Phosphorus Solubilization by Bacillus Species
Abstract
1. Introduction
2. Results
2.1. Growth of Bacillus
2.2. Concentration of Phosphorus (Expressed as P2O5)
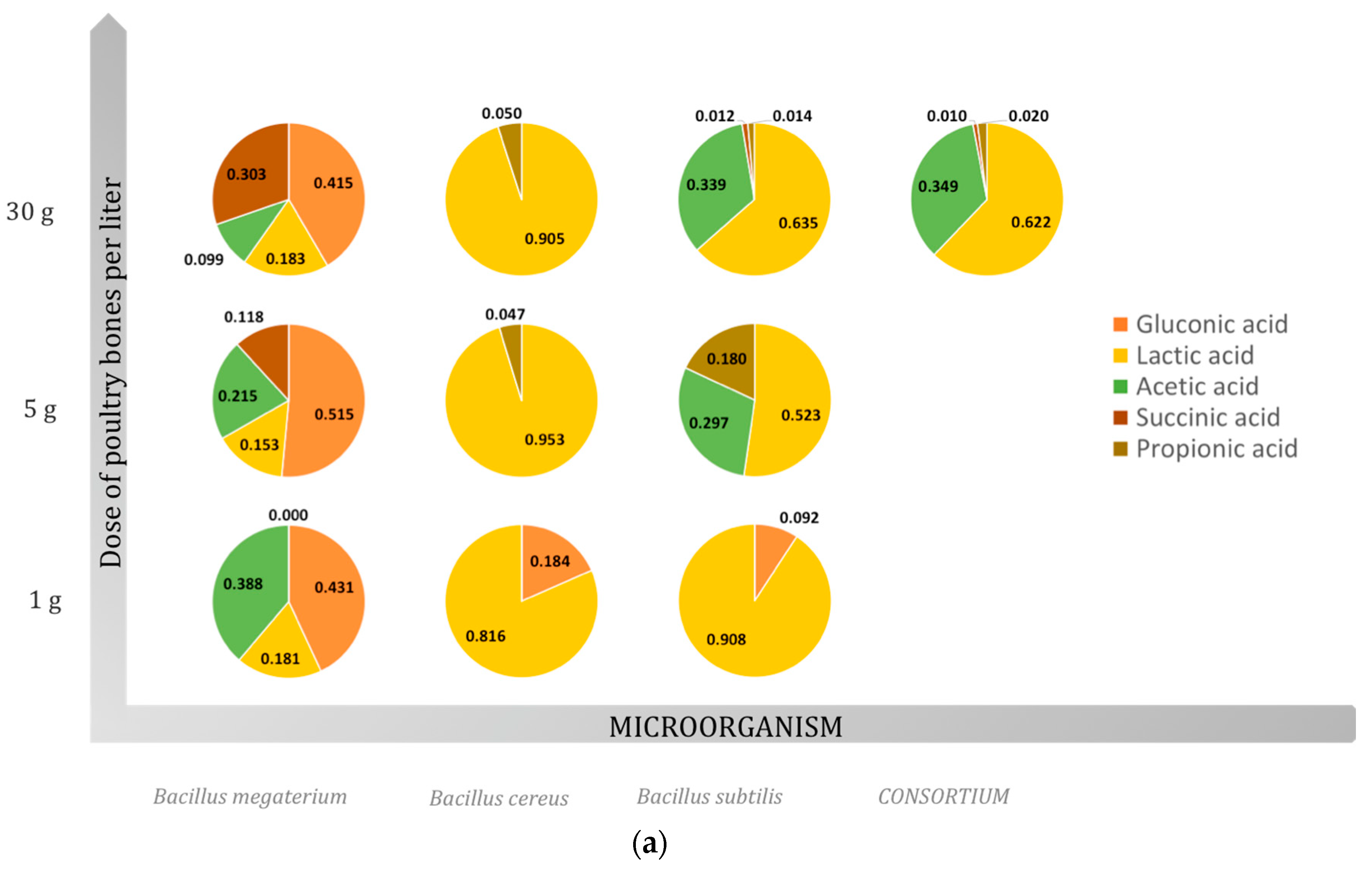
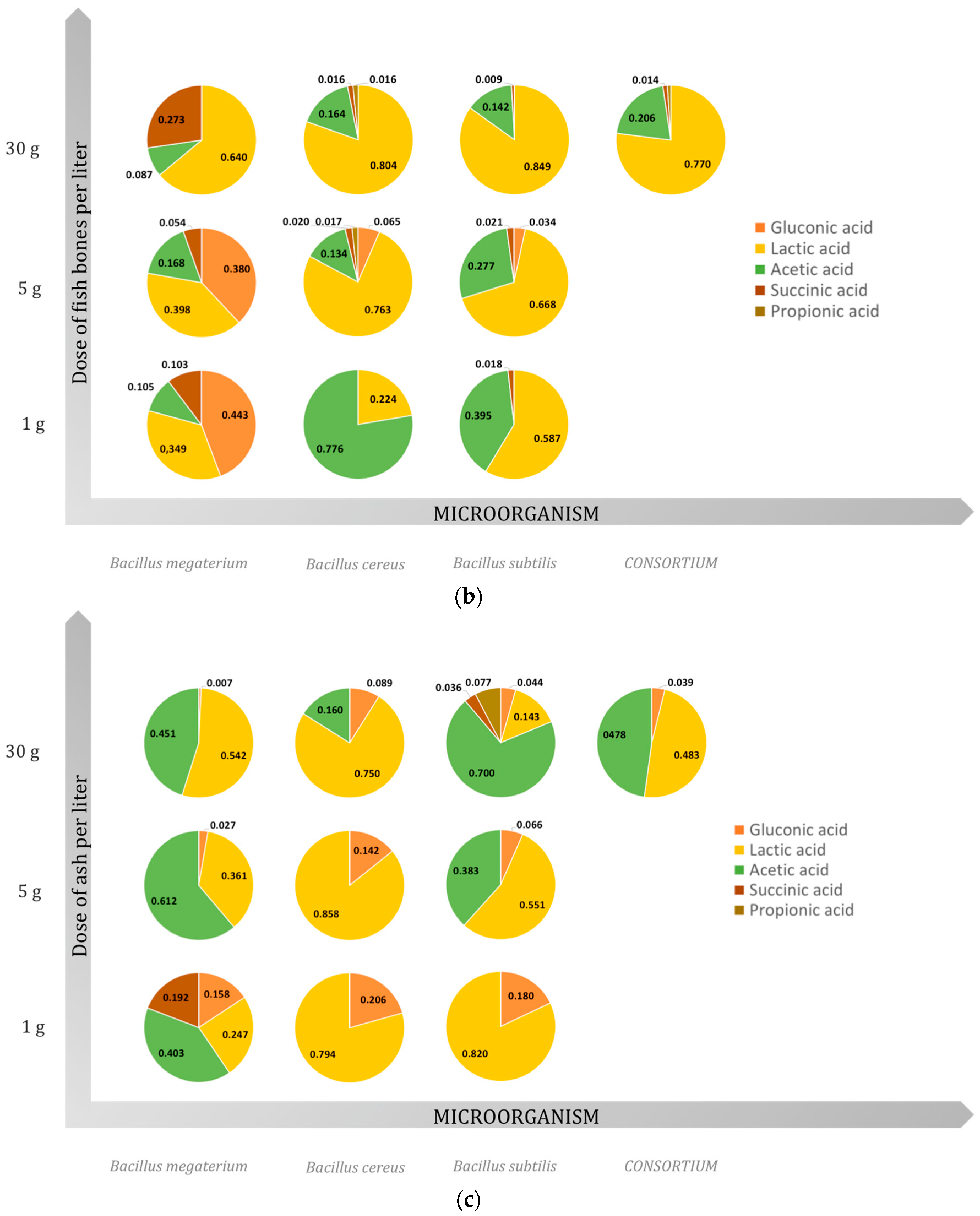
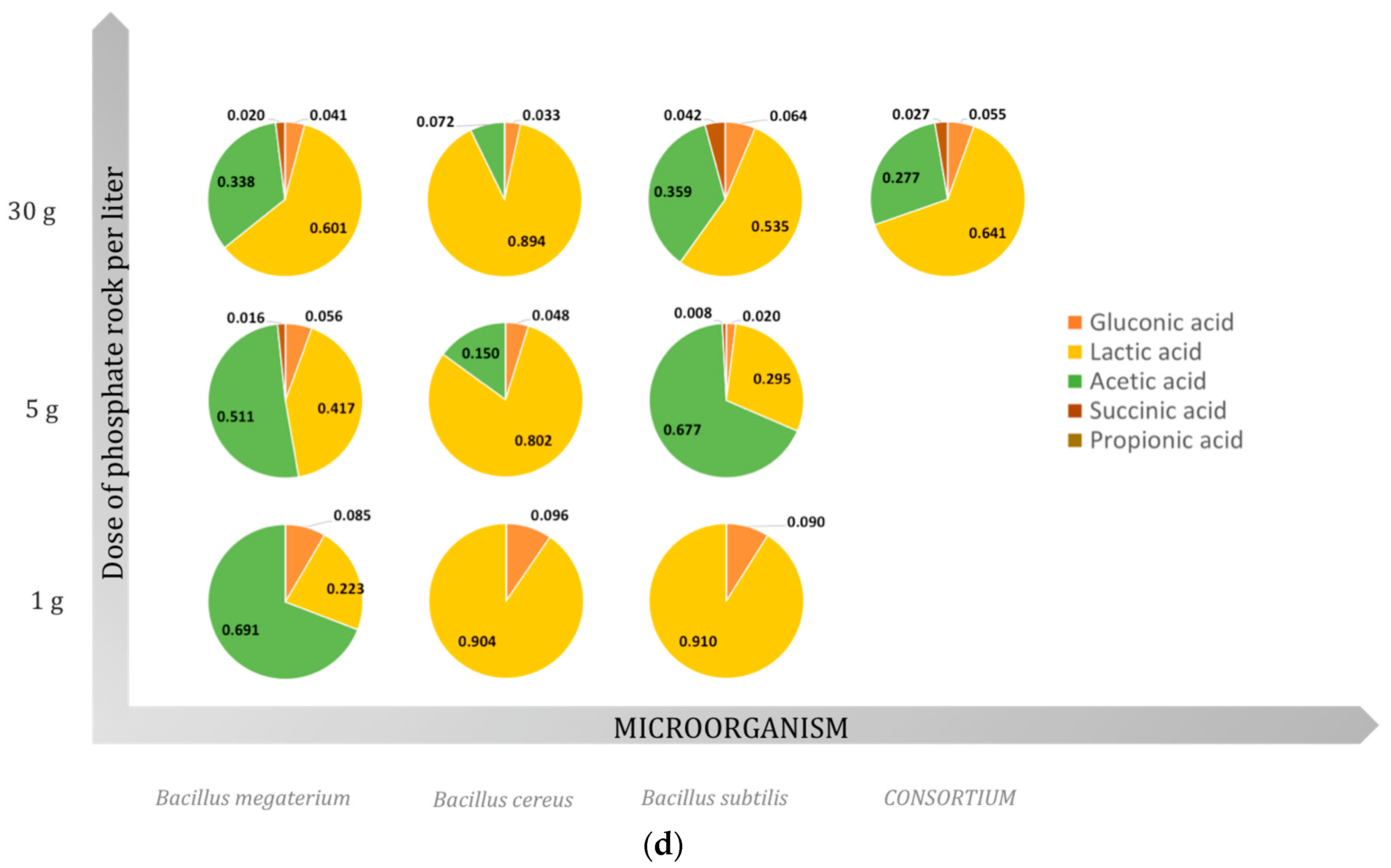
2.3. Organic Acids
