Olfactory Impact of Terpene Alcohol on Terpenes Aroma Expression in Chrysanthemum Essential Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Aromatic Recombination
2.3. Terpenes and Terpene Alcohols Analyses
2.3.1. Solid Phase Micro Extraction (SPME) Condition
2.3.2. Gas Chromatography−Mass Spectrometer Analyses
2.4. Electronic Nose
2.5. Olfactory Analyses
2.5.1. Laboratorial Environment
2.5.2. Addition and Omission Test
2.5.3. Olfactory Thresholds Test
2.5.4. Descriptive Testing Methods
2.6. Statistical Analysis
3. Results and Discussion
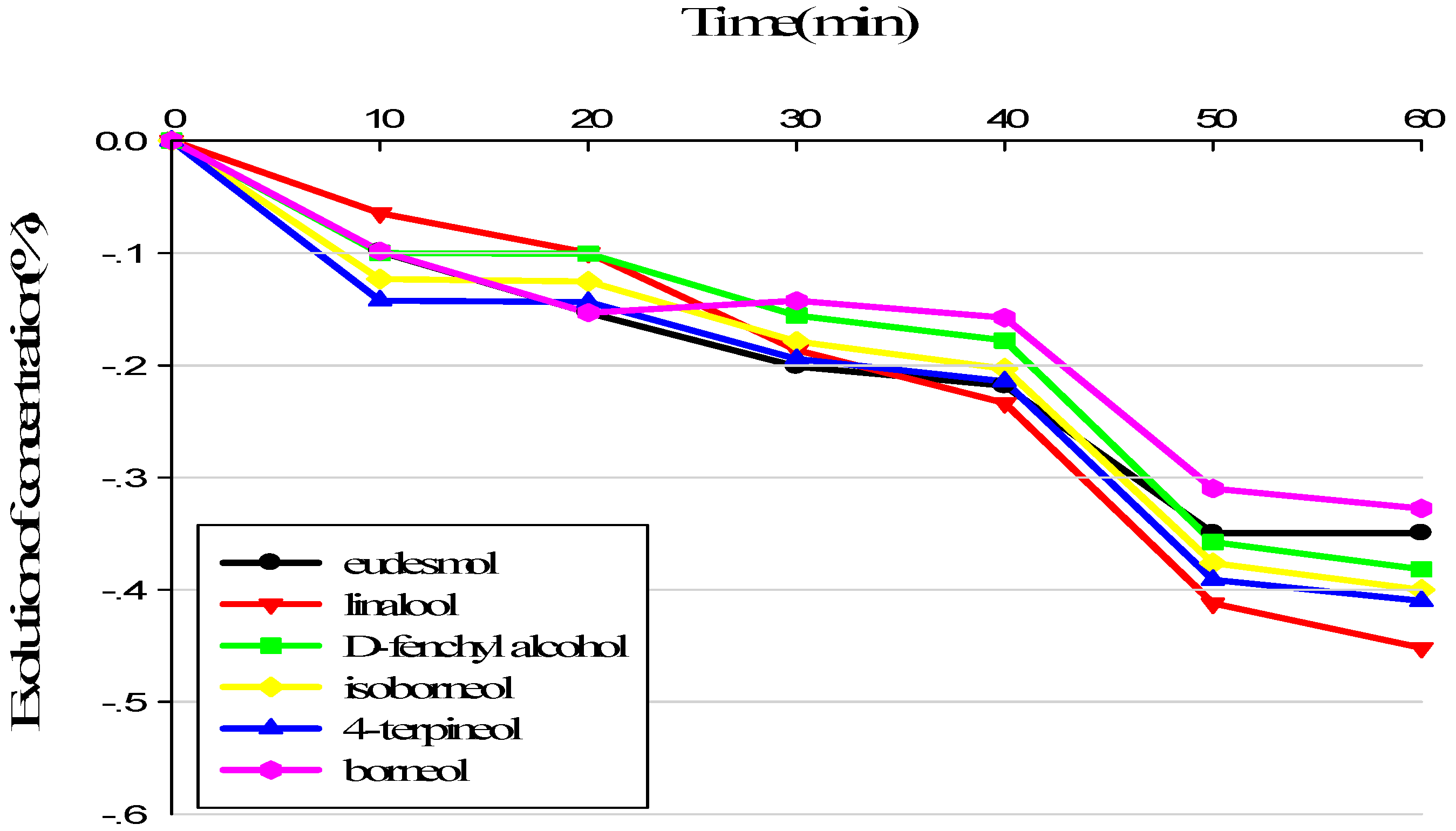
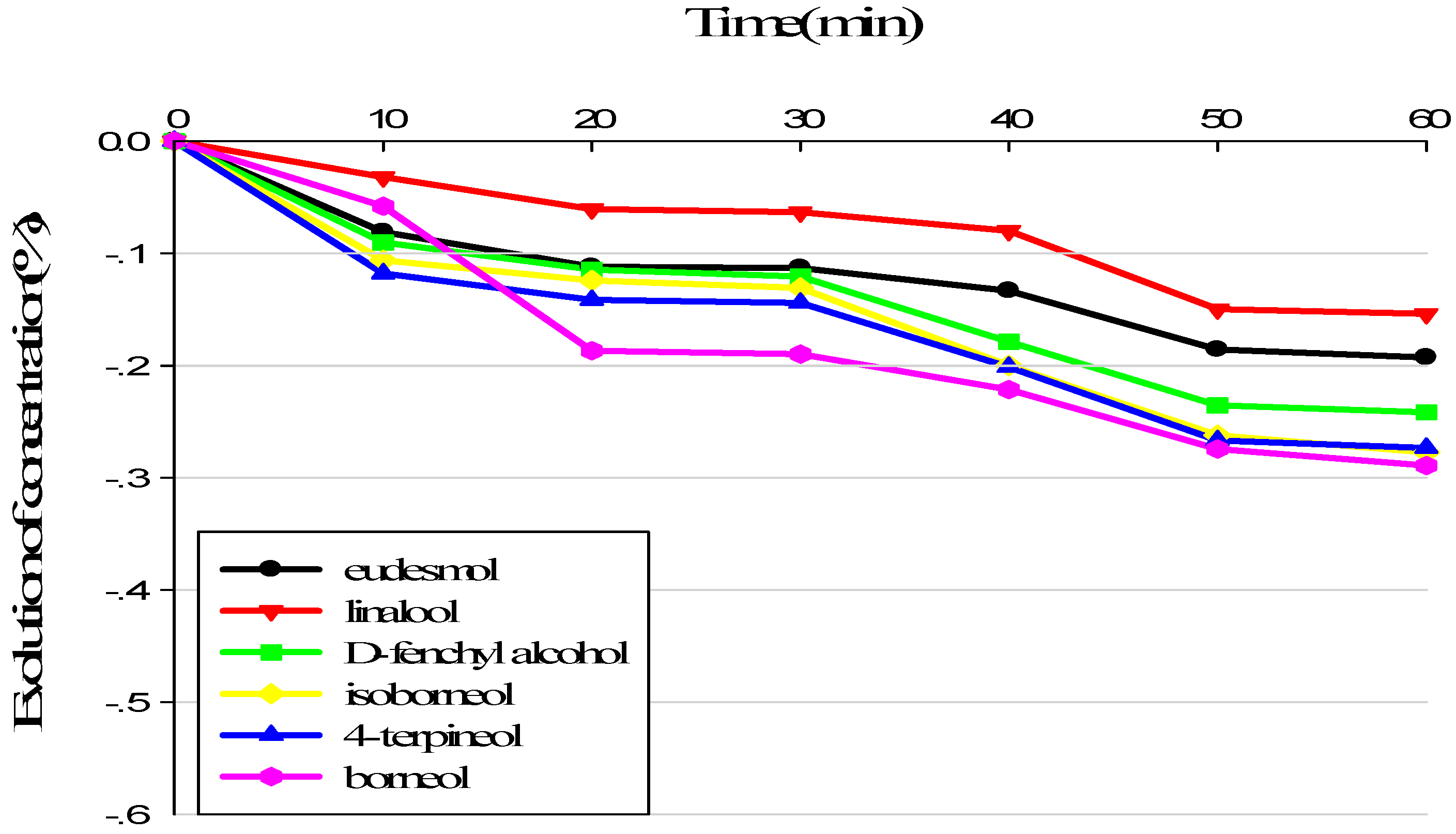
