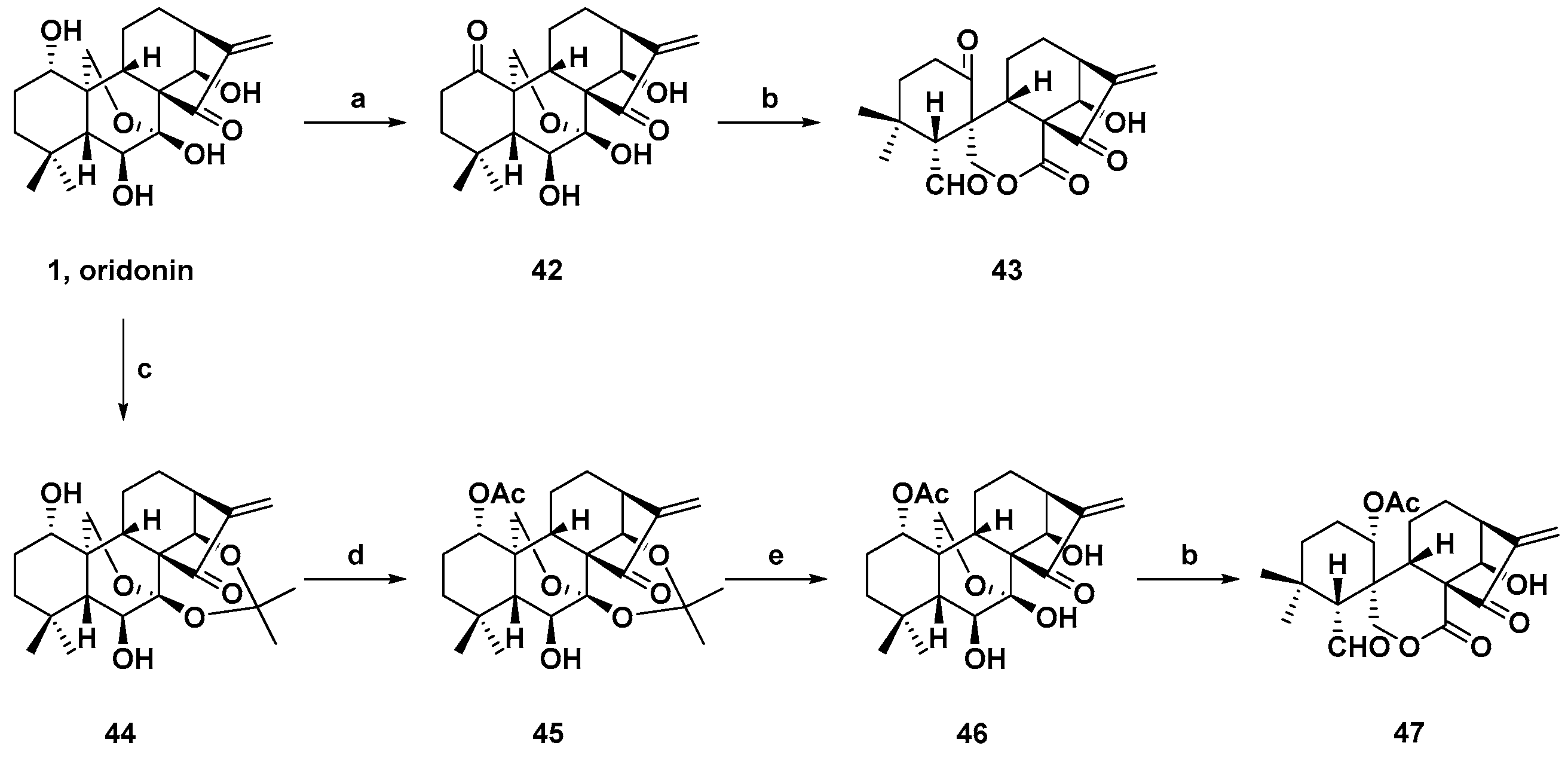
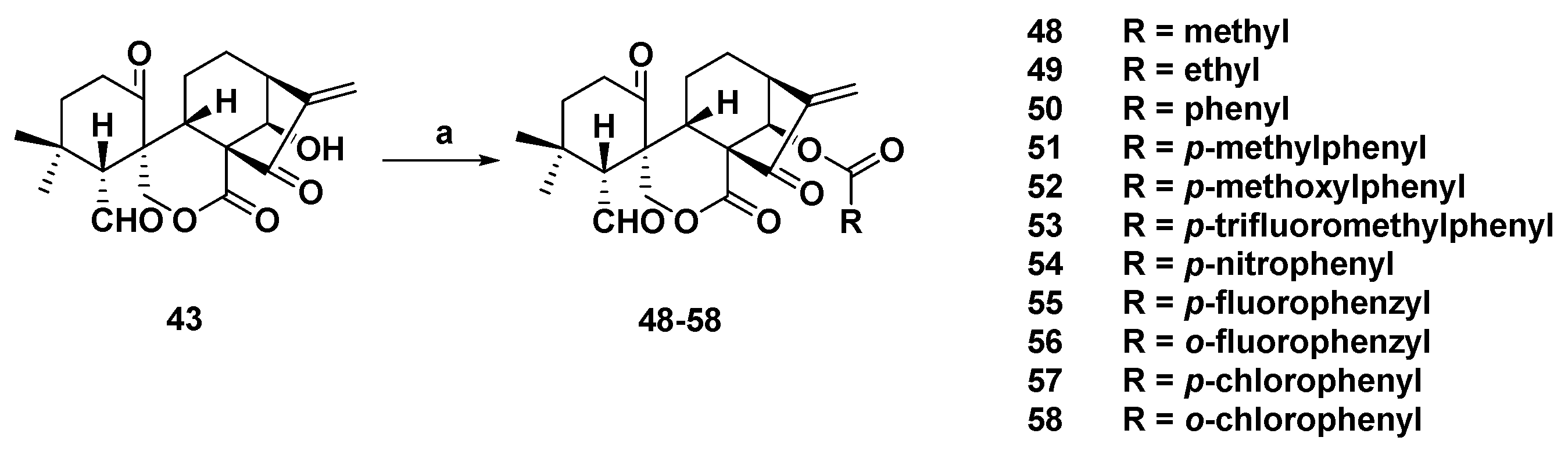
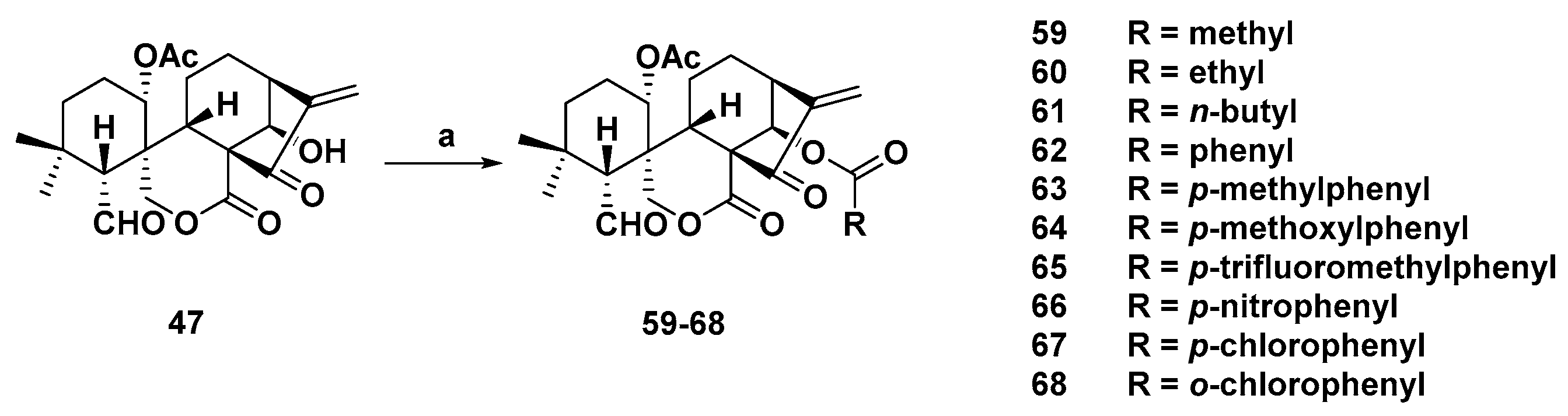
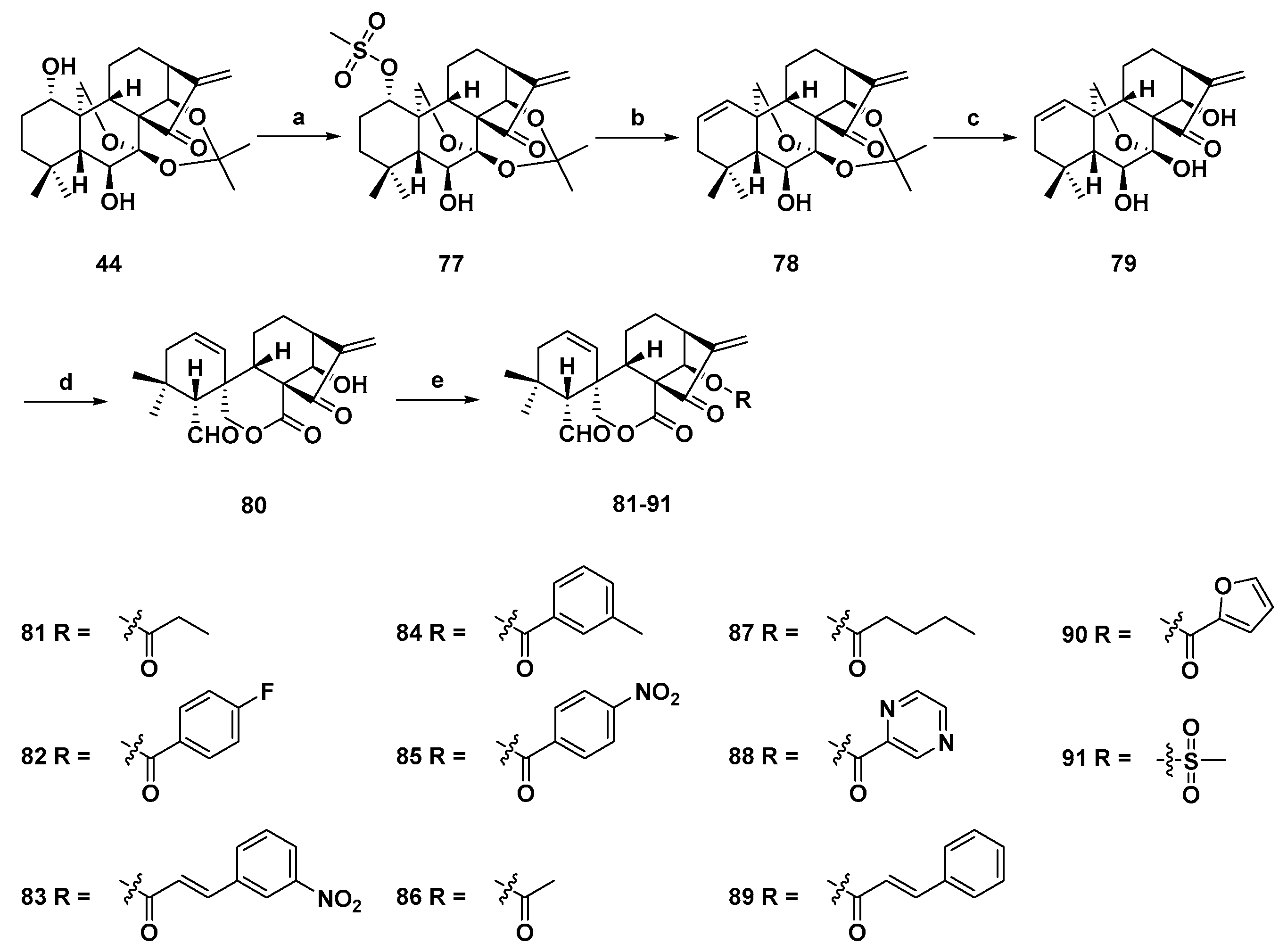
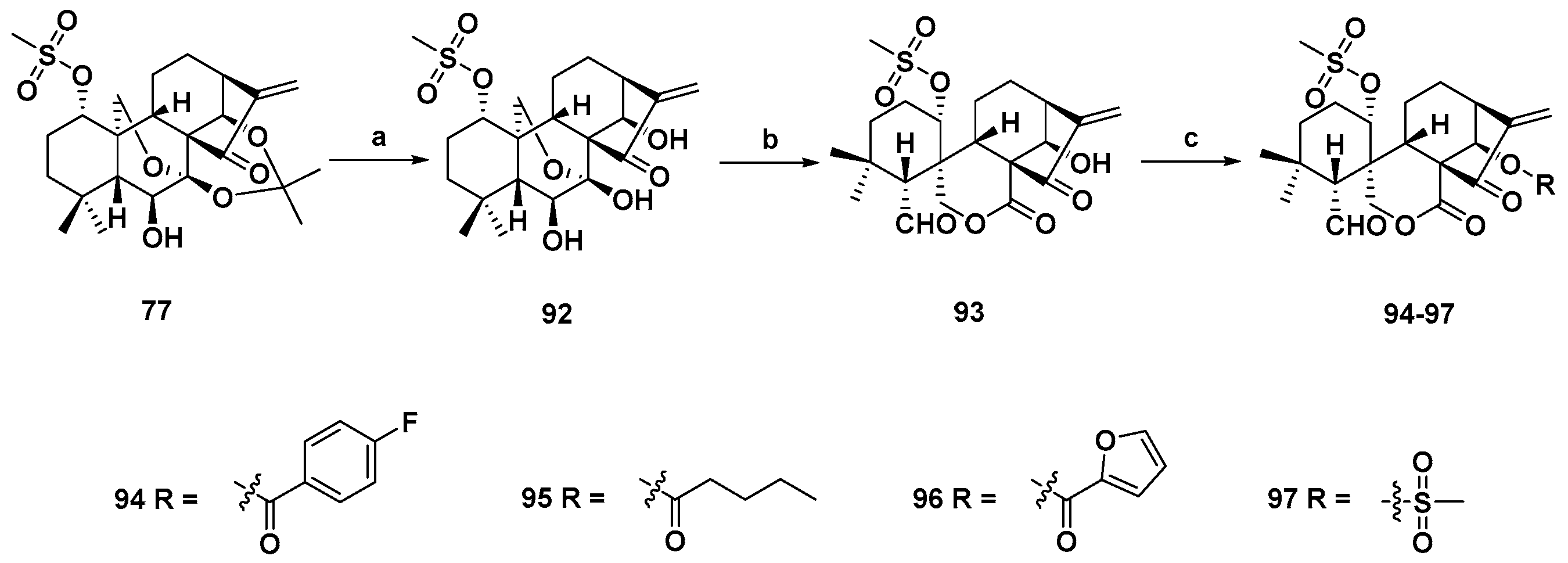
3. Synthetic Spirolactone-Type Diterpenoid Derivatives
4. Conclusions
Funding
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- DeCorte, B.L. Underexplored opportunities for natural products in drug discovery. J. Med. Chem. 2016, 59, 9295–9304. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hu, X.; Han, T.; Liao, J.; Xiao, W.; Xu, S.; Li, Z.; Wang, Z.; Hua, H.; Xu, J. NO-releasing enmein-type diterpenoid derivatives with selective antiproliferative activity and effects on apoptosis-related proteins. Molecules 2016, 21, 1193. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Sashidhara, K.V. Lipid lowering agents of natural origin: An account of some promising chemotypes. Eur. J. Med. Chem. 2017, 140, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bai, L.; He, J.; Zhong, L.; Duan, X.; Ouyang, L.; Zhu, Y.; Wang, T.; Zhang, Y.; Shi, J. Recent advances in discovery and development of natural products as source for anti-Parkinson’s disease lead compounds. Eur. J. Med. Chem. 2017, 141, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Vishwakarma, R.A.; Bharate, S.B. Natural alkaloids as P-gp inhibitors for multidrug resistance reversal in cancer. Eur. J. Med. Chem. 2017, 138, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Xu, F.; Gao, X.; Han, T.; Li, J.; Pan, H.; Zang, L.; Li, D.; Li, Z.; Uchita, T.; et al. Nitric oxide-releasing derivatives of brefeldin A as potent and highly selective anticancer agents. Eur. J. Med. Chem. 2017, 136, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Li, J.; Xue, J.; Li, H.; Xu, F.; Cheng, K.; Li, D.; Li, Z.; Gao, M.; Hua, H. Scutellarin derivatives as apoptosis inducers: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2017, 135, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, J.; Wang, M.; Xu, S.; Liu, W.; Zang, L.; Li, Z.; Hua, H.; Xu, J.; Li, D. Novel enmein-type diterpenoid hybrids coupled with nitrogen mustards: Synthesis of promising candidates for anticancer therapeutics. Eur. J. Med. Chem. 2018, 146, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Tian, K.; Pan, H.; Liu, Y.; Xu, F.; Li, Z.; Uchita, T.; Gao, M.; Hua, H.; Li, D. Novel hybrids of brefeldin A and nitrogen mustards with improved antiproliferative selectivity: Design, synthesis and antitumor biological evaluation. Eur. J. Med. Chem. 2018, 150, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Fujita, E.; Nagao, Y.; Kaneko, K.; Nakazawa, S.; Kuroda, H. The antitumor and antibacterial activity of the Isodon diterpenoids. Chem. Pharm. Bull. 1976, 24, 2118–2127. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhan, R.; Du, X.; Su, J.; Li, X.N.; Yang, J.H.; Zhang, H.B.; Li, Y.; Sun, H.D.; Li, G.P.; et al. Cytotoxic ent-kaurane diterpenoids from Isodon henryi. Chem. Pharm. Bull. 2011, 59, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Y.; Fu, Y.H.; Liu, B.; Liu, Z.C.; Li, D.P.; Liang, D.J.; Zhang, W.; Cao, Y.G.; Zhang, N.S.; Zhang, X.C.; et al. Stevioside suppressed inflammatory cytokine secretion by downregulation of NF-κB and MAPK signaling pathways in LPS-stimulated RAW264.7 cells. Inflammation 2012, 35, 1669–1675. [Google Scholar]
- Kubota, T.; Matsuura, T.; Tsutsui, T.; Uyeo, S.; Takahashi, M.; Irie, H.; Numata, A.; Fujita, T.; Okamoto, T.; Natsume, M.; et al. The constitution and stereochemistry of enmein. Tetrahedron Lett. 1964, 20, 1243–1256. [Google Scholar] [CrossRef]
- Osawa, K.; Yasuda, H.; Maruyama, T.; Morita, H.; Takeya, K.; Itokawa, H.; Okuda, K. An investigation of diterpenes from the leaves of Rabdosia trichocarpa and their antibacterial activity against oral microorganisms. Chem. Pharm. Bull. 1994, 42, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Isobe, T.; Nitoda, T.; Kubo, I. Melanogenesis inhibitors from Rabdosia japonica. Phytomedicine 2012, 19, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.G.; Ung, C.O.; Feng, Z.L.; Huang, L.; Hu, H. Naturally occurring diterpenoid dimers: Source, biosynthesis, chemistry and bioactivities. Planta Med. 2016, 82, 1309–1328. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ding, C.; Ye, N.; Liu, Z.; Wold, E.A.; Chen, H.; Wild, C.; Shen, Q.; Zhou, J. Discovery and development of natural product oridonin inspired anticancer agents. Eur. J. Med. Chem. 2016, 122, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, H.; Xu, F.; Gao, X.; Li, J.; Xu, S.; Zhang, D.; Wu, X.; Xu, J.; Hua, H.; et al. Diterpenoid lead stevioside and its hydrolysis products steviol and isosteviol: Biological activity and structural modification. Eur. J. Med. Chem. 2018, 156, 885–906. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Nakamura, S.; Kojima, N.; Hasei, T.; Yamashita, M.; Watanabe, T.; Matsuda, H. Antimutagenic activity of ent-kaurane diterpenoids from the aerial parts of Isodon japonicus. Tetrahedron Lett. 2017, 58, 3574–3578. [Google Scholar] [CrossRef]
