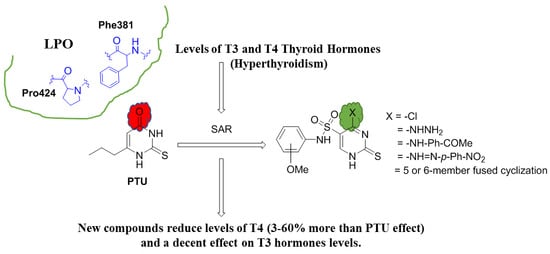
Design, Synthesis, Molecular Modeling, and Biological Evaluation of Novel Thiouracil Derivatives as Potential Antithyroid Agents
Abstract
1. Introduction
2. Results and Discussion
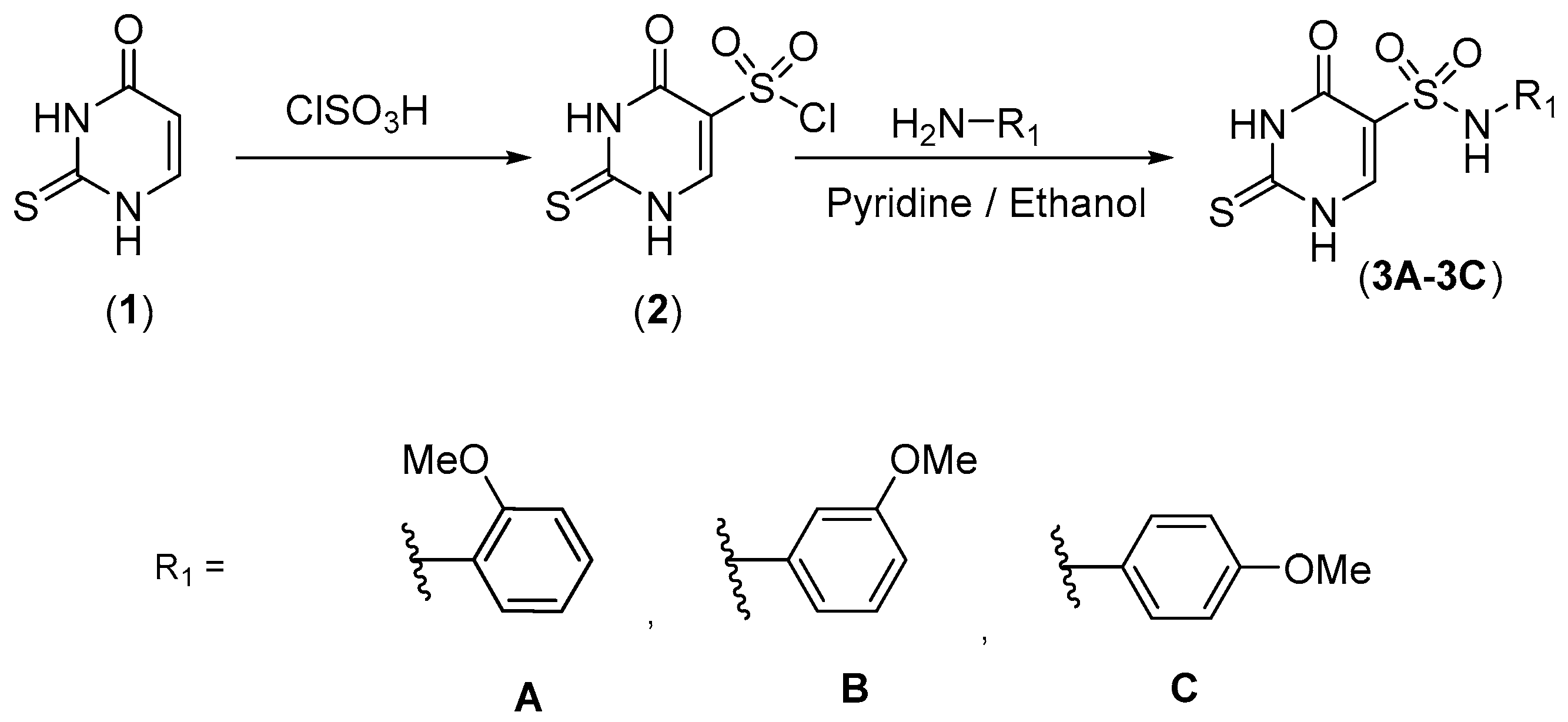
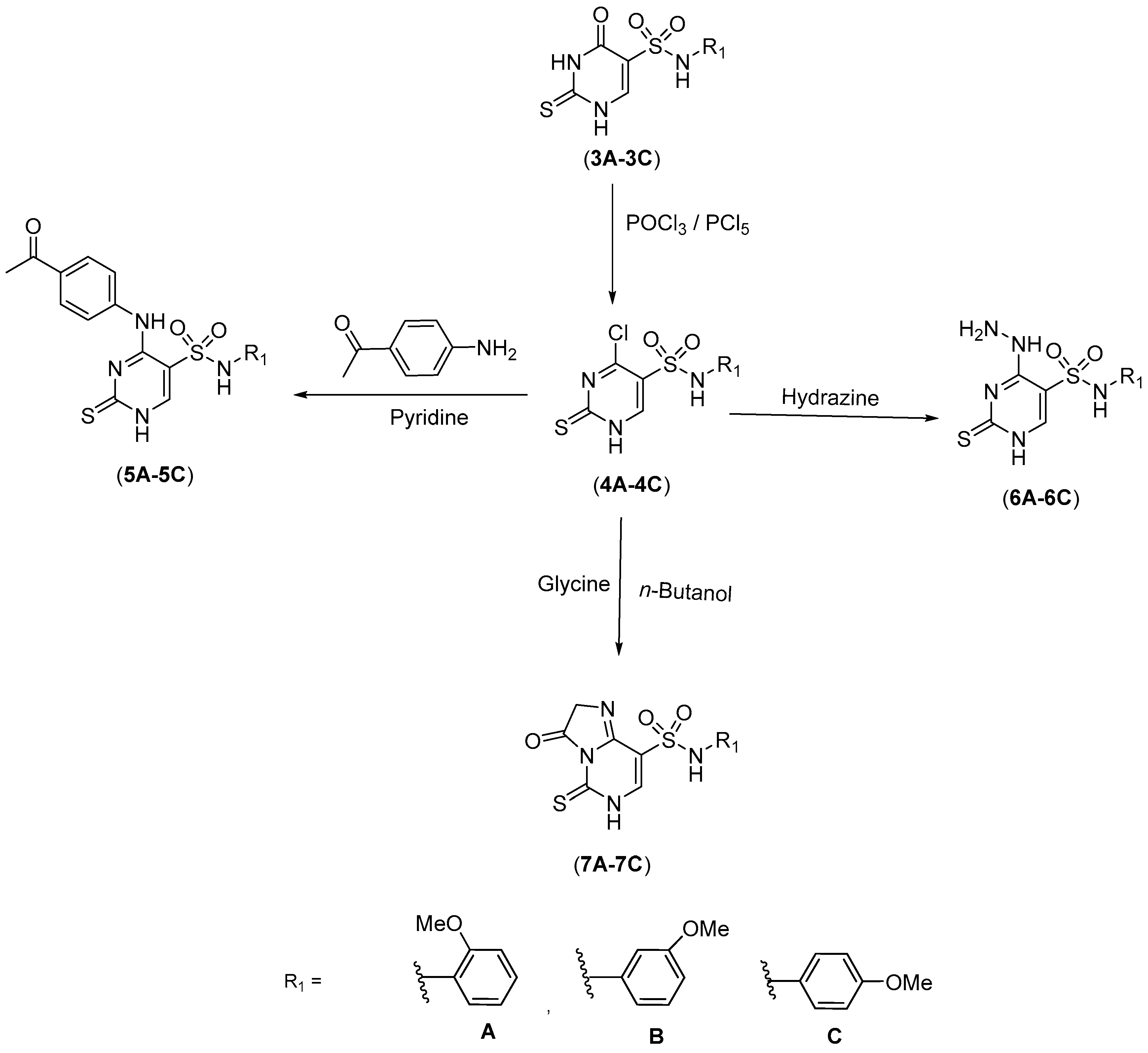
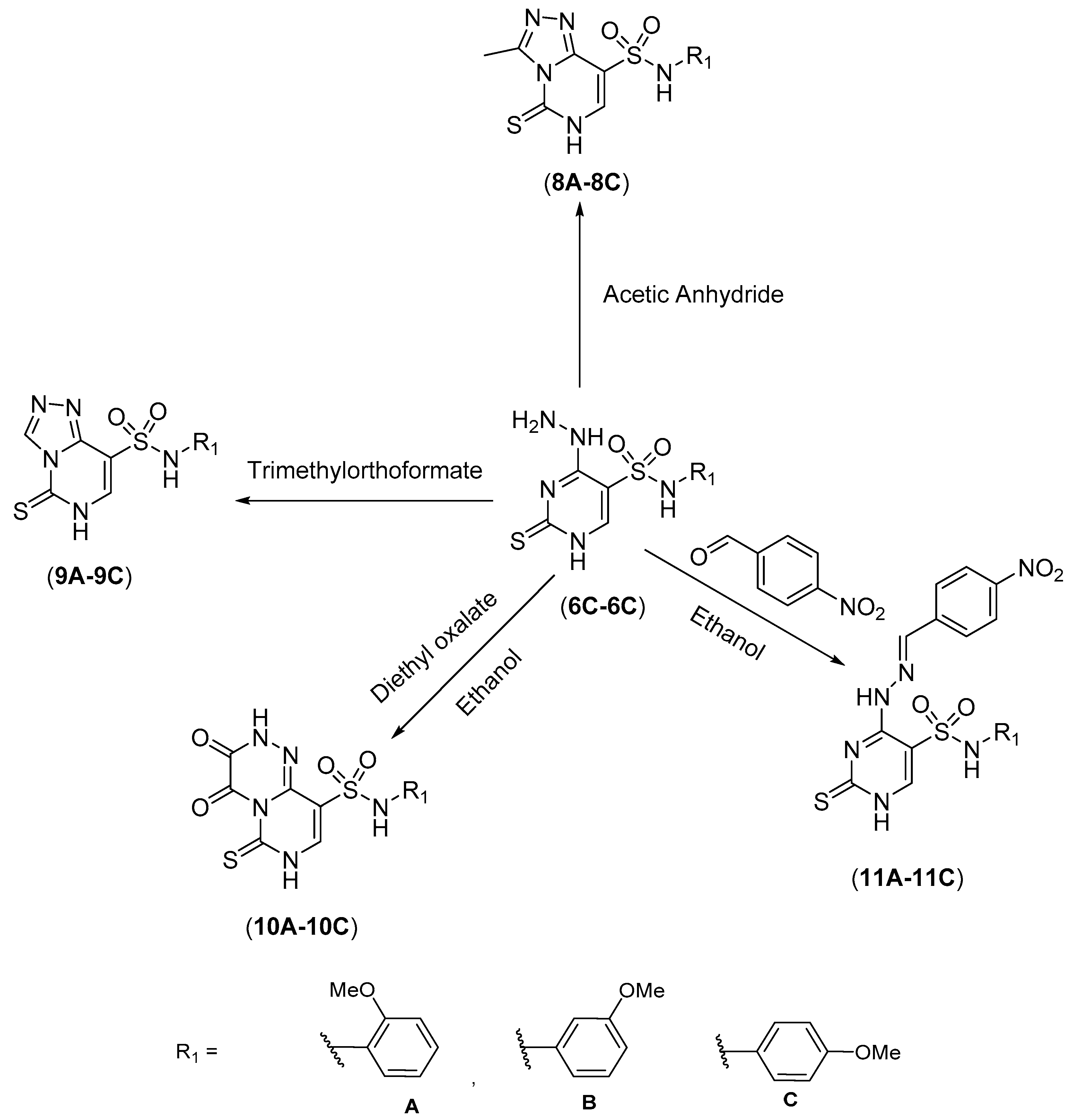
2.1. Chemistry
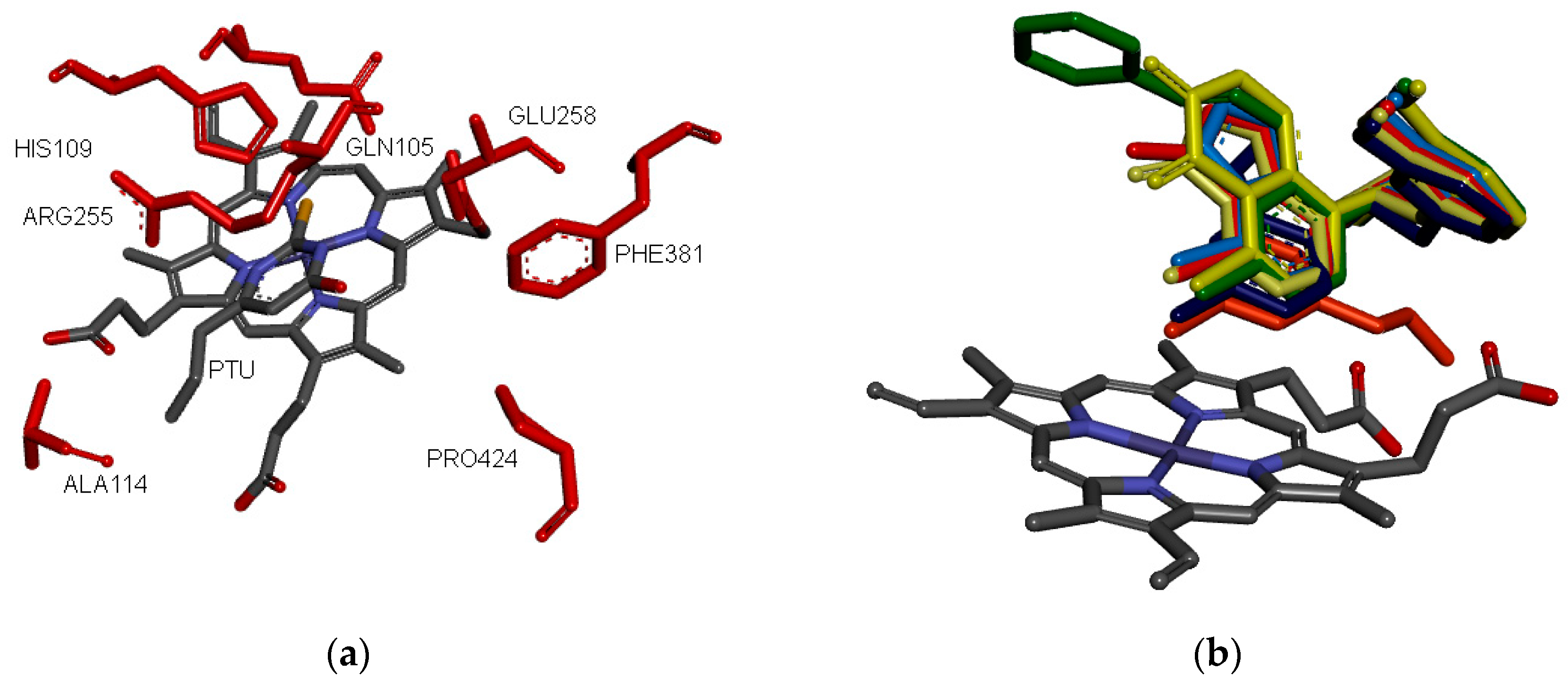
2.2. Molecular Modeling and Binding Mode Prediction
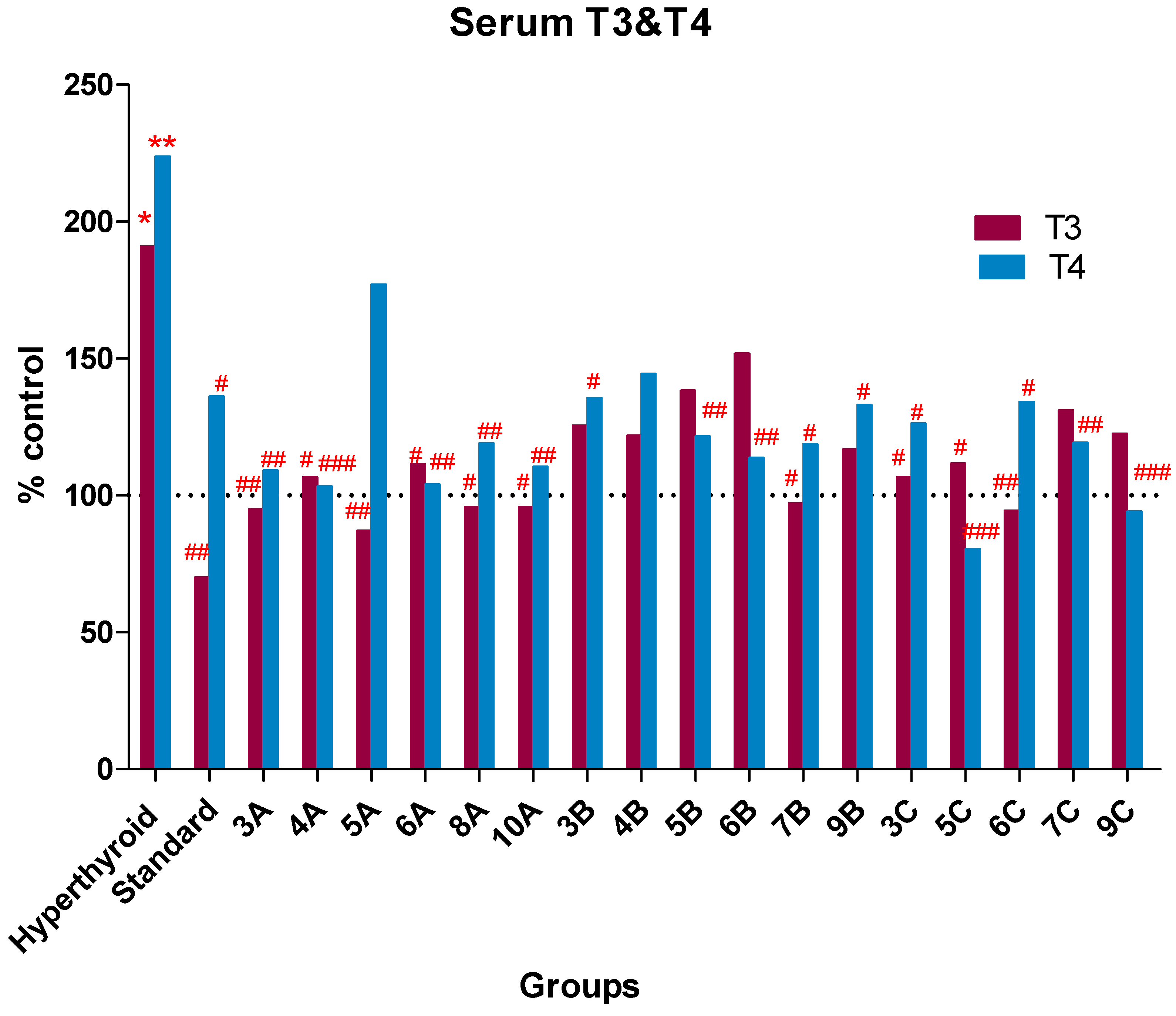
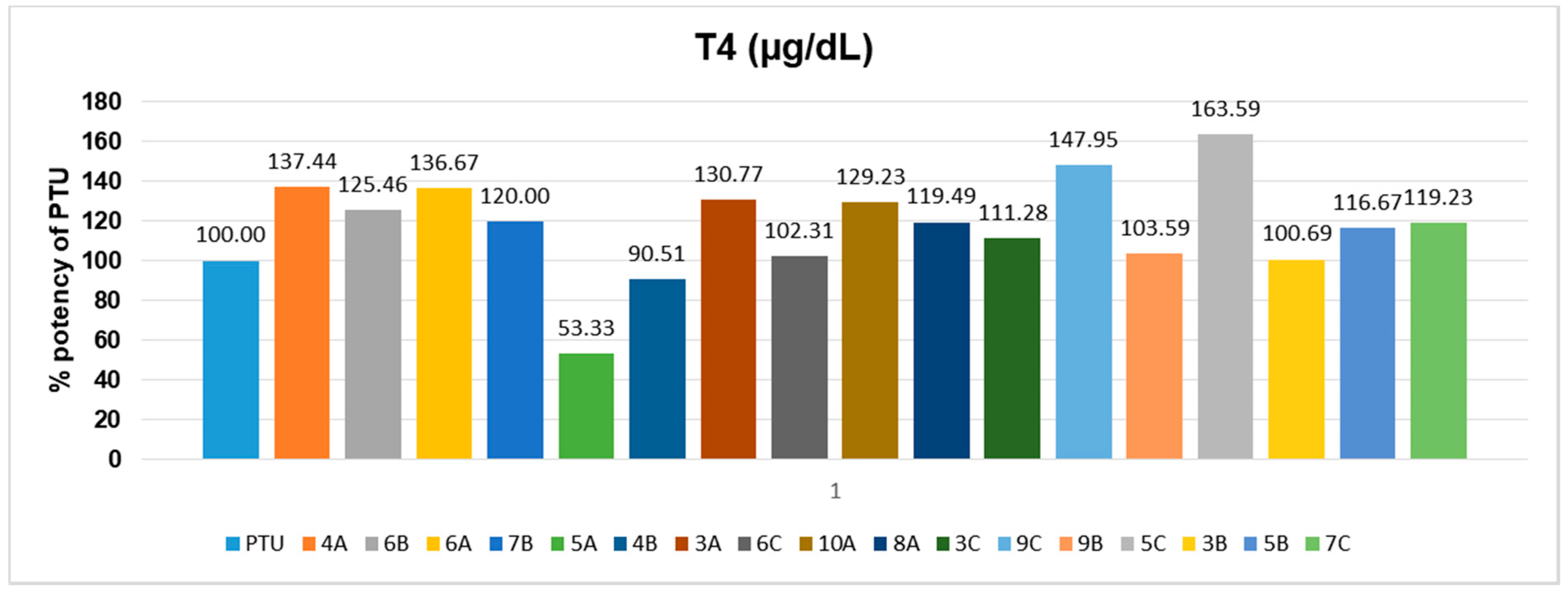
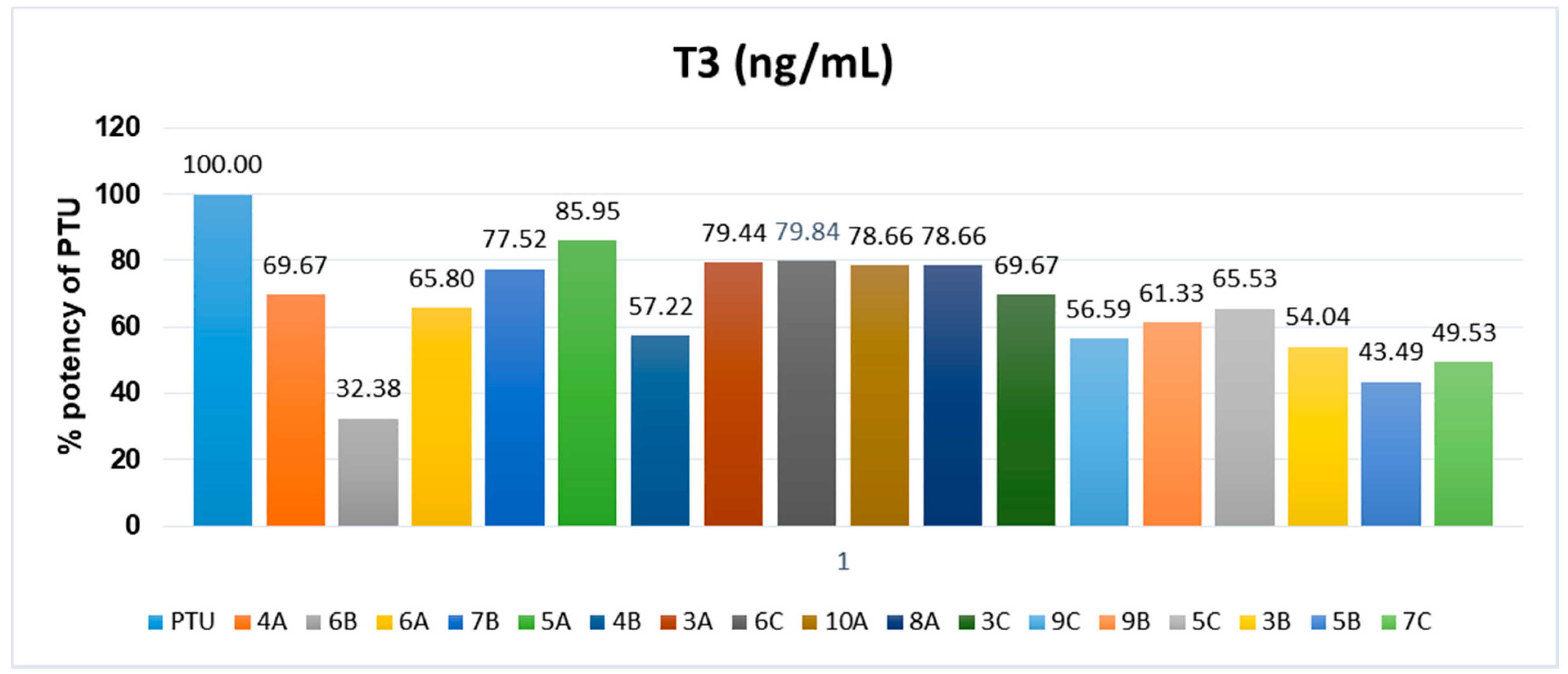
2.3. Biological Screening
3. Materials and Methods
3.1. General Experimental Methods
3.2. Synthesis of N-(2,3 or 4-Methoxyphenyl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-sulphonamides (3A–3C)
3.3. Synthesis of 4-Chloro-N-(2,3 or 4-methoxyphenyl)-2-thioxo-1,2-dihydropyrimidine-5-sulphonamide (4A–4C)
3.4. Synthesis of 4-(4-acetylphenyl) amino]-N-(2,3or4-methoxyphenyl)-2-thioxo-1,2-dihydropyrimidine-5-sulphonamides (5A–5C)
3.5. Synthesis of 4-Hydrazinyl-N-(2,3 or 4-methoxyphenyl)-2-thioxo-1,2-dihydropyrimidine-5-sulphonamides (6A–6C)
3.6. Synthesis of N-(2,3 or 4-methoxyphenyl)-3-oxo-5-thioxo-1,2,3,5,6,8a-hexahydroimidazo[1,2-c] pyrimidine-8-sulphonamides (7A–7C)
3.7. Synthesis of N-(2,3 or 4-methoxyphenyl)-3-methyl-5-thioxo-5,6-dihydro [1,2,4] triazolo[4,3-c] pyrimidine-8-sulphonamides (8A–8C)
3.8. Synthesis of 8-(N-(2,3 or 4-methoxyphenyl)-5-thioxo-5,6-dihydro [1,2,4] triazolo [4,3-c] pyrimidine-8-sulphonamides (9A–9C)
3.9. Synthesis of N-(2,3 or 4-methoxyphenyl)-3,4-dioxo-6-thioxo-3,4,6,7-tetrahydro-2H-pyrimido[6,1-c] [1,2,4] triazine-5-sulphonamides (10A–10C)