3.1. Evolution of Sample Composition during Sensory Analysis
3.2. Organoleptic Effect of Terpene Alcohols on Quantitative Perception
3.2.1. Addition and Omission Tests
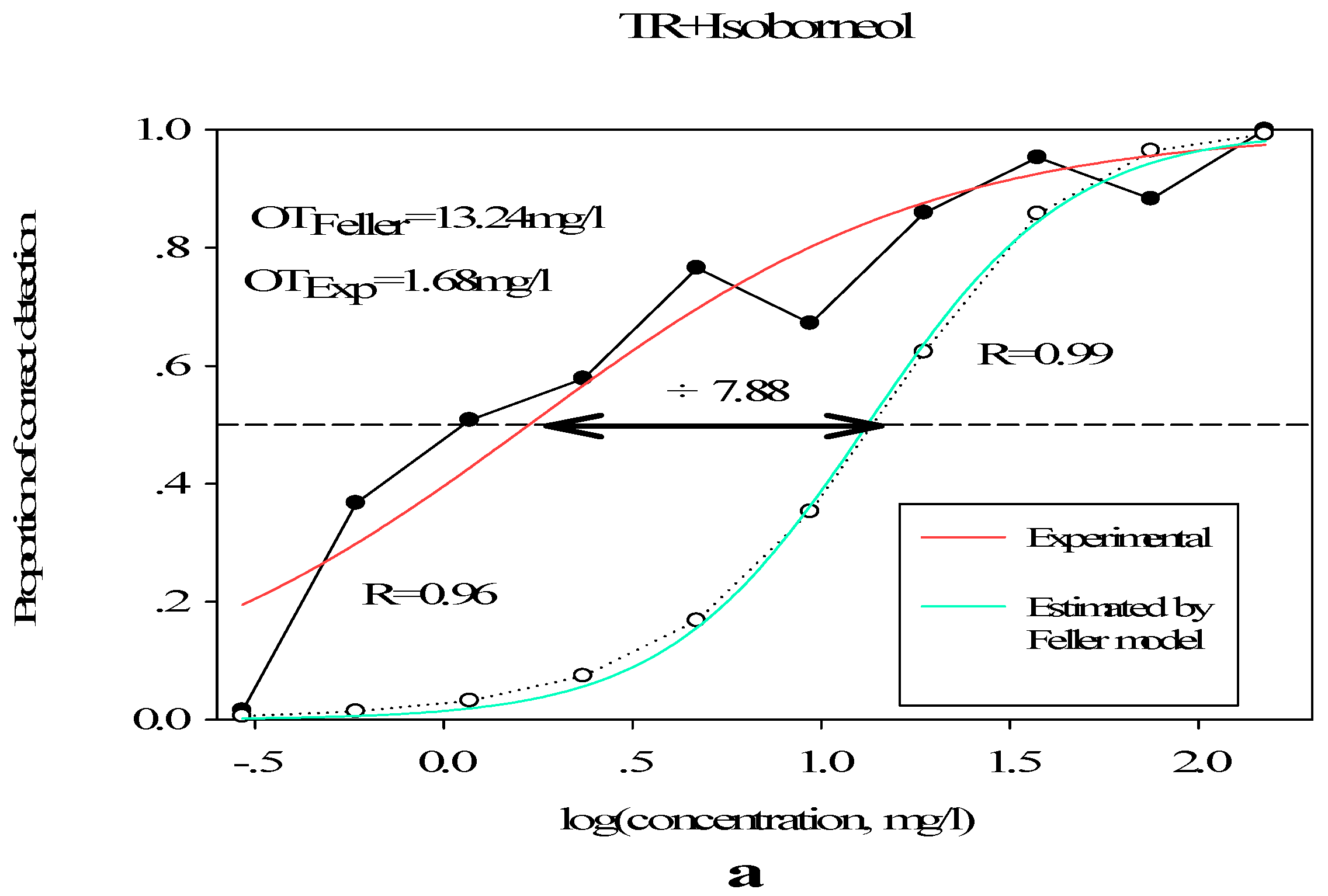