2.3.1. Gluconic Acid
2.3.2. Lactic Acid
2.3.3. Acetic Acid
2.3.4. Succinic Acid
2.3.5. Propionic Acid
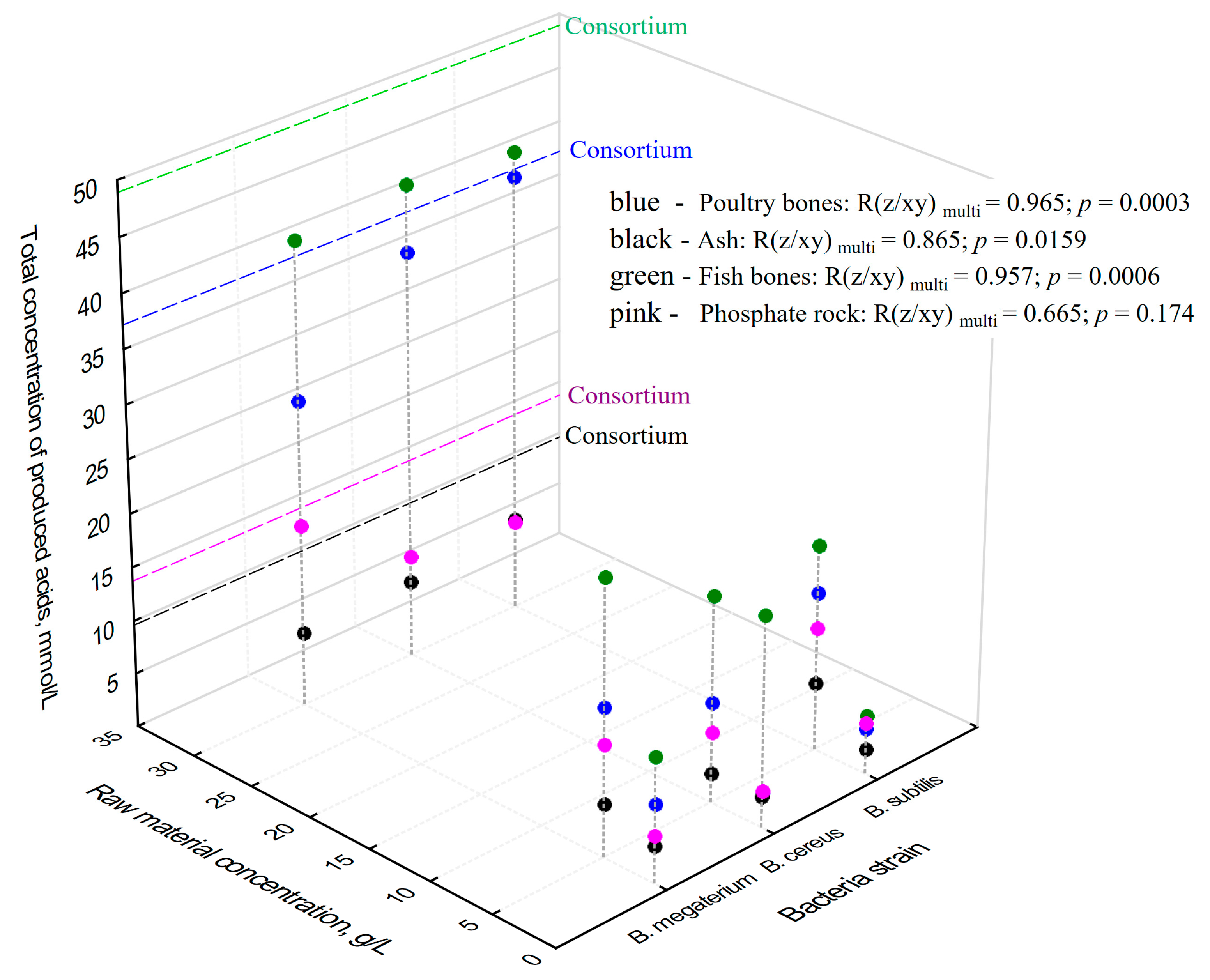
2.4. Total Concentrations of Produced Organic Acids vs. Concentrations of P2O5
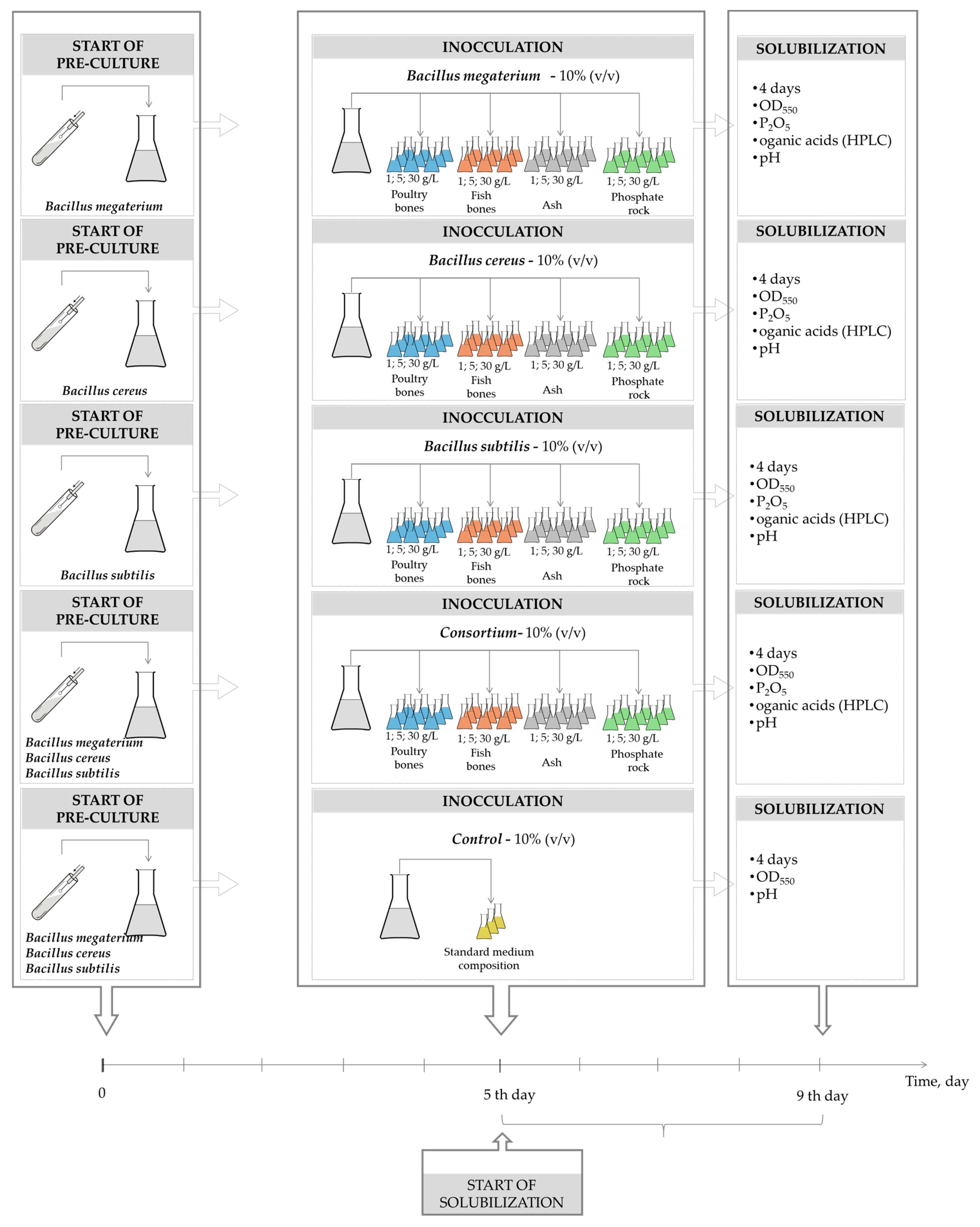
3. Materials and Methods
3.1. Bacteria and Culture Medium
3.2. Phosphate Source
3.3. Experimental and Analytical Methods
3.4. Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Report on Critical Raw Materials for the EU; Report of the Ad Hoc Working Group on Defining Critical Raw Materials; Ref. Ares (2015) 1819503; European Commission: Luxembourg, 2014.