- Kampan, N.C.; Madondo, M.T.; McNally, O.M.; Quinn, M.; Plebanski, M. Paclitaxel and its evolving role in the management of ovarian cancer. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Guido, G.; Massimo, P.; Nunzio, O.; Pietro, P.; Marianna, V.; Tania, D.R.; Luigi, C.; Giuseppe, T.; Mario, R.D. Nano albumin bound paclitaxel in pancreatic cancer: Current evidences and future directions. World J. Gastroent. 2017, 23, 5875–5886. [Google Scholar]
- Gupta, N.; Hatoum, H.; Dy, G.K. First line treatment of advanced non-small-cell lung cancer—Specific focus on albumin bound paclitaxel. Int. J. Nanomed. 2014, 9, 209–221. [Google Scholar]
- Wang, S.Q.; Wang, C.; Chang, L.M.; Zhou, K.R.; Wang, J.W.; Yu, K.; Yang, D.X.; Shi, H.G.; Wang, R.; Shi, X.L.; et al. Geridonin and paclitaxel act synergistically to inhibit the proliferation of gastric cancer cells through ROS-mediated regulation of the PTEN/PI3K/Akt pathway. Oncotarget 2016, 7, 72990–73002. [Google Scholar] [CrossRef] [PubMed]
- Fujita, E.; Fujita, T.; Shibuya, M. Diterpenoid constituents of Isodon trichocarpus and Isodon japonicus (terpenoids IV). Tetrahedron Lett. 1966, 7, 3153–3162. [Google Scholar] [CrossRef]
- Fujita, E.; Fujita, T.; Katayama, H.; Shibuya, M. Oridonin, a new diterpenoid from Isodon species. Chem. Commun. 1967, 101, 252–254. [Google Scholar] [CrossRef]
- Wu, J.; Ding, Y.; Chen, C.H.; Zhou, Z.; Ding, C.; Chen, H.; Zhou, J.; Chen, C. A new oridonin analog suppresses triple-negative breast cancer cells and tumor growth via the induction of death receptor 5. Cancer Lett. 2016, 380, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhou, J.C.; Wu, K.J.; Huang, J.; Ding, Y.; Yun, E.J.; Wang, B.; Ding, C.Y.; Hernandez, E.; Santoyo, J.; et al. Targeting XBP1-mediated β-catenin expression associated with bladder cancer with newly synthetic Oridonin analogues. Oncotarget 2016, 7, 56842–56854. [Google Scholar] [PubMed]
- Li, D.; Han, T.; Liao, J.; Hu, X.; Xu, S.; Tian, K.; Gu, X.; Cheng, K.; Li, Z.; Hua, H.; et al. Oridonin, a promising ent-kaurane diterpenoid lead compound. Int. J. Mol. Sci. 2016, 17, 1395. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, P.; Zhu, H.; Shi, X.; Zhao, C.; Wang, N.; Zhang, L. A sensitive analysis method for 7 diterpenoids in rat plasma by liquid chromatography-electrospray ionization mass spectrometry and its application to pharmacokinetic study of Isodon serra extract. J. Chromatogr. A 2011, 1218, 7771–7780. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.H.; Zhang, Y.; Xu, Z.H.; Liu, J.W.; Jia, W. Immunosuppressive ent-kaurene diterpenoids from Isodon serra. Helv. Chim. Acta 2004, 87, 3160–3166. [Google Scholar] [CrossRef]