3.10. Synthesis of N-(2,3 or 4-Methoxyphenyl)-4-[(2E)-2-(4-nitrobenzylidene) hydrazinyl]-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-ulphonamides (11A–11C)
3.11. Animals
3.12. Induction of Hyperthyroidism
3.13. Experimental Design
3.14. Data Presentation and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PTU | 6-n-propyl-2-thiouracil |
| LPO | mammalian lactoperoxidase |
| TPO | thyroid peroxidase |
| T4 | prohormone thyroxine |
| T3 | triiodothyronine thyroid hormone |
| POCl3 | phosphorus oxychloride |
| PCl5 | Phosphorus pentachloride |
| Ac2O | Acetic anhydride |
References
- Gillam, M.P.; Sidhaye, A.R.; Lee, E.J.; Rutishauser, J.; Stephan, C.W.; Kopp, P. Functional Characterization of Pendrin in a Polarized Cell System: Evidence for Pendrin-mediated Apical Iodide Efflux. J. Biol. Chem. 2004, 279, 13004–13010. [Google Scholar] [CrossRef] [PubMed]
- Kopp, P. Mutations in the Pendred Syndrome (PDS/SLC26A) Gene: An Increasingly Complex Phenotypic Spectrum from Goiter to Thyroid Hypoplasia. J. Clin. Endocrinol. Metab. 2014, 99, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Soldin, O.P.; Aschner, M. Effects of Manganese on Thyroid Hormone Homeostasis: Potential Links. Neurotoxicology 2007, 28, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Brent, G.A. Mechanisms of Thyroid Hormone Action. J. Clin. Invest. 2012, 122, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Brix, K.; Führer, D.; Biebermann, H. Molecules Important for Thyroid Hormone Synthesis and action—Known Facts and Future Perspectives. Thyroid Res. 2011, 4, S9. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.R. The Immune System as a Regulator of Thyroid Hormone Activity. Exp. Biol. Med. (Maywood) 2006, 231, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Fekete, C.; Lechan, R.M. Negative Feedback Regulation of Hypophysiotropic thyrotropin-releasing Hormone (TRH) Synthesizing Neurons: Role of Neuronal Afferents and Type 2 Deiodinase. Front. Neuroendocrinol. 2007, 28, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, K.; Ichikawa, K.; Sakurai, A.; Suzuki, S.; Takeda, T.; Kobayashi, M.; Miyamoto, T.; Arai, M.; Nagasawa, T. Administration of Thyroxine in Treated Graves’ Disease. Effects on the Level of Antibodies to thyroid-stimulating Hormone Receptors and on the Risk of Recurrence of Hyperthyroidism. N. Engl. J. Med. 1991, 324, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Hodak, S.P. Hyperthyroidism. Endocrin. Metab. Clin. 2007, 36, 617–656. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.S. Antithyroid drugs. N. Engl. J. Med. 2005, 352, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, R.P.; Taurog, A.; Dorris, M.L. Mechanism of iodide-dependent Catalytic Activity of Thyroid Peroxidase and Lactoperoxidase. J. Biol. Chem. 1984, 259, 197–205. [Google Scholar] [PubMed]
- Davidson, B.; Soodak, M.; Neary, J.T.; Strout, H.V.; Kieffer, J.D.; Mover, H.; Maloof, F. The Irreversible Inactivation of Thyroid Peroxidase by Methylmercaptoimidazole, Thiouracil, and Propylthiouracil in Vitro and Its Relationship to in Vivo Findings. Endocrinology 1978, 103, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Basosi, R.; Niccolai, N.; Rossi, C. Coordination Behaviour of Antithyroid Drugs Against the Fe(I)(NO)2 Group in Solution: ESR and FT-NMR Study. Biophys. Chem. 1978, 8, 61–69. [Google Scholar] [CrossRef]