- European Commission. Closing the Loop: Commission Adopts Ambitious New Circular Economy Package to Boost Competitiveness, Create Jobs and Generate Sustainable Growth. Available online: http://europa.eu/rapid/press-release_IP-15-6203_en.htm (accessed on 27 February 2018).
- Waste Atlas. Interactive Map with Visualized Waste Management Data, Global Waste Clock. Available online: http://www.atlas.d-waste.com (accessed on 27 February 2018).
- Schroder, J.J.; Cordell, D.; Smit, A.L.; Rosemarin, A. Sustainable Use of Phosphorus; Plant Research International: Wageningen, The Netherlands, 2010; Volume 357. [Google Scholar]
- Meena, V.S.; Maurya, B.R.; Meena, S.K.; Meena, R.K.; Kumar, A.; Verma, J.P.; Singh, N.P. Can Bacillus Species Enhance Nutrient Availability in Agricultural Soils? In Bacilli and Agrobiotechnology; Islam, M., Rahman, M., Pandey, P., Jha, C., Aeron, A., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhuang, W.; Li, N.N.; Tang, C.L.; Ying, H.J. Efficient biosynthesis of d-ribose using a novel co-feeding strategy in Bacillus subtilis without acid formation. Lett. Appl. Microbiol. 2017, 64, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Awasthi, R.; Tewari, R.; Nayyar, H. Synergy between Plants and P-Solubilizing Microbes in soils: Effects on Growth and Physiology of Crops. IRJM 2011, 2, 484–503. [Google Scholar]
- Haichar, F.Z.; Santaella, C.; Heulin, T.; Achouak, W. Root exudates mediated interactions below ground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Lanoue, A.; Strecker, T.; Scheu, S.; Steinauer, K.; Thakur, M.P.; Mommer, L. Root biomass and exudates link plant diversity with soil bacterial and fungal biomass. Sci. Rep. 2017, 7, 44641. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wang, G.; Farooq, T.H.; Li, Q.; Zou, X.; Ma, X. Low phosphorus and competition affect Chinese fir cutting growth and root organic acid content: Does neighboring root activity aggravate P nutrient deficiency? J. Soils Sediments 2017, 17, 2775–2785. [Google Scholar] [CrossRef]
- Krishnaraj, P.U.; Dahale, S. Mineral Phosphate Solubilization: Concepts and Prospects in Sustainable Agriculture. Proc. Indian Natl. Sci. Acad. 2014, 80, 389–405. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Saeid, A.; Dobrowolska-Iwanek, J.; Chojnacka, K. Utilization of microorganisms in the solubilization of low-quality phosphorus raw material. Ecol. Eng. 2016, 89, 109–113. [Google Scholar] [CrossRef]
- Saeid, A.; Labuda, M.; Chojnacka, K.; Górecki, H. Valorization of bones to liquid phosphorus fertilizer by microbial solubilization. Waste Biomass Valoriz. 2014, 5, 265–272. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Saeid, A.; Chojnacka, K.; Górecki, H. New generation of phosphate fertilizer from bones, produced by bacteria. Open Chem. 2015, 13, 951–958. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Saeid, A.; Chojnacka, K.; Górecki, H. Production of phosphate biofertilizers from bones by phosphate-solubilizing bacteria Bacillus megaterium. Open Chem. 2015, 13, 1063–1070. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Saeid, A.; Samoraj, M.; Chojnacka, K. Solid-state solubilization of bones by B. megaterium in spent mushroom substrate as a medium for a phosphate enriched substrate. J. Chem. Technol. Biotechnol. 2017, 92, 1397–1405. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Saeid, A.; Chojnacka, K. In situ solubilization of phosphorus bearing raw materials by Bacillus megaterium. Eng. Life Sci. 2017, 17, 749–758. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Saeid, A.; Malinowski, P.; Chojnacka, K. Valorization of phosphorus secondary raw materials by Acidithiobacillus ferrooxidans. Molecules 2017, 22, 473. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Kiran, S.; Gulati, A.; Singh, B.; Tewari, R. Isolation and identification of phosphate solubilizing bacteria able to enhance the growth and aloin-A biosynthesis of Aloe barbadensis Miller. Microbiol. Res. 2012, 167, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Labuda, M.; Saeid, A.; Chojnacka, K.; Górecki, H. Zastosowanie Bacillus megaterium w solubilizacji fosforu. Przem. Chem. 2012, 91, 837–840. (In Polish) [Google Scholar]