- Takeda, Y.; Fujita, T.; Sun, H.D.; Minami, Y.; Ochi, M.; Chen, C.C. Revision of the structures of isodonal, rabdolasional and related diterpenoids. Chem. Pharm. Bull. 1990, 38, 1877–1880. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Wu, Z.W. The crystal and molecular structure of Rabdophyllin G. Acta Chim. Sin. 1984, 42, 645–649. [Google Scholar]
- Fujita, E.; Fuji, K.; Sai, M.; Node, M. The structure of trichorabdal B and its transformation into a novel skeleton; X-ray crystal structures. J. Chem. Soc. Chem. Commun. 1981, 621, 899–900. [Google Scholar] [CrossRef]
- Node, M.; Sai, M.; Fujita, E.; Fuji, K. Antitumor diterpenoids from Rabdosia trichocappa: Trichorabdal E, F, and H and G Acetate. Heterocycles 1984, 22, 1701–1704. [Google Scholar]
- Chen, Y.Z.; Li, Y.Z.; Yue, J.M. Diterpenoids from Rabdosia Gaponica var. Glaucocalyx. J. Nat. Prod. 1989, 52, 886–887. [Google Scholar] [CrossRef]
- Node, M.; Sai, M.; Fujita, E.; Fuji, K. Terpenoids. LII.: The structures of trichorabdal F, trichorabdal G acetate, and trichorabdal H. Acomment on the structure of shikodonin. Chem. Pharm. Bull. 1989, 37, 1470–1471. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, H.J.; Sun, H.D. Diterpenoids from Rabdosia Setschwanensis. Phytochemistry 1990, 29, 2591–2595. [Google Scholar]
- Takeda, Y.; Ichihara, T. Isolongirabdiol, a new diterpenoid from Rabdosia Longituba. J. Nat. Prod. 1990, 53, 138–142. [Google Scholar] [CrossRef]
- Takeda, Y.; Ikawa, A.; Matsumoto, T.; Terao, H.; Otsuka, H. Diterpenoid constituents of Rabdosia Longituba. Planta Med. 1992, 58, 470–471. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Ikawa, A.; Matsumoto, T.; Terao, H.; Otsuka, H. Diterpenoids having ent-kaurene and ent-spiro-seco-kaurene skeletons from Rabdosia Longituba. Phytochemistry 1992, 31, 1687–1690. [Google Scholar] [CrossRef]
- Takeda, Y.; Ichihara, T.; Otsuka, H.; Kido, M. Longirabdolactone and longirabdacetal, 6,7-seco-ent-kaurenoids from Rabdosia Longituba. Phytochemistry 1993, 33, 643–646. [Google Scholar] [CrossRef]
- Takeda, Y.; Futatsuishi, Y.; Ichihara, T.; Matsumoto, T.; Terao, H.; Terada, H.; Otsuka, H. Rabdokaurins C and D, two new diterpenoids from Rabdosia Longituba. Chem. Pharm. Bull. 1993, 41, 685–687. [Google Scholar] [CrossRef]
- Shen, X.Y.; Isogai, A.; Furihata, K.; Lin, Z.W.; Sun, H.D.; Suzuki, A. 6,7-seco-ent-Kaurane diterpenoid from Rabdosia Eriocalyx. Phytochemistry 1994, 35, 820–821. [Google Scholar]
- Chen, S.N.; Yue, J.M.; Chen, S.Y.; Lin, Z.W.; Qin, G.W.; Sun, H.D.; Chen, Y.Z. Diterpenoids from Isodon eriocalyx. J. Nat. Prod. 1999, 62, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.M.; Li, S.H.; Zhao, Q.S.; Lin, Z.W.; Sun, H.D.; Lu, Y.; Wang, C.; Zheng, Q.T. Two novel ent-kaurane diterpenoids isolated from Isodon eriocalyx var. laxiflora. Tetrahedron Lett. 2002, 43, 661–664. [Google Scholar] [CrossRef]