- Azizi, F.; Amouzegar, A.; Mehran, L.; Alamdari, S.; Subekti, I.; Vaidya, B.; Poppe, K.; Sarvghadi, F.; Luis, T.S., Jr.; Akamizu, T. Management of Hyperthyroidism During Pregnancy in Asia. Endocr. J. 2014, 61, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Burch, H.B.; Burman, K.D.; Cooper, D.S. A 2011 Survey of Clinical Practice Patterns in the Management of Graves’ Disease. J. Clin. Endocrinol. Metab. 2012, 97, 4549–4558. [Google Scholar] [CrossRef] [PubMed]
- Andersson, L.A.; Bylkas, S.A.; Wilson, A.E. Spectral Analysis of Lactoperoxidase: Evidence a Common Heme in Mmammalian Peroxidases. J. Biol. Chem. 1996, 271, 3406–3412. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, T.J.; Davey, C.A.; Fenna, R.E. X-ray Crystal Structure and Characterization of Halide-binding Sites of Human Myeloperoxidase at 1.8 Å Resolution. J. Biol. Chem. 2000, 275, 11964–11971. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.A.; Zou, J.Y.; Cowan, S.W.; Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. 1991, 47, 110–119. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, N.; Sharma, S.; Shin, K.; Takase, M.; Kaur, P.; Srinivasan, A.; Singh, T.P. Inhibition of Lactoperoxidase by Its Own Catalytic Product: Crystal Structure of the Hypothiocyanate-Inhibited Bovine Lactoperoxidase at 2.3-Å Resolution. Biophys. J. 2009, 96, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, I.A.; Singh, A.K.; Singh, N.; Sinha, M.; Singh, S.B.; Bhushan, A.; Kaur, P.; Srinivasan, A.; Sharma, S.; Singh, T.P. Structural Evidence of Substrate Specificity in Mammalian Peroxidases: Structure of the Thiocyanate Complex with Lactoperoxidase and Its Interactions at 2.4 Å Resolution. J. Biol. Chem. 2009, 284, 14849–14856. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, N.; Sinha, M.; Bhushan, A.; Kaur, P.; Srinivasan, A.; Sharma, S.; Singh, T. Binding Modes of Aromatic Ligands to Mammalian Heme Peroxidases with Associated Functional Implications: Crystal Structures of Lactoperoxidase Complexes with Acetylsalicylic Acid, Salicylhydroxamic Acid, and Benzylhydroxamic Acid. J. Biol. Chem. 2009, 284, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Singh, A.; Kushwaha, G.S.; Singh, A.K.; Kaur, P.; Sharma, S.; Singh, T.P. Mode of Binding of the Antithyroid Drug Propylthiouracil to Mammalian Haem Peroxidases. Erratum. Acta Crystallogr. F Struct. Biol. Commun. 2015, 71, 804. [Google Scholar] [CrossRef] [PubMed]
- Sing, R.P.; Singh, A.; Sirohi, H.V.; Singh, A.K.; Kaur, P.; Sharma, S.; Singh, T.P. Dual binding mode of antithyroid drug methimazole to mammalian heme peroxidases—Structural determination of the lactoperoxidase–methimazole complex at 1.97 Å resolution. FEBS Open Biol. 2016, 6, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, N.; Tiwari, A.; Sinha, M.; Kushwaha, G.S.; Kaur, P.; Srinivasan, A.; Sharma, S.; Singh, T.P. First Structural Evidence for the Mode of Diffusion of Aromatic Ligands and Ligand-Induced Closure of the Hydrophobic Channel in Heme Peroxidases. J. Biol. Inorg. Chem. 2010, 15, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Mugesh, G. Novel Thyroid Hormone Analogues, Enzyme Inhibitors and Mimetics, and Their Action. Mol. Cell. Endocrinol. 