- Sabiha-Javied, M.T.; Chaudhry, M.M.; Tufail, M.; Irfan, N. Heavy metal pollution from phosphate rock used for the production of fertilizer in Pakistan. Microchem. J. 2009, 91, 94–99. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kawahata, H.; Gupta, L.P.; Itougac, M.; Sakakibarac, H.; Ohta, H.; Komai, T.; Onoe, Y. Chemistry of fly ash and cyclone ash leachate from waste materials and effects of ash leachates on bacterial growth, nitrogen-transformation activity, and metal accumulation. J. Hazard. Mater. 2009, 165, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Pande, A.; Pandey, P.; Mehra, S.; Singh, M.; Kaushik, S. Phenotypic and genotypic characterization of phosphate solubilizing bacteria and their efficiency on the growth of maize. J. Genet. Eng. Biotechnol. 2017, 15, 379–391. [Google Scholar] [CrossRef]
- Mardad, I.; Serrano, A.; Soukri, A. Solubilization of inorganic phosphate and production of organic acids by bacteria isolated from a Moroccan mineral phosphate deposit. Afr. J. Microbiol. Res. 2013, 7, 626–635. [Google Scholar]
- Singh, C.P.; Amberger, A. Organic acids and phosphorus solubilization in straw composted with rock phosphate. Bioresour. Technol. 1998, 63, 13–16. [Google Scholar] [CrossRef]
- Reyes, I.; Baziramakenga, R.; Bernier, L.; Antoun, H. Solubilization of phosphate rocks and minerals by a wild-type strain and two UV-induced mutants of Penicillium rugulosum. Soil Biol. Biochem. 2001, 33, 1741–1747. [Google Scholar] [CrossRef]
- Lin, T.F.; Huang, H.I.; Shen, F.T.; Young, C.C. The protons of gluconic acid are the major factor responsible for the dissolution of tricalcium phosphate by Burkholderia cepacia CC-Al74. Bioresour. Technol. 2006, 97, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- Hayat, W.; Aman, H.; Irshad, U.; Azeem, M.; Nazir, R. Analysis of ecological attributes of bacterial phosphorus solubilizers, native to pine forests of Lower Himalaya. Appl. Soil Ecol. 2017, 112, 51–59. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Y.; Shi, M.; Cao, Z.; Wei, Z. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour. Technol. 2018, 247, 190–199. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Bacteria Strain | Raw Materials | Monod | Haldane | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Unit | Value ± SD | p-Value | R2 | χ2 | Parameter | Unit | Value ± SD | p-Value | R2 | χ2 | ||
| B. megaterium | Bones | μmax | h−1 | 0.110 ± 0.001 * | 0.000 | 0.999 | 0.0000169 | ||||||
| Ks | g/L | 0.113 ± 0.014 * | 0.016 | ||||||||||
| Ash | μmax | h−1 | 0.129 ± 0.018 * | 0.019 | 0.964 | 0.00656 | |||||||
| Ks | g/L | 0.833 ± 0.594 | 0.296 | ||||||||||
| Fish bones | μmax | h−1 | 0.124 ± 0.007 * | 0.003 | 0.996 | 0.00103 | |||||||
| Ks | g/L | 1.874 ± 0.426 * | 0.048 | ||||||||||
| Phosphate rock | μmax | h−1 | 0.117 ± 0.000 * | 0.000 | 0.996 | 0.000 | |||||||
| Ks | g/L | 0.100 ± 0.001 * | 0.000 | ||||||||||
| B. cereus | Bones | μmax | h−1 | 0.123 ± 0.001 * | 0.000 | 0.999 | 0.000007 | ||||||
| Ks | g/L | 0.0465 ± 0.0077 * | 0.026 | ||||||||||
| Ash | μmax | h−1 | 0.135 ± 0.010 * | 0.005 | 0.989 | 0.00197 | |||||||
| Ks | g/L | 0.182 ± 0.155 | 0.363 | ||||||||||
| Fish bones | μmax | h−1 | 0.116 ± 0.001 * | 0.000 | 0.999 | 0.000050 | |||||||