- Li, L.M.; Li, G.Y.; Li, S.H.; Weng, Z.Y.; Xiao, W.L.; Han, Q.B.; Ding, L.S.; Lou, L.G.; Sun, H.D. Cytotoxic ent-kauranoids from Isodon parvifolius. Chem. Biodivers. 2006, 3, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Li, L.M.; Li, G.Y.; Ding, L.S.; Lei, C.; Yang, L.B.; Zhao, Y.; Weng, Z.Y.; Li, S.H.; Huang, S.X.; Xiao, W.L.; et al. Sculponins A–C, three new 6,7-seco-ent-kauranoids from Isodon sculponeatus. Tetrahedron Lett. 2007, 48, 9100–9103. [Google Scholar] [CrossRef]
- Yang, L.B.; Huang, S.X.; Li, L.M.; Zhao, Y.; Lei, C.; Xiao, W.L.; Pu, J.X.; Han, Q.B.; Sun, H.D. ent-Kaurane diterpenoids from Isodon japonicus. Helv. Chim. Acta 2007, 90, 2375–2379. [Google Scholar] [CrossRef]
- Zhang, J.X.; Wang, Y.X.; He, Z.A.; Yan, F.L. Diterpenoids from Isodon excisoides. J. Chem. Res. 2009, 1, 35–37. [Google Scholar] [CrossRef]
- Zhang, J.X.; Wang, Y.X.; He, Z.A.; Yan, F.L.; Sun, H.D. Two new ent-kaurane diterpenoids from Isodon excisoides. Chin. Chem. Lett. 2009, 20, 201–203. [Google Scholar] [CrossRef]
- Li, X.N.; Pu, J.X.; Du, X.; Lou, L.G.; Li, L.M.; Huang, S.X.; Zhao, B.; Zhang, M.; He, F.; Luo, X.; et al. Structure and cytotoxicity of diterpenoids from Isodon eriocalyx. J. Nat. Prod. 2010, 73, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.J.; Yan, F.L.; Hai, G.F.; Hou, R.J.; Ding, M.M.; Bai, Y.X. Two new diterpenoids and other constituents from Isodon rubescens. Fitoterapia 2011, 82, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Q.; Xuan, L.J. ent-6,7-Secokaurane diterpenoids from Rabdosia serra and their cytotoxic activities. Phytochemistry 2016, 122, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Fujita, E.; Shibuya, M.; Nakamura, S.; Okada, Y.; Fujita, T. Total synthesis of enmein. J. Chem. Soc. Chem. Commun. 1972, 19, 1107. [Google Scholar] [CrossRef]
- Fujita, E.; Shibuya, M.; Nakamura, S.; Okada, Y.; Fujita, T. Terpenoids. Part XXVIII. Total synthesis of enmein. J. Chem. Soc. Perkin Trans. 1974, 1, 165–177. [Google Scholar] [CrossRef]
- George, A.; Lewis, N.M. Conversion of gibberellic acid into the b-ring seco-kaurenoid, longirabdolactone. Aust. J. Chem. 2003, 56, 805–809. [Google Scholar]
- Cha, J.Y.; Yeoman, J.T.; Reisman, S.E. A concise total synthesis of (−)-maoecrystal Z. J. Am. Chem. Soc. 2011, 133, 14964–14967. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, J.T.S.; Cha, J.Y.; Mak, V.W.; Reisman, S.E. A unified strategy for the synthesis of (−)-maoecrystal Z, (−)-trichorabdal A, and (−)-longikaurin E. Tetrahedron 2014, 70, 4070–4088. [Google Scholar] [CrossRef]