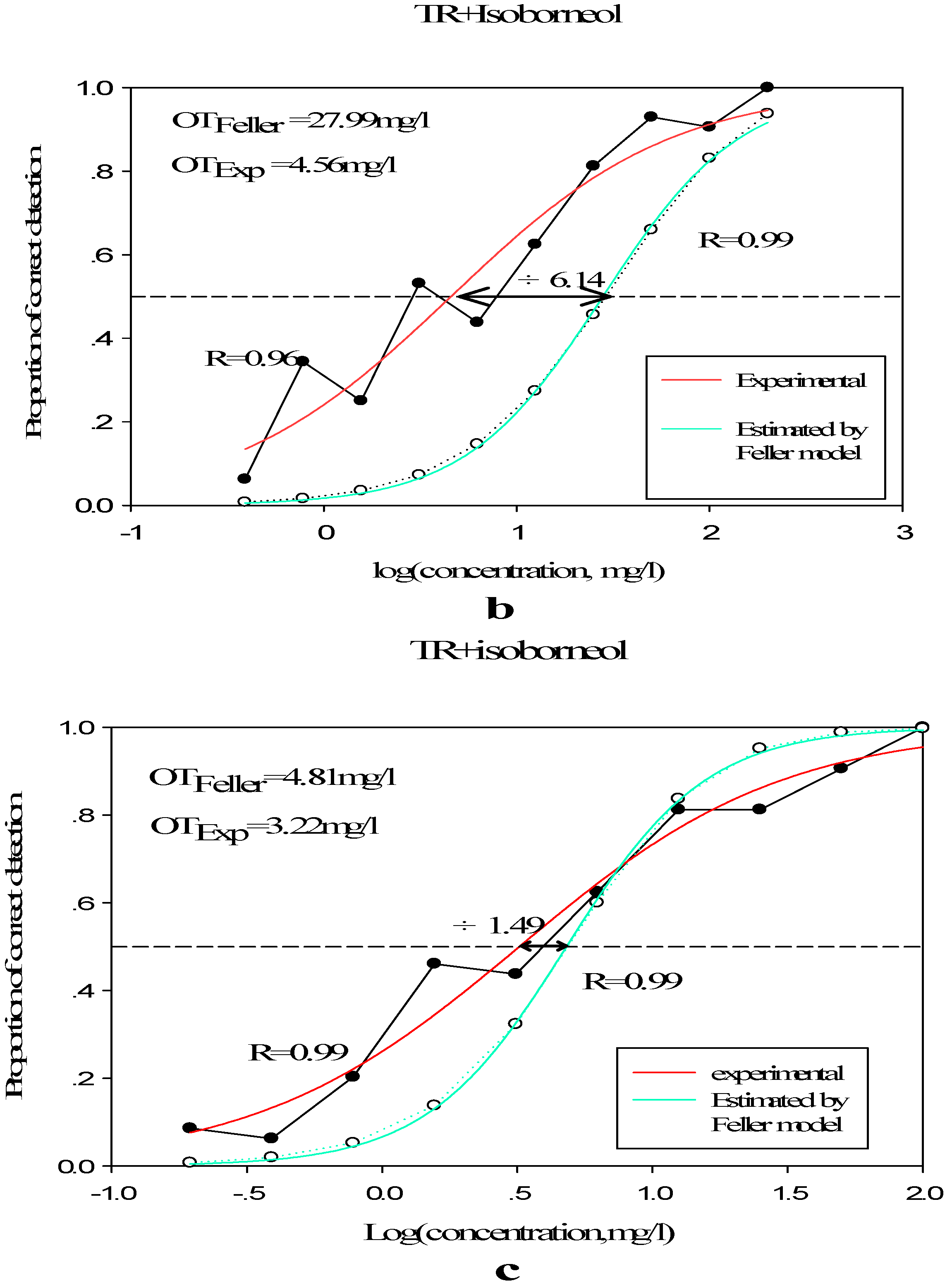
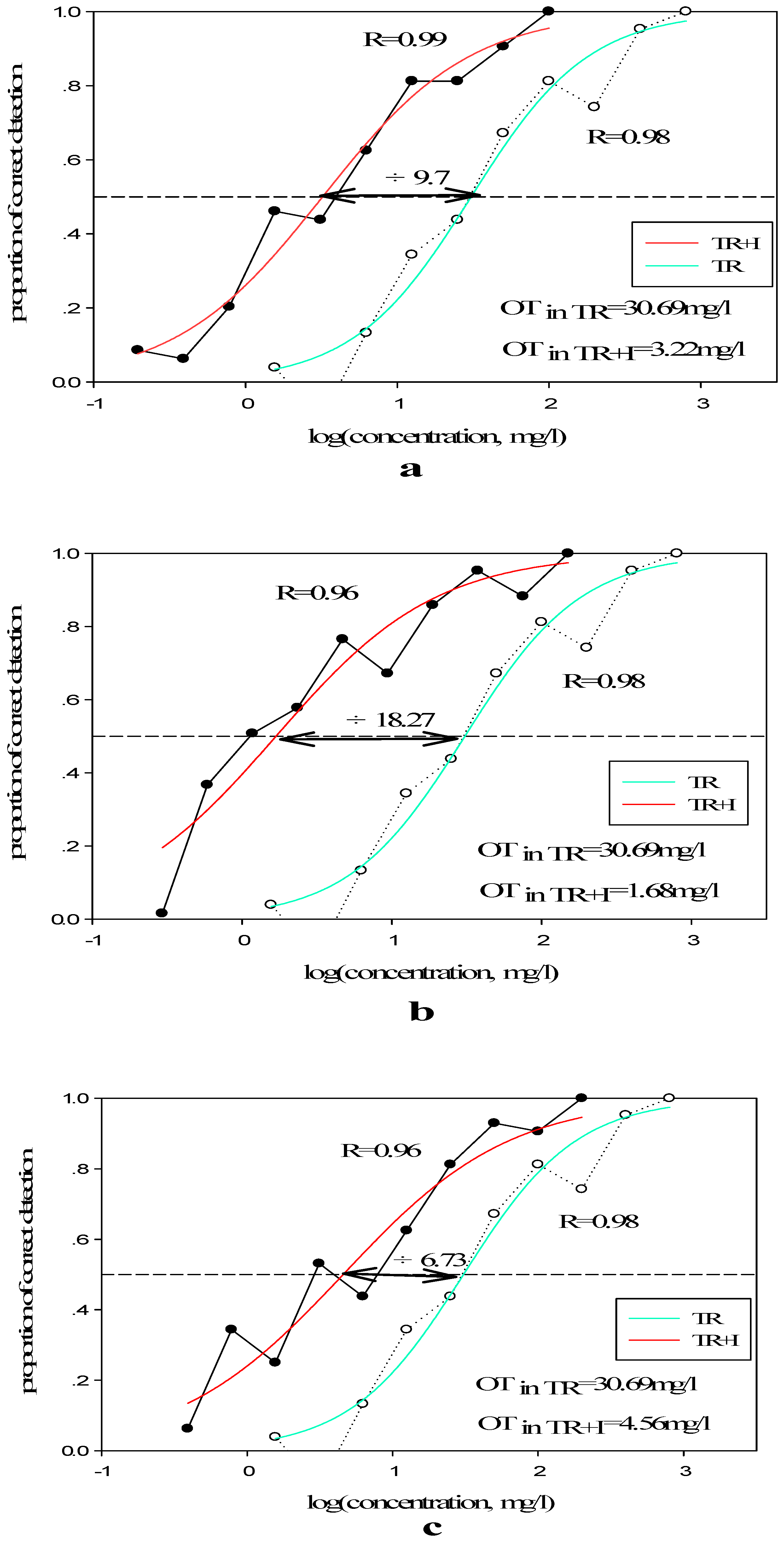
3.2.2. Olfactory Threshold of Terpene Alcohols in Different Matrixes
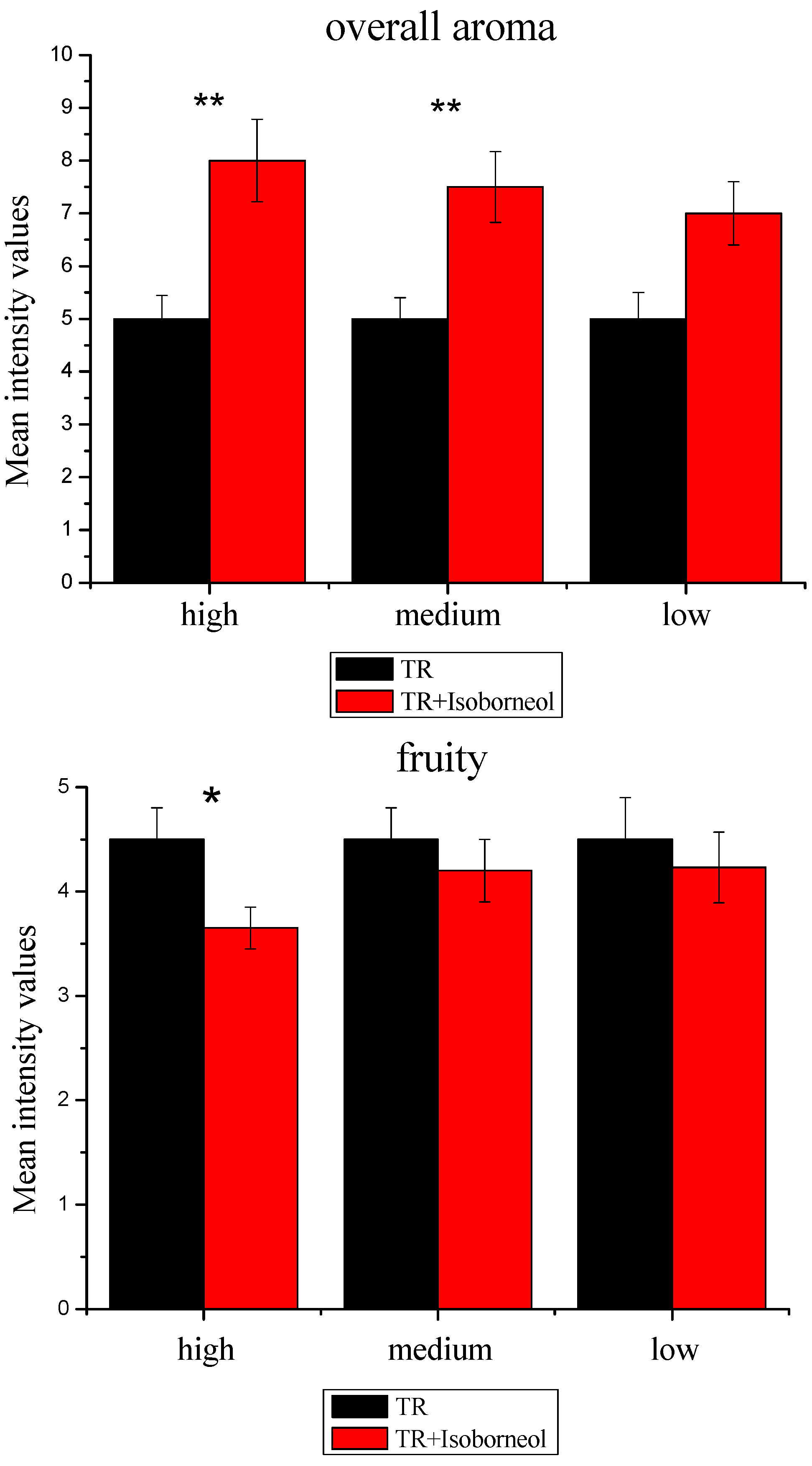
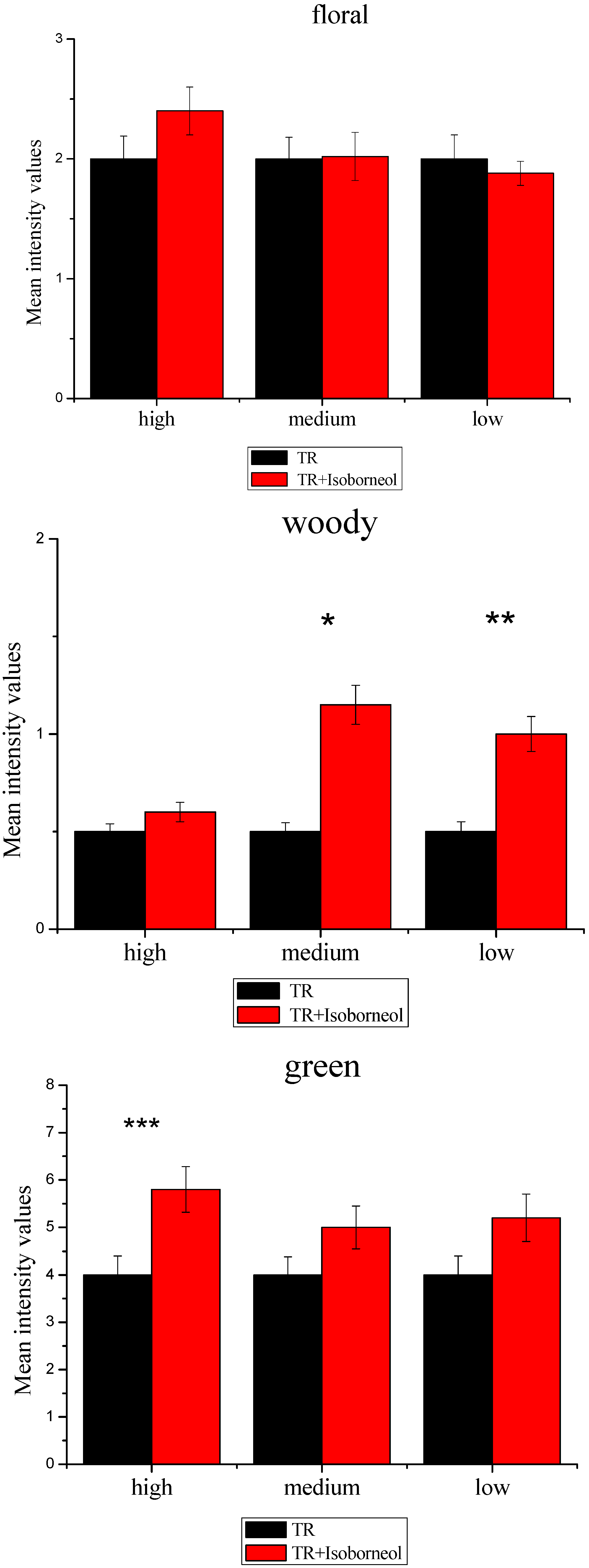