2017, 458, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Manna, D.; Roy, G.; Mugesh, G. Antithyroid Drugs and Their Analogues: Synthesis, Structure, and Mechanism of Action. Acc. Chem. Res. 2013, 46, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Langer, M.; Kratz, F.; Rothen-Rutishauser, B.; Wunderli-Allenspach, H.; Beck-Sickinger, A.G. Novel Peptide Conjugates for Tumor-Specific Chemotherapy. J. Med. Chem. 2001, 44, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Alyar, S.; Karacan, N. Synthesis, Characterization, Antimicrobial Activity and structure-activity Relationships of New Aryldisulfonamides. J. Enzyme Inhib. Med. Chem. 2009, 24, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Fathalla, O.A.; Zaghary, W.A.; Radwan, H.H.; Awad, S.M.; Mohamed, M.S. Synthesis of New 2-thiouracil-5-sulfonamide derivatives with Biological Activity. Arch. Pharm. Res. 2002, 25, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Kar, A. Evaluation of the Antithyroid, Antioxidative and Antihyperglycemic Activity of Scopoletin from Aegle marmelos leaves in Hyperthyroid Rats. Phytother. Res. 2006, 20, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Kar, A. Synergistic interactions of Aegle marmelos leaf, Emblica officinalis fruit and Ocimum sanctum leaf extracts in the regulation of hyperthyroidism and/or hyperglycemia. Orient. Pharm. Exp. Med. 2004, 4, 37–43. [Google Scholar]