| Ks | g/L | 0.220 ± 0.028 * | 0.016 | ||||||||||
| Phosphate rock | μmax | h−1 | 0.119 ± 0.016 * | 0.004 | 0.942 | 0.0129 | μmax | h−1 | 0.144 ± 0.038 | 0.064 | 0.959 | 0.009 | |
| Ks | g/L | 0.438 ± 0.384 | 0.373 | ||||||||||
| Ks | g/L | 0.252 ± 0.207 | 0.310 | KI | g/L | 85.9 ± 119 | 0.546 | ||||||
| B. subtilis | Bones | μmax | h−1 | 0.126 ± 0.003 * | 0.000 | 0.998 | 0.000207 | ||||||
| Ks | g/L | 0.0947 ± 0.0456 | 0.173 | ||||||||||
| Ash | μmax | h−1 | 0.127 ± 0.007 * | 0.003 | 0.993 | 0.00112 | |||||||
| Ks | g/L | 0.112 ± 0.109 | 0.412 | ||||||||||
| Fish bones | μmax | h−1 | 0.120 ± 0.011 * | 0.008 | 0.984 | 0.00253 | |||||||
| Ks | g/L | 0.525 ± 0.285 | 0.207 | ||||||||||
| Phosphate rock | μmax | h−1 | 0.104 ± 0.018 * | 0.009 | 0.998 | 0.0181 | μmax | h−1 | 0.129 ± 0.045 | 0.101 | 0.935 | 0.013 | |
| Ks | g/L | 0.233 ± 0.253 | 0.425 | Ks | g/L | 0.442 ± 0.496 | 0.467 | ||||||
| KI | g/L | 69.3 ± 109 | 0.590 | ||||||||||
| Bacteria Strain | Dose | Bones | Fish Bones | Ash | Phosphate Rock | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Value ± SD | p-Value | R2 | χ2 | Value ± SD | p-Value | R2 | χ2 | Value ± SD | p-Value | R2 | χ2 | Value ± SD | p-Value | R2 | χ2 | ||
| B. megaterium | 1 g/L | (mg/L) | 133 ± 2 * | 0.000 | 0.999 | 0.117 | 213 ± 6 | 0.001 | 0.987 | 0.987 | 47.5 ± 1.7 * | 0.001 | 0.995 | 0.258 | 37.0 ± 1.5 * | 0.002 | 0.990 | 0.0819 |
| b (day) | 3.14 ± 0.18 * | 0.003 | 0.999 ± 0.199 | 0.037 | 1.94 ± 0.31 * | 0.024 | 0.628 ± 0.082 * | 0.016 | ||||||||||
| k (1/day) | 0.0399 ± 0.0020 * | 0.002 | 0.0896 ± 0.0252 | 0.071 | 0.0391 ± 0.0065 * | 0.026 | 0.0216 ± 0.0056 | 0.061 | ||||||||||
| 5 g/L | (mg/L) | 270 ± 11 * | 0.002 | 0.998 | 2.54 | 483 ± 5 | 0.000 | 0.998 | 0.128 | 85.4 ± 1.7 * | 0.000 | 0.999 | 0.0676 | 33.4 ± 1.4 * | 0.002 | 0.994 | 0.102 | |
| b (day) | 7.25 ± 0.99 * | 0.018 | 0.565 ± 0.028 | 0.002 | 3.26 ± 0.14 * | 0.002 | 1.31 ± 0.16 * | 0.014 | ||||||||||
| k (1/day) | 0.0287 ± 0.0029 * | 0.009 | 0.0304 ± 0.0026 | 0.007 | 0.0238 ± 0.0012 * | 0.003 | 0.0257 ± 0.0047 * | 0.032 | ||||||||||
| 30 g/L | (mg/L) | 279 ± 6 * | 0.000 | 0.999 | 0.591 | 433 ± 12 | 0.001 | 0.985 | 0.775 | 43.6 ± 1.2 * | 0.001 | 0.999 | 0.0376 | 29.5 ± 3.2 * | 0.011 | 0.982 | 0.338 | |
| b (day) | 73.5 ± 6.4 * | 0.007 | 0.408 ± 0.061 | 0.022 | 1.56 ± 0.08 * | 0.003 | 1.61 ± 0.35 * | 0.043 | ||||||||||
| k (1/day) | 0.0355 ± 0.0012 * | 0.001 | 0.0284 ± 0.0082 | 0.074 | 0.0200 ± 0.0018 * | 0.008 | 0.0206 ± 0.0073 | 0.106 | ||||||||||
| B. cereus | 1 g/L | (mg/L) | 67.8 ± 2.86 * | 0.002 | 0.970 | 0.345 | 221 ± 5 | 0.001 | 0.996 | 0.241 | 33.5 ± 0.7 * | 0.000 | 0.998 | 0.0388 | 35.1 ± 1.8 * | 0.003 | 0.993 | 0.999 |
| b (day) | 0.523 ± 0.122 | 0.051 | 0.834 ± 0.073 | 0.008 | 1.95 ± 0.18 * | 0.008 | 1.09 ± 0.13 * | 0.014 | ||||||||||
| k (1/day) | 0.0362 ± 0.0144 | 0.128 | 0.0279 ± 0.0040 | 0.020 | 0.0410 ± 0.0038 * | 0.009 | 0.0203 ± 0.0044 * | 0.043 | ||||||||||
| 5 g/L | (mg/L) | 76.8 ± 4.9 * | 0.004 | 0.975 | 1.29 | 393 ± 16 | 0.002 | 0.987 | 3.23 | 53.1 ± 3.3 * | 0.004 | 0.993 | 0.524 | 33.9 ± 2.8 * | 0.007 | 0.994 | 0.131 | |
| b (day) | 1.33 ± 0.392 | 0.077 | 1.93 ± 0.55 | 0.073 | 3.06 ± 0.58 * | 0.034 | 1.57 ± 0.20 * | 0.016 | ||||||||||
| k (1/day) | 0.0404 ± 0.0141 | 0.104 | 0.0890 ± 0.0223 | 0.057 | 0.0285 ± 0.0056 * | 0.037 | 0.0163 ± 0.0037 * | 0.047 | ||||||||||
| 30 g/L | (mg/L) | 42.6 ± 4.25 * | 0.010 | 0.976 | 2.21 | 405 ± 5 | 0.000 | 0.998 | 0.347 | 42.7 ± 2.8 * | 0.004 | 0.991 | 0.210 | 31.5 ± 6.4 * | 0.038 | 0.981 | 0.330 | |
| b (day) | 3.33 ± 1.43 | 0.145 | 1.2364 ± 0.105419 | 0.007 | 1.41 ± 0.20 * | 0.020 | 1.63 ± 0.46 | 0.071 | ||||||||||
| k (1/day) | 0.0363 ± 0.0138 | 0.119 | 0.0855 ± 0.0087 | 0.010 | 0.0214 ± 0.0052 | 0.054 | 0.0145 ± 0.0067 | 0.164 | ||||||||||