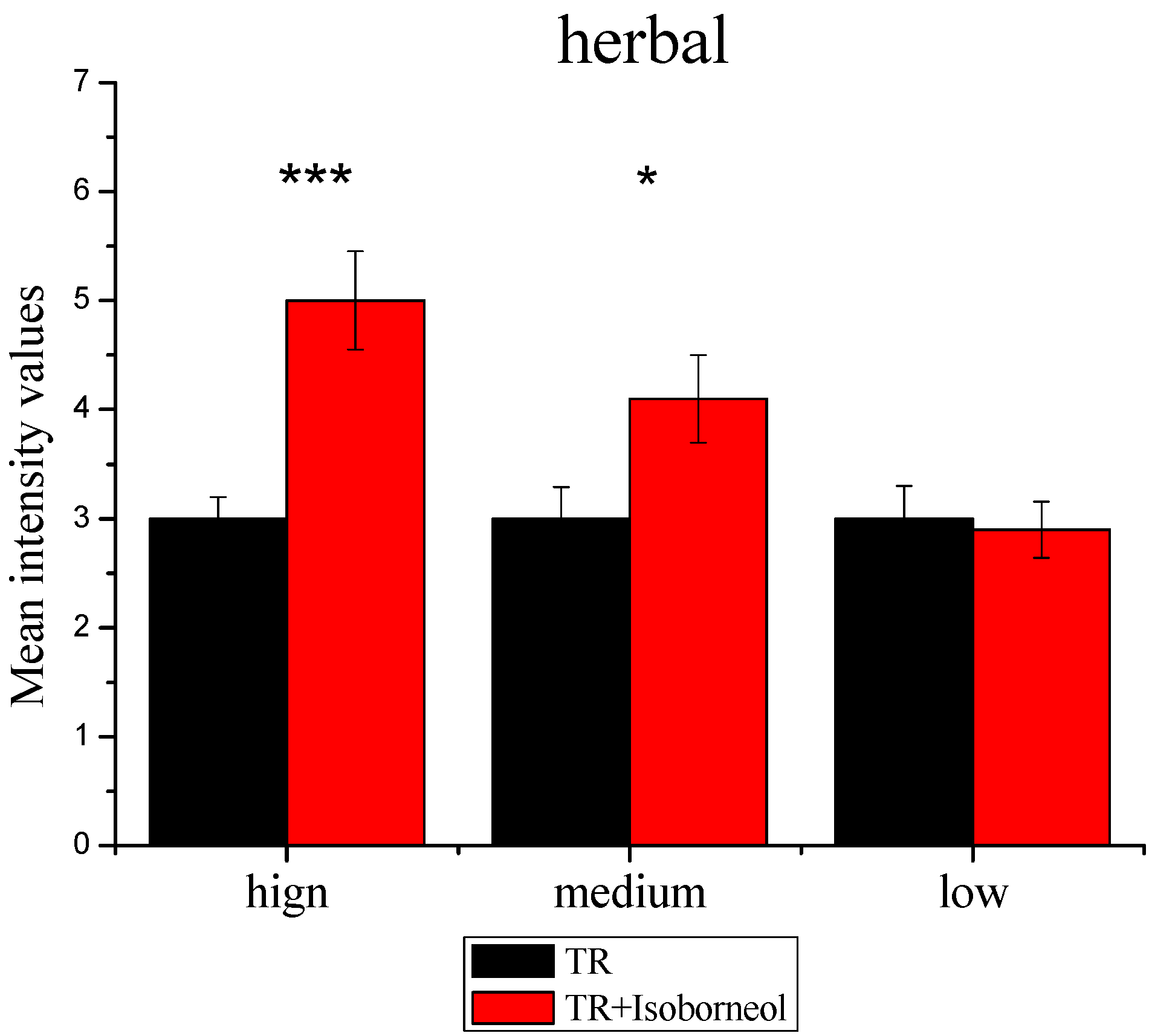
3.2.3. Sensory Effect of Terpene Alcohols on Aroma Expression
3.3. Sensory Effect of Terpene Alcohols on Qualitative Aroma Perception