- Nakashima, T.; Taurog, A.; Riesco, G. Mechanism of Action of Thioureylene Antithyroid Drugs: Factors Affecting Intrathyroidal Metabolism of Propylthiouracil and Methimazole in Rats. Endocrinology 1978, 103, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Abrams, G.M.; Larsen, P.R. Triiodothyronine and Thyroxine in the Serum and Thyroid Glands of Iodine-Deficient Rats. J. Clin. Investig. 1973, 52, 2522–2531. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, M.; Lau, R.; Wasser, H.L.; Nelson, A.M.; Kuma, K.; Hershman, J.M. Thyroid T4 5′-deiodinase Activity in Normal and Abnormal Human Thyroid Glands. Metabolism 1984, 33, 332–336. [Google Scholar] [CrossRef]
- Braverman, L.E.; Ingbar, S.H.; Sterling, K. Conversion of Thyroxine (T4) to triiodothyronine (T3) in Athyreotic Human Subjects. J. Clin. Investig. 1970, 49, 855–864. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds listed in the main text of the manuscript are available from the authors. |
| Groups | Serum T3 (ng/mL) | Serum T4 (µg/dL) |
|---|---|---|
| Control | 60.5 ± 5.08 | 4.45 ± 1.1 |
| Hyperthyroid group | 115.5 ± 22.47 * | 9.96 ± 1.28 ** |
| Standard (PTU 10 mg/kg) | 42.4 ± 1.44 ## | 6.06 ± 0.58 # |
| Compound 3A | 57.43 ± 1.43 ## | 4.86 ± 0.48 ## |
| Compound 4A | 64.57 ± 3.96 # | 4.60 ± 0.24 ### |
| Compound 5A | 52.67 ± 2.42 ## | 7.88 ± 0.29 |
| Compound 6A | 67.4 ± 1.86 # | 4.63 ± 0.67 ## |
| Compound 8A | 58 ± 3.72 # | 5.3 ± 0.4 ## |
| Compound 10A | 58 ± 4.16 # | 4.92 ± 0.28 ## |
| Compound 3B | 76 ± 2.77 | 6.03 ± 0.36 # |
| Compound 4B | 73.67 ± 3.43 | 6.43 ±1.25 |