- Behera, T.K.; Nurul Islam, S.K.; Singh, V. Maoecrystal V: A formidable synthetic challenge. J. Chem. Sci. 2013, 125, 1301–1314. [Google Scholar] [CrossRef]
- Lazarski, K.E.; Moritz, B.J.; Thomson, R.J. The total synthesis of Isodon diterpenes. Angew. Chem. 2014, 53, 10588–10599. [Google Scholar] [CrossRef] [PubMed]
- Riehl, P.S.; DePorre, Y.C.; Armaly, A.M.; Groso, E.J.; Schindler, C.S. New avenues for the synthesis of ent-Kaurene diterpenoids. Tetrahedron 2015, 71, 6629–6650. [Google Scholar] [CrossRef]
- Sun, H.D.; Huang, S.X.; Han, Q.B. Diterpenoids from Isodon species and their biological activities. Nat. Prod. Rep. 2006, 23, 673–698. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, W.G.; Sun, H.D.; Pu, J.X. Diterpenoids from Isodon species: An update. Nat. Prod. Rep. 2017, 34, 1090–1140. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.D.; Lin, Z.W.; Niu, F.D.; Lin, L.Z.; Chai, H.B.; John, M.P.; Geoffrey, A.C. Cytotoxic ent-kaurene diterpenoids from three Isodon species. Phytochemistry 1995, 38, 437–442. [Google Scholar] [CrossRef]
- Sun, H.D.; Lin, Z.W.; Niu, F.D.; Shen, P.Q.; Pan, L.T.; Lin, L.Z.; Geoffrey, A.C. Diterpenoids from Isodon Eriocalyx var. Laxiflora. Phytochemistry 1995, 38, 1451–1455. [Google Scholar] [CrossRef]
- Jiang, B.; Mei, S.X.; Zhao, A.H.; Sun, H.D.; Lu, Y.; Zheng, Q.T. Diterpenoids from Isodon sculponeatus. Chin. J. Chem. 2002, 20, 887–890. [Google Scholar] [CrossRef]
- Na, Z.; Xiang, W.; Niu, X.M.; Mei, S.X.; Lin, Z.W.; Li, C.M.; Sun, H.D. Diterpenoids from Isodon enanderianus. Phytochemistry 2002, 60, 55–60. [Google Scholar] [CrossRef]
- Niu, X.M.; Li, S.H.; Li, M.L.; Zhao, Q.S.; Mei, S.X.; Na, Z.; Wang, S.J.; Lin, Z.W.; Sun, H.D. Cytotoxic ent-kaurene diterpenoids from Isodon eriocalyx var. laxiflora. Planta Med. 2002, 68, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.B.; Zhao, A.H.; Zhang, J.X.; Lu, Y.; Zhang, L.L.; Zheng, Q.T.; Sun, H.D. Cytotoxic constituents of Isodon rubescens var. lushiensis. J. Nat. Prod. 2003, 66, 1391–1394. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Pu, J.X.; Xiao, W.L.; Zhao, Y.; Gao, X.M.; Li, X.N.; Zhang, H.B.; Wang, Y.Y.; Li, Y.; Sun, H.D. Cytotoxic ent-kaurane diterpenoids from Isodon rubescens var. lushiensis. J. Nat. Prod. 2010, 73, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.B.; Li, M.L.; Li, S.H.; Mou, Y.K.; Lin, Z.W.; Sun, H.D. ent-Kaurane diterpenoids from Isodon rubescens var. Lushanensis. Chem. Pharm. Bull. 2003, 51, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.H.; Wen, Z.Y.; Xu, G.; Xiao, W.L.; Peng, L.Y.; Lin, Z.W.; Sun, H.D. Cytotoxic ent-kaurane diterpenoids from Isodon eriocalyx. Chem. Biodivers. 