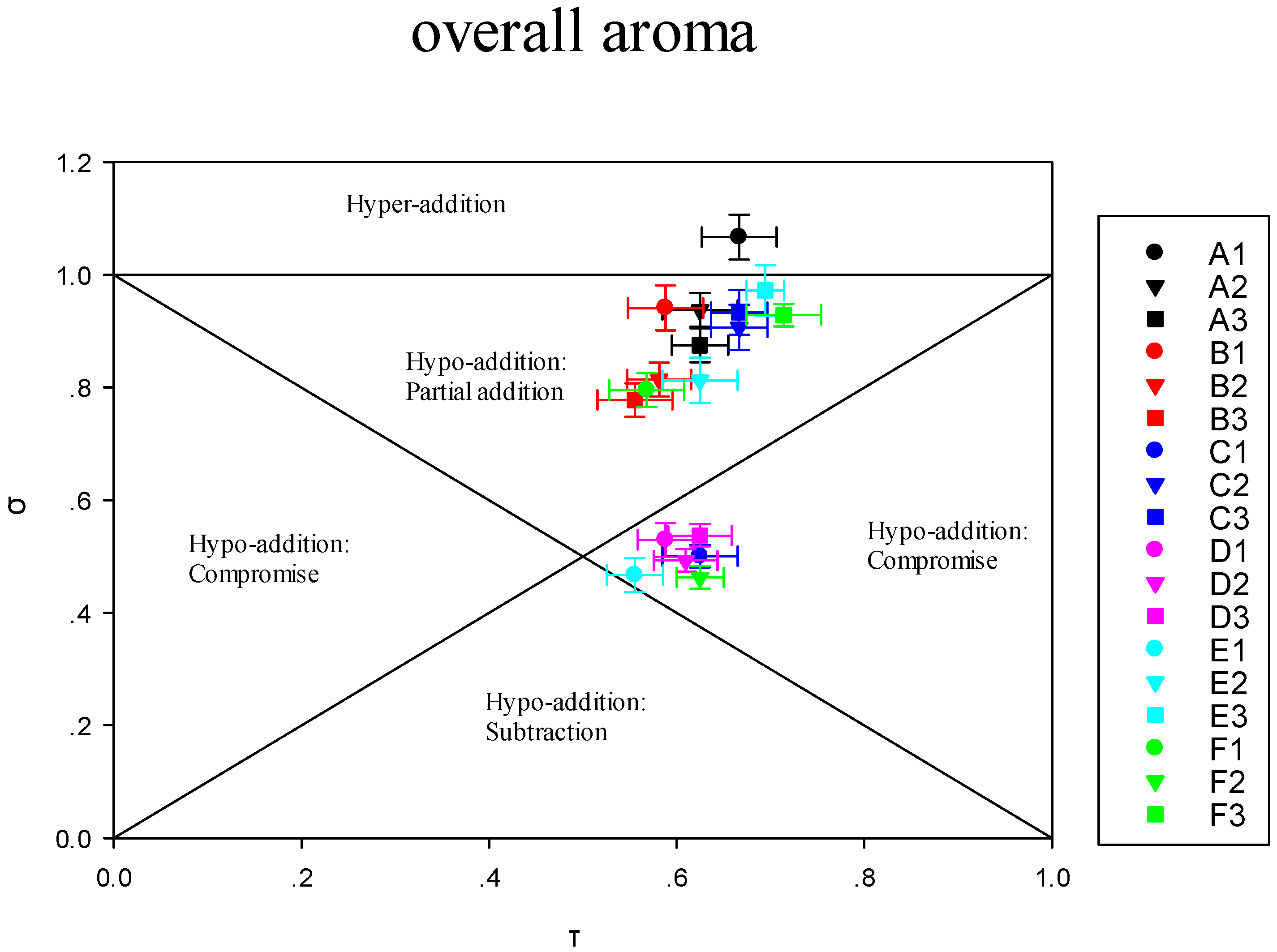
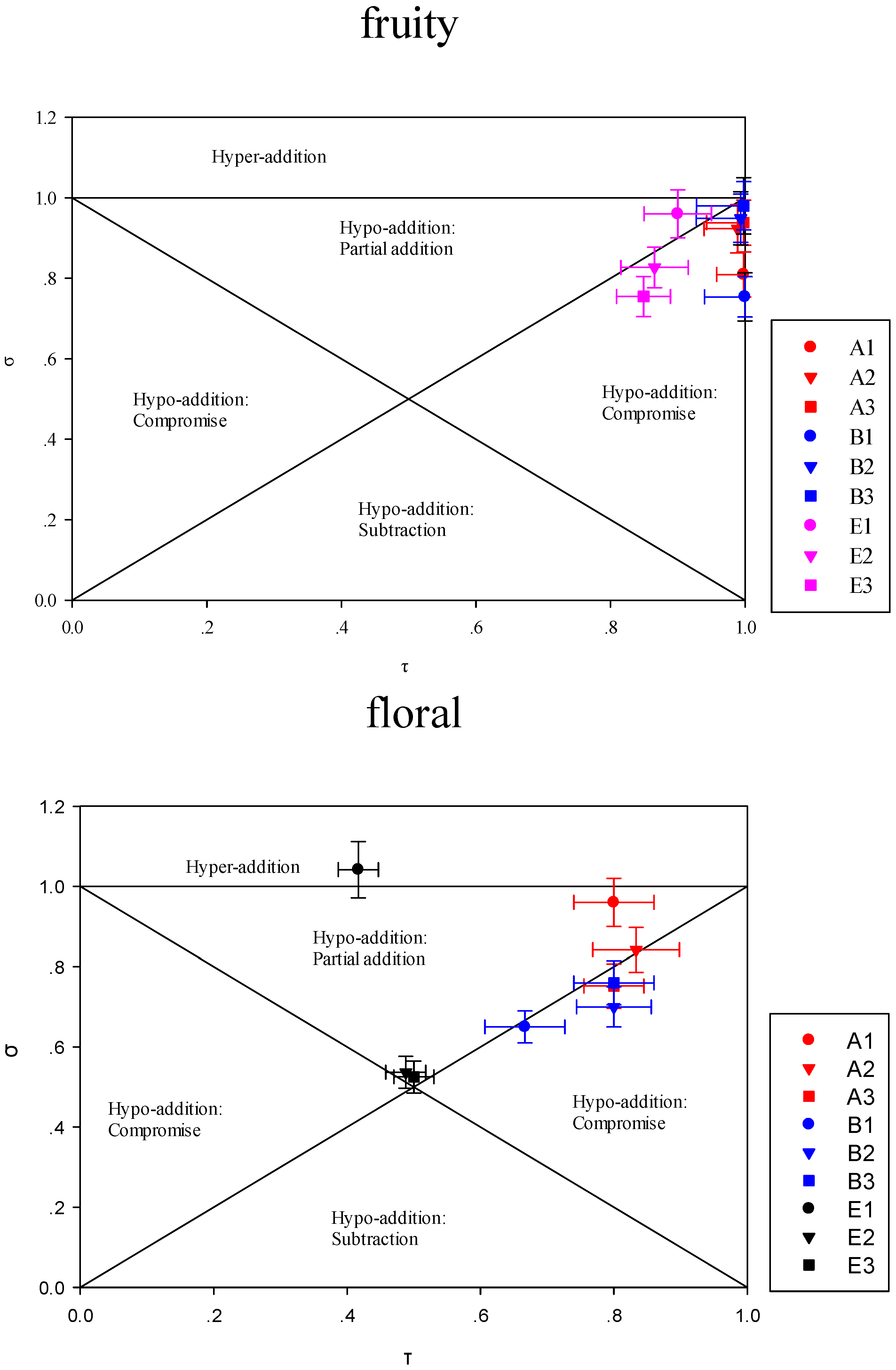
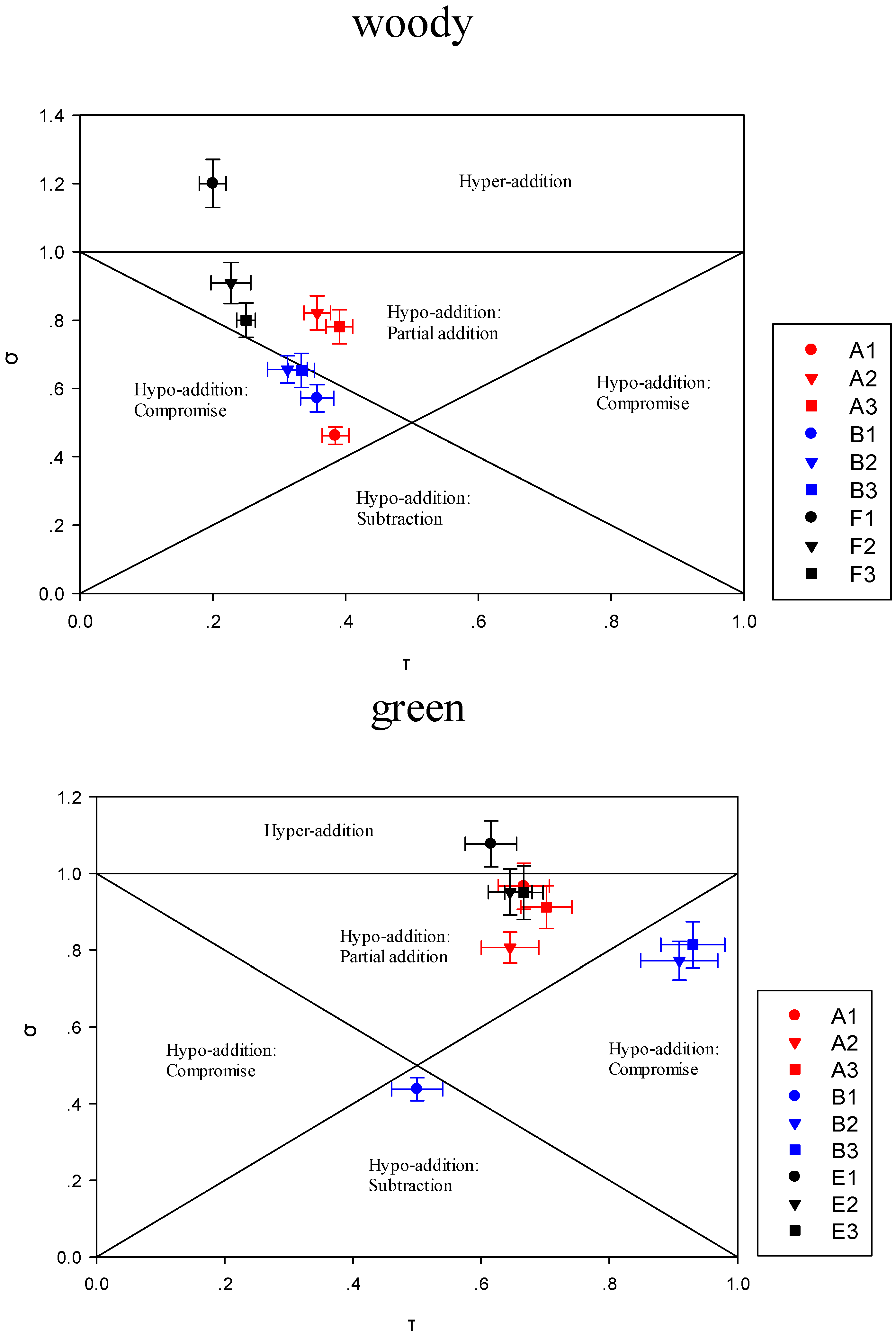
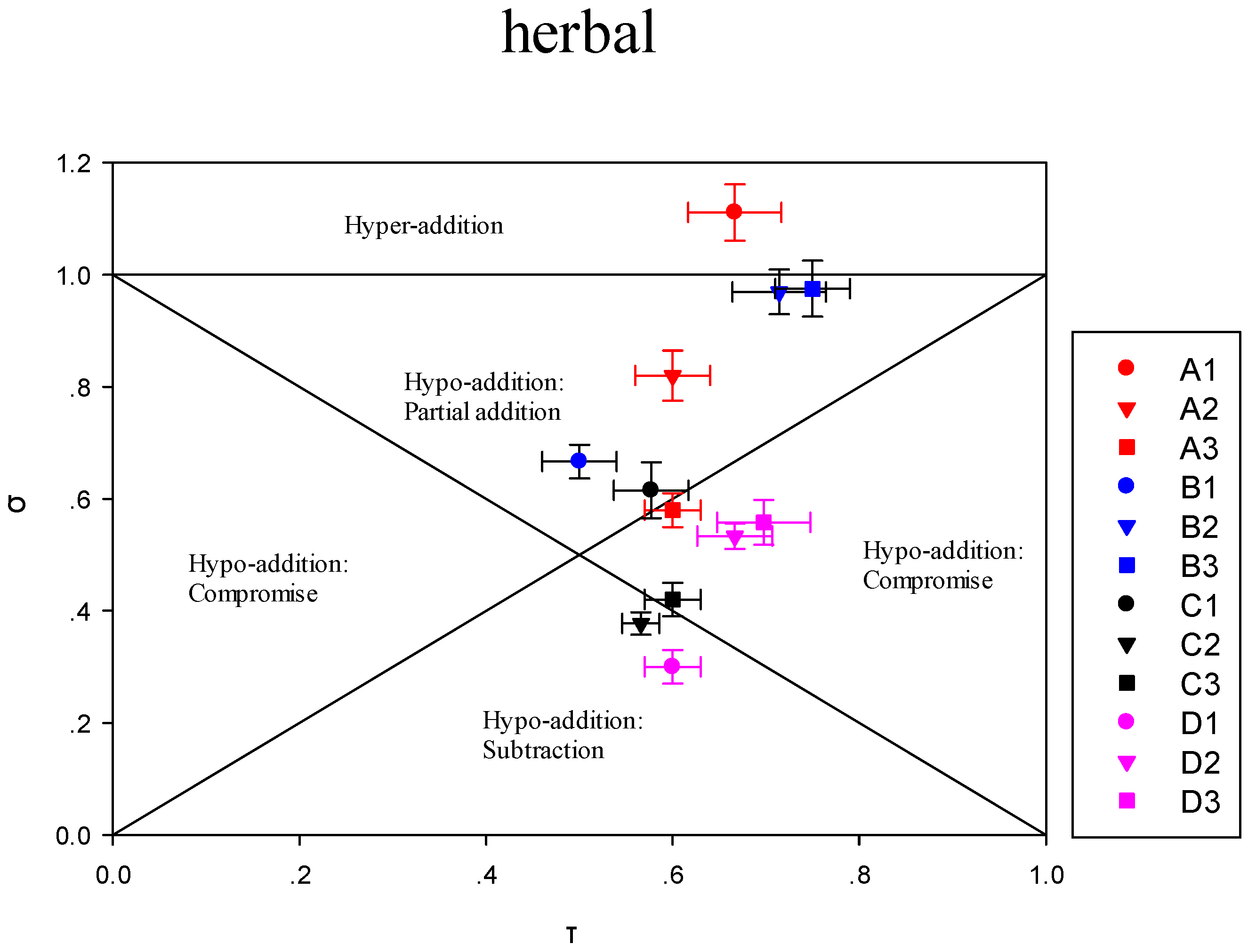
3.3.1. Impact of the Addition of One or More Terpene Alcohols on Terpenes Recombination