| Compound 5B | 83.71 ± 4.61 | 5.41 ± 0.51 ## |
| Compound 6B | 91.83 ± 6.84 | 5.06 ± 0.502 ## |
| Compound 7B | 58.83 ± 3.49 # | 5.28 ± 0.63 # |
| Compound 9B | 70.67 ± 3.35 | 5.92 ± 0.61 # |
| Compound 3C | 64.57 ± 1.94 # | 5.62 ± 0.83 # |
| Compound 5C | 67.6 ± 3.91 # | 3.58 ± 0.35 ### |
| Compound 6C | 57.14 ± 2.52 ## | 5.97 ± 0.69 # |
| Compound 7C | 79.29 ± 4.79 | 5.31 ± 0.49 ## |
| Compound 9C | 74.13 ± 3.91 | 4.19 ± 0.61 ### |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, S.M.; Zohny, Y.M.; Ali, S.A.; Mahgoub, S.; Said, A.M. Design, Synthesis, Molecular Modeling, and Biological Evaluation of Novel Thiouracil Derivatives as Potential Antithyroid Agents. Molecules 2018, 23, 2913. https://doi.org/10.3390/molecules23112913
Awad SM, Zohny YM, Ali SA, Mahgoub S, Said AM. Design, Synthesis, Molecular Modeling, and Biological Evaluation of Novel Thiouracil Derivatives as Potential Antithyroid Agents. Molecules. 2018; 23(11):2913. https://doi.org/10.3390/molecules23112913
Chicago/Turabian StyleAwad, Samir M., Yasser M. Zohny, Sahar A. Ali, Shahenda Mahgoub, and Ahmed M. Said. 2018. "Design, Synthesis, Molecular Modeling, and Biological Evaluation of Novel Thiouracil Derivatives as Potential Antithyroid Agents" Molecules 23, no. 11: 2913. https://doi.org/10.3390/molecules23112913
APA StyleAwad, S. M., Zohny, Y. M., Ali, S. A., Mahgoub, S., & Said, A. M. (2018). Design, Synthesis, Molecular Modeling, and Biological Evaluation of Novel Thiouracil Derivatives as Potential Antithyroid Agents. Molecules, 23(11), 2913. https://doi.org/10.3390/molecules23112913