| B. subtilis | 1 g/L | (mg/L) | 79.4 ± 13.3 * | 0.027 | 0.993 | 0.445 | 242 ± 26 | 0.011 | 0.962 | 4.09 | 42.1 ± 1.2 * | 0.001 | 0.996 | 0.0860 | 33.1 ± 2.8 * | 0.007 | 0.977 | 0.293 |
| b (day) | 2.12 ± 0.44 * | 0.041 | 0.981 ± 0.285 | 0.075 | 1.06 ± 0.10 * | 0.009 | 1.03 ± 0.23 * | 0.047 | ||||||||||
| k (1/day) | 0.0136 ± 0.0039 | 0.076 | 0.0221 ± 0.0115 | 0.194 | 0.0273 ± 0.0041 * | 0.022 | 0.0224 ± 0.0089 | 0.127 | ||||||||||
| 5 g/L | (mg/L) | 56.6 ± 3.6 * | 0.004 | 0.944 | 0.791 | 427 ± 10 | 0.001 | 0.993 | 0.886 | 36.6 ± 1.5 * | 0.002 | 0.988 | 0.274 | 32.0 ± 5.3 * | 0.026 | 0.964 | 0.382 | |
| b (day) | 0.668 ± 0.235 | 0.105 | 0.842 ± 0.112 | 0.017 | 1.34 ± 0.28 * | 0.040 | 0.939 ± 0.298 | 0.088 | ||||||||||
| k (1/day) | 0.0429 ± 0.0229 | 0.202 | 0.0522 ± 0.0095 | 0.031 | 0.0453 ± 0.0106 | 0.051 | 0.0155 ± 0.0096 | 0.247 | ||||||||||
| 30 g/L | (mg/L) | 70.2 ± 34.5 | 0.179 | 0.958 | 1.504 | 428 ± 14 | 0.001 | 0.984 | 2.27 | 51.0 ± 2.9 * | 0.003 | 0.985 | 0.665 | 41.2 ± 6.8 * | 0.026 | 0.994 | 0.0622 | |
| b (day) | 1.79 ± 1.17 | 0.266 | 0.900 ± 0.195 | 0.044 | 1.65 ± 0.41 | 0.056 | 1.16 ± 0.32 | 0.068 | ||||||||||
| k (1/day) | 0.0118 ± 0.0098 | 0.353 | 0.0894 ± 0.0294 | 0.093 | 0.0384 ± 0.0106 | 0.069 | 0.00983 ± 0.00341 | 0.102 | ||||||||||
| Consortium | 30 g/L | (mg/L) | 106 ± 4 * | 0.002 | 0.992 | 0.719 | 414 ± 9 | 0.001 | 0.994 | 0.934 | 27.3 ± 1.3 * | 0.002 | 0.978 | 0.106 | 36.7 ± 6.9 * | 0.034 | 0.989 | 0.0836 |
| b (day) | 2.24 ± 0.469 * | 0.041 | 1.08 ± 0.15 | 0.018 | 0.549 ± 0.104 * | 0.034 | 0.918 ± 0.323 | 0.105 | ||||||||||
| k (1/day) | 0.0430 ± 0.0085 * | 0.037 | 0.0680 ± 0.0114 | 0.027 | 0.0248 ± 0.0090 | 0.111 | 0.00973 ± 0.00470 | 0.174 | ||||||||||
| Bacteria Strain | Raw Material | Dose g/L | Gluconic Acid | 2-Hydroxypropanoic Acid (Lactic Acid) | Acetic Acid | Butanedoic Acid (Succinic Acid) | Propionic Acid | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Con. ± SD | f(dose) = Con. | Con. ± SD | f(dose) = Con. | Con. ± SD | f(dose) = Con. | Con. ± SD | f(dose) = Con. | Con. ± SD | f(dose) = Con. | ||||||||
| R | p | R | p | R | p | R | p | R | p | ||||||||
| B. megaterium | PB | 1 | 3.15 ± 0.03 | 0.940 | 0.222 | 1.32 ± 0.03 * | 0.998 | 0.043 | 2.83 ± 0.06 | −0.463 | 0.694 | <LOQ | - | - | <LOQ | - | - |
| 5 | 7.13 ± 0.19 | 2.12 ± 0.18 * | 2.97 ± 0.02 | 1.63 ± 0.03 | <LOQ | ||||||||||||
| 30 | 11.8 ± 0.1 | 5.20 ± 0.07 * | 2.82 ± 0.02 | 8.63 ± 0.01 | <LOQ | ||||||||||||
| A | 1 | 0.542 ± 0.049 | −0.725 | 0.483 | 0.849 ± 0.034 | 0.981 | 0.124 | 1.38 ± 0.05 | 0.636 | 0.562 | 0.660 ± 0.023 | - | - | <LOQ | - | - | |
| 5 | 0.135 ± 0.004 | 1.78 ± 0.03 | 3.01 ± 0.06 | <LOQ | <LOQ | ||||||||||||
| 30 | 0.051 ± 0.006 | 3.71 ± 0.08 | 3.09 ± 0.17 | <LOQ | <LOQ | ||||||||||||
| FB | 1 | 5.14 ± 0.20 | - | - | 4.05 ± 0.09 | 0.992 | 0.080 | 1.22 ± 0.06 | 0.469 | 0.689 | 1.19 ± 0.01 | 0.994 | 0.071 | <LOQ | - | - | |
| 5 | 9.71 ± 0.09 | 10.1 ± 0.2 | 4.28 ± 0.07 | 1.39 ± 0.02 | <LOQ | ||||||||||||
| 30 | <LOQ | 27.5 ± 0.1 | 3.75 ± 0.02 | 11.8 ± 0.02 | <LOQ | ||||||||||||
| PR | 1 | 0.375 ± 0.046 | 0.853 | 0.350 | 0.992 ± 0.054 | 0.971 | 0.153 | 3.06 ± 0.04 | 0.709 | 0.498 | <LOQ | - | - | <LOQ | - | - | |
| 5 | 0.579 ± 0.502 | 4.34 ± 0.65 | 5.32 ± 0.62 | 0.169 ± 0.014 | <LOQ | ||||||||||||
| 30 | 0.697 ± 0.041 | 10.2 ± 0.1 | 5.72 ± 0.11 | 0.331 ± 0.018 | <LOQ | ||||||||||||
| B. cereus | PB | 1 | 0.608 ± 0.037 | - | - | 2.69 ± 0.40 * | 0.999 | 0.031 | <LOQ | - | - | <LOQ | - | - | <LOQ | - | - |
| 5 | <LOQ | 8.91 ± 0.48 * | <LOQ | <LOQ | 0.439 ± 0.007 | ||||||||||||
| 30 | <LOQ | 36.0 ± 0.4 * | <LOQ | <LOQ | 1.89 ± 0.015 | ||||||||||||
| A | 1 | 0.589 ± 0.026 | 0.522 | 0.650 | 2.26 ± 0.18 | 0.995 | 0.062 | <LOQ | - | - | <LOQ | - | - | <LOQ | - | - | |