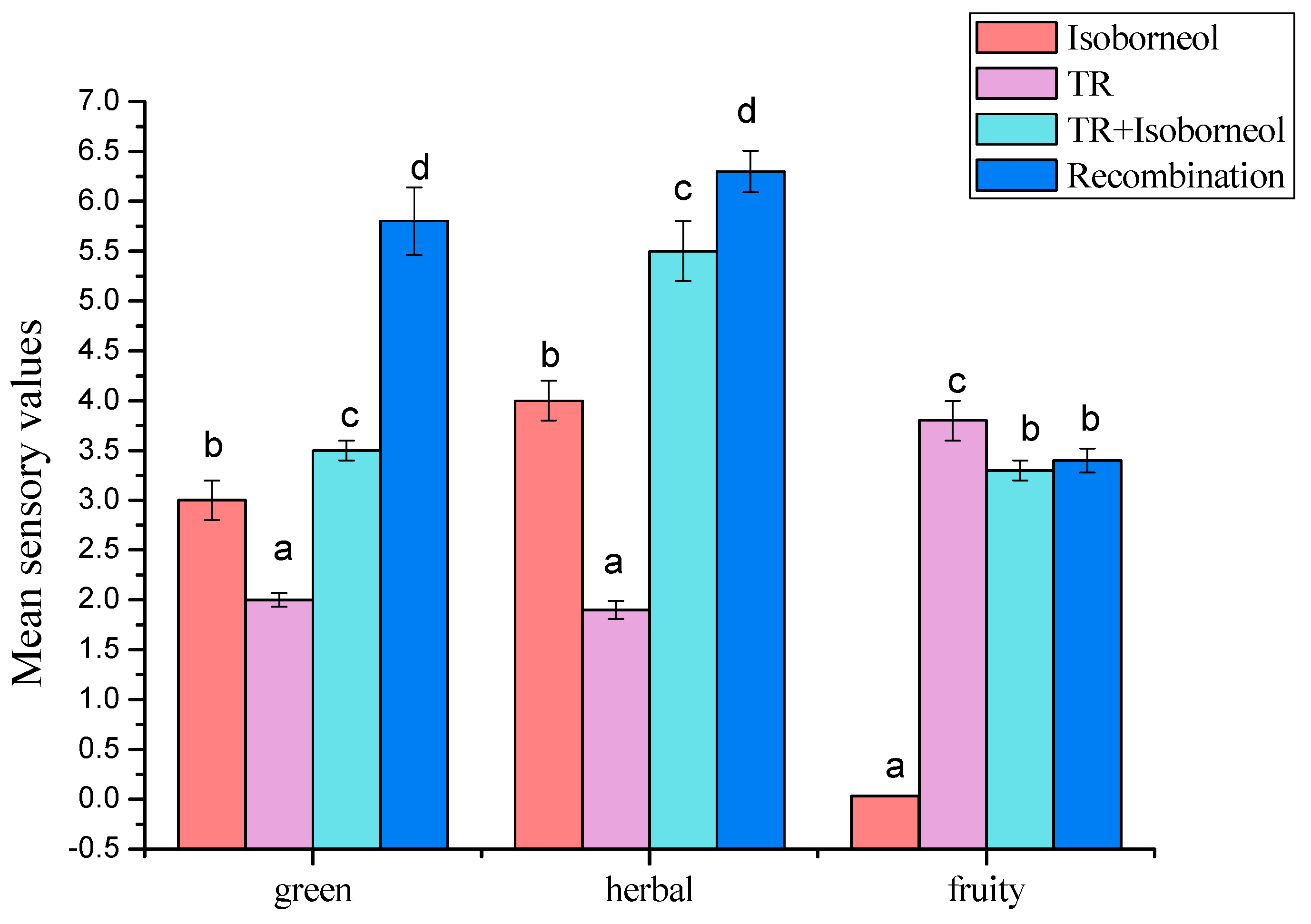
3.3.2. Modulation of Sensory Attributes by Terpene Alcohols Addition
3.4. The Contribution of Isoborneol to Overall Flavor
3.4.1. Sensory Analysis