2005, 2, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.B.; Cheung, S.; Tai, J.; Qiao, C.F.; Song, J.Z.; Tso, T.F.; Sun, H.D.; Xu, H.X. Maoecrystal Z, a cytotoxic diterpene from Isodon eriocalyx with a unique skeleton. Org. Lett. 2006, 8, 4727–4730. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.S.; Lee, S.A.; Han, X.H.; Hwang, J.S.; Lee, C.; Lee, D.; Hong, J.T.; Kim, Y.; Lee, H.; Hwang, B.Y. ent-Kaurane diterpenoids from Isodon japonicus. J. Nat. Prod. 2008, 71, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Li, L.M.; Li, G.Y.; Pu, J.X.; Xiao, W.L.; Ding, L.S.; Sun, H.D. ent-Kaurane and cembrane diterpenoids from Isodon sculponeatus and their cytotoxicity. J. Nat. Prod. 2009, 72, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pu, J.X.; Weng, Z.Y.; Zhao, Y.; Zhao, Y.; Xiao, W.L.; Sun, H.D. 6,7-seco-ent-Kaurane diterpenoids from Isodon sculponeatus with cytotoxic activity. Chem. Biodivers. 2010, 7, 2888–2896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Pu, J.X.; Wang, Y.Y.; He, F.; Zhao, Y.; Li, X.N.; Luo, X.; Xiao, W.L.; Li, Y.; Sun, H.D. Four new ent-kauranoids from Isodon rubescens var. lushanensis and data reassignment of dayecrystal B. Chem. Pharm. Bull. 2010, 58, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.M.; Luo, X.; Pu, J.X.; Wu, Y.L.; Zhao, Y.; Yang, L.B.; He, F.; Li, X.N.; Xiao, W.L.; Chen, G.Q.; et al. Antiproliferative diterpenoids from the leaves of Isodon rubescens. Planta Med. 2011, 77, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Du, X.; Peng, G.; Shi, Y.M.; Wang, W.G.; Zhan, R.; Kong, L.M.; Li, X.N.; Li, Y.; Pu, J.X.; et al. Ternifolide A, a new diterpenoid possessing a rare macrolide motif from Isodon ternifolius. Org. Lett. 2012, 14, 3210–3213. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Wang, W.G.; Zhou, M.; Wu, H.Y.; Zhan, R.; Du, X.; Pu, J.X.; Sun, H.D. 6,7-seco-ent-Kaurane diterpenoids from Isodon sculponeatus and their bioactivity. Chin. Chem. Lett. 2014, 25, 541–544. [Google Scholar] [CrossRef]
- Tanaka, N.; Tsuji, E.; Sakai, K.; Gonoi, T.; Kobayashi, J. Hikiokoshins A–I, diterpenes from the leaves of Isodon japonicus. Phytochemistry 2014, 102, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.G.; Yan, B.C.; Li, X.N.; Du, X.; Wu, H.Y.; Zhan, R.; Li, Y.; Pu, J.X.; Sun, H.D. 6,7-seco-ent-Kaurane-type diterpenoids from Isodon eriocalyx var. laxiflora. Tetrahedron 2014, 70, 7445–7453. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.; Xu, S.; Cai, H.; Yao, H.; Zhang, Y.; Jiang, J.; Xu, J. The conversion of oridonin to spirolactone-type or enmein-type diterpenoid: Synthesis and biological evaluation of ent-6,7-seco-oridonin derivatives as novel potential anticancer agents. Eur. J. Med. Chem. 2012, 52, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cai, H.; Jiang, B.; Liu, G.; Wang, Y.; Wang, L.; Yao, H.; Wu, X.; Sun, Y.; Xu, J. Synthesis of spirolactone-type diterpenoid derivatives from kaurene-type oridonin with improved antiproliferative effects and their apoptosis-inducing activity in human hepatoma Bel-7402 cells. Eur. J. Med. Chem. 2013, 59, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Han, T.; Tian, K.; Tang, S.; Xu, S.; Hu, X.; Wang, L.; Li, Z.; Hua, H.; Xu, J. Novel nitric oxide-releasing spirolactone-type diterpenoid derivatives with in vitro synergistic anticancer activity as apoptosis inducer. Bioorg. Med. Chem. Lett. 2016, 26, 4191–4196. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yao, H.; Hu, M.; Li, D.; Zhu, Z.; Xie, W.; Yao, H.; Wu, L.; Chen, Z.S.; Xu, J. 6,7-seco-ent-Kauranoids derived from oridonin as potential anticancer agents. J. Nat. Prod. 2017, 80, 2391–2398. [Google Scholar] [CrossRef] [PubMed]

| Compound | IC50 [µM] | ||||
|---|---|---|---|---|---|
| K562 | MGC-803 | CaEs-17 | Bel-7402 | MCF-7 | |
| 51 | 1.27 | 2.24 | 1.05 | 1.54 | / a |
| 68 | 0.39 | 1.28 | 0.60 | 1.39 | / a |
| 76d | 1.74 | 1.16 | 3.75 | 0.86 | / a |
| 82 | 0.69 | 2.20 | / a | 1.80 | 0.68 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Jiao, R.; Mu, J.; Xu, S.; Li, X.; Wang, X.; Li, Z.; Xu, J.; Hua, H.; Li, D. Bioactive Natural Spirolactone-Type 6,7-seco-ent-Kaurane Diterpenoids and Synthetic Derivatives. Molecules 2018, 23, 2914. https://doi.org/10.3390/molecules23112914
Li H, Jiao R, Mu J, Xu S, Li X, Wang X, Li Z, Xu J, Hua H, Li D. Bioactive Natural Spirolactone-Type 6,7-seco-ent-Kaurane Diterpenoids and Synthetic Derivatives. Molecules. 2018; 23(11):2914. https://doi.org/10.3390/molecules23112914
Chicago/Turabian StyleLi, Haonan, Runwei Jiao, Jiahui Mu, Shengtao Xu, Xu Li, Xianhua Wang, Zhanlin Li, Jinyi Xu, Huiming Hua, and Dahong Li. 2018. "Bioactive Natural Spirolactone-Type 6,7-seco-ent-Kaurane Diterpenoids and Synthetic Derivatives" Molecules 23, no. 11: 2914. https://doi.org/10.3390/molecules23112914
APA StyleLi, H., Jiao, R., Mu, J., Xu, S., Li, X., Wang, X., Li, Z., Xu, J., Hua, H., & Li, D. (2018). Bioactive Natural Spirolactone-Type 6,7-seco-ent-Kaurane Diterpenoids and Synthetic Derivatives. Molecules, 23(11), 2914. https://doi.org/10.3390/molecules23112914