| 5 | 0.393 ± 0.008 | 2.37 ± 0.05 | <LOQ | <LOQ | <LOQ | ||||||||||||
| 30 | 0.628 ± 0.008 | 5.26 ± 0.10 | 1.12 ± 0.05 | <LOQ | <LOQ | ||||||||||||
| FB | 1 | <LOQ | - | - | 4.38 ± 0.06 | 0.980 | 0.128 | 15.2 ± 0.3 | −0.273 | 0.824 | <LOQ | - | - | <LOQ | - | - | |
| 5 | 1.24 ± 0.06 | 14.6 ± 0.1 | 2.57 ± 0.06 | 0.390 ± 0.108 | 0.334 ± 0.008 | ||||||||||||
| 30 | <LOQ | 35.5 ± 0.3 | 7.25 ± 0.04 | 0.709 ± 0.022 | 0.690 ± 0.044 | ||||||||||||
| PR | 1 | 0.329 ± 0.015 | −0.669 | 0.533 | 3.10 ± 0.19 | 0.959 | 0.184 | <LOQ | - | - | <LOQ | - | - | <LOQ | - | - | |
| 5 | 0.318 ± 0.001 | 5.28 ± 0.10 | 0.985 ± 0.076 | <LOQ | <LOQ | ||||||||||||
| 30 | 0.316 ± 0.005 | 8.48 ± 0.09 | 0.685 ± 0.024 | <LOQ | <LOQ | ||||||||||||
| B. subtilis | PB | 1 | 0.391 ± 0.021 | - | - | 3.84 ± 0.07 * | 0.999 | 0.024 | <LOQ | - | - | <LOQ | - | - | <LOQ | - | - |
| 5 | <LOQ | 7.73 ± 0.06 * | 4.39 ± 0.19 | <LOQ | 2.67 ± 0.05 | ||||||||||||
| 30 | <LOQ | 25.9 ± 0.9 * | 13.8 ± 0.8 | 0.499 ± 0.037 | 0.565 ± 0.019 | ||||||||||||
| A | 1 | 0.422 ± 0.001 * | −0.997 | 0.045 | 1.93 ± 0.08 | −0.651 | 0.549 | <LOQ | - | - | <LOQ | - | - | <LOQ | - | - | |
| 5 | 0.413 ± 0.018 * | 3.46 ± 0.01 | 2.41 ± 0.08 | <LOQ | <LOQ | ||||||||||||
| 30 | 0.376 ± 0.007 * | 1.22 ± 0.02 | 5.96 ± 0.07 | 0.302 ± 0.007 | 0.652 ± 0.055 | ||||||||||||
| FB | 1 | <LOQ | - | - | 3.22 ± 0.04 | 0.988 | 0.098 | 2.17 ± 0.08 | 0.749 | 0.461 | 0.097 ± 0.011 | 0.565 | 0.618 | <LOQ | - | - | |
| 5 | 0.646 ± 0.014 | 12.8 ± 0.1 | 5.30 ± 0.06 | 0.409 ± 0.020 | <LOQ | ||||||||||||
| 30 | <LOQ | 36.7 ± 0.4 | 6.11 ± 0.21 | 0.391 ± 0.056 | <LOQ | ||||||||||||
| PR | 1 | 0.426 ± 0.024 | −0.655 | 0.545 | 4.31 ± 0.05 | −0.071 | 0.954 | <LOQ | - | - | <LOQ | - | - | <LOQ | - | - | |
| 5 | 0.228 ± 0.001 | 3.39 ± 0.16 | 7.79 ± 0.28 | 0.097 ± 0.004 | <LOQ | ||||||||||||
| 30 | 0.212 ± 0.003 | 3.90 ± 0.13 | 3.92 ± 0.08 | 0.234 ± 0.017 | <LOQ | ||||||||||||
| Consortium | PB | 30 | <LOQ | - | - | 23.1 ± 0.2 | - | - | 12.9 ± 0.3 | - | - | 0.356 ± 0.060 | - | - | 0.751 ± 0.010 | - | - |
| A | 30 | 0.370 ± 0.019 | 4.63 ± 0.17 | 4.58 ± 0.26 | <LOQ | <LOQ | |||||||||||
| FB | 30 | <LOQ | 37.7 ± 0.1 | 10.1 ± 0.5 | 0.668 ± 0.003 | 0.485 ± 0.015 | |||||||||||
| FR | 30 | 0.315 ± 0.027 | 7.99 ± 0.15 | 5.17 ± 0.23 | 0.255 ± 0.005 | <LOQ | |||||||||||
| Bacteria | Dose (g/L) | R | p-Value | R2 | |
|---|---|---|---|---|---|
| B. cereus | 1 | = −11.2 + 10.7 · Cacids | 0.985 | 0.014 | 0.971 |
| 5 | = −76.3 + 22.7 · Cacids | 0.936 | 0.064 | 0.876 | |
| 30 | = −31.6 + 6.6 · Cacids | 0.688 | 0.312 | 0.473 | |
| B. megaterium | 1 | = −40.3 + 22.1 · Cacids | 0.982 | 0.0177 | 0.965 |
| 5 | = −77.1 + 21.6 · Cacids | 0.922 | 0.0777 | 0.851 | |
| 30 | = −88.2 + 11.9 · Cacids | 0.953 | 0.0468 | 0.909 | |
| B. subtilis | 1 | = −98.6 + 47.1 · Cacids | 0.647 | 0.353 | 0.419 |
| 5 | = −224 + 28 · Cacids | 0.787 | 0.213 | 0.619 | |
| 30 | = −13.4 + 6.4 · Cacids | 0.663 | 0.337 | 0.439 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeid, A.; Prochownik, E.; Dobrowolska-Iwanek, J. Phosphorus Solubilization by Bacillus Species. Molecules 2018, 23, 2897. https://doi.org/10.3390/molecules23112897
Saeid A, Prochownik E, Dobrowolska-Iwanek J. Phosphorus Solubilization by Bacillus Species. Molecules. 2018; 23(11):2897. https://doi.org/10.3390/molecules23112897
Chicago/Turabian StyleSaeid, Agnieszka, Ewelina Prochownik, and Justyna Dobrowolska-Iwanek. 2018. "Phosphorus Solubilization by Bacillus Species" Molecules 23, no. 11: 2897. https://doi.org/10.3390/molecules23112897
APA StyleSaeid, A., Prochownik, E., & Dobrowolska-Iwanek, J. (2018). Phosphorus Solubilization by Bacillus Species. Molecules, 23(11), 2897. https://doi.org/10.3390/molecules23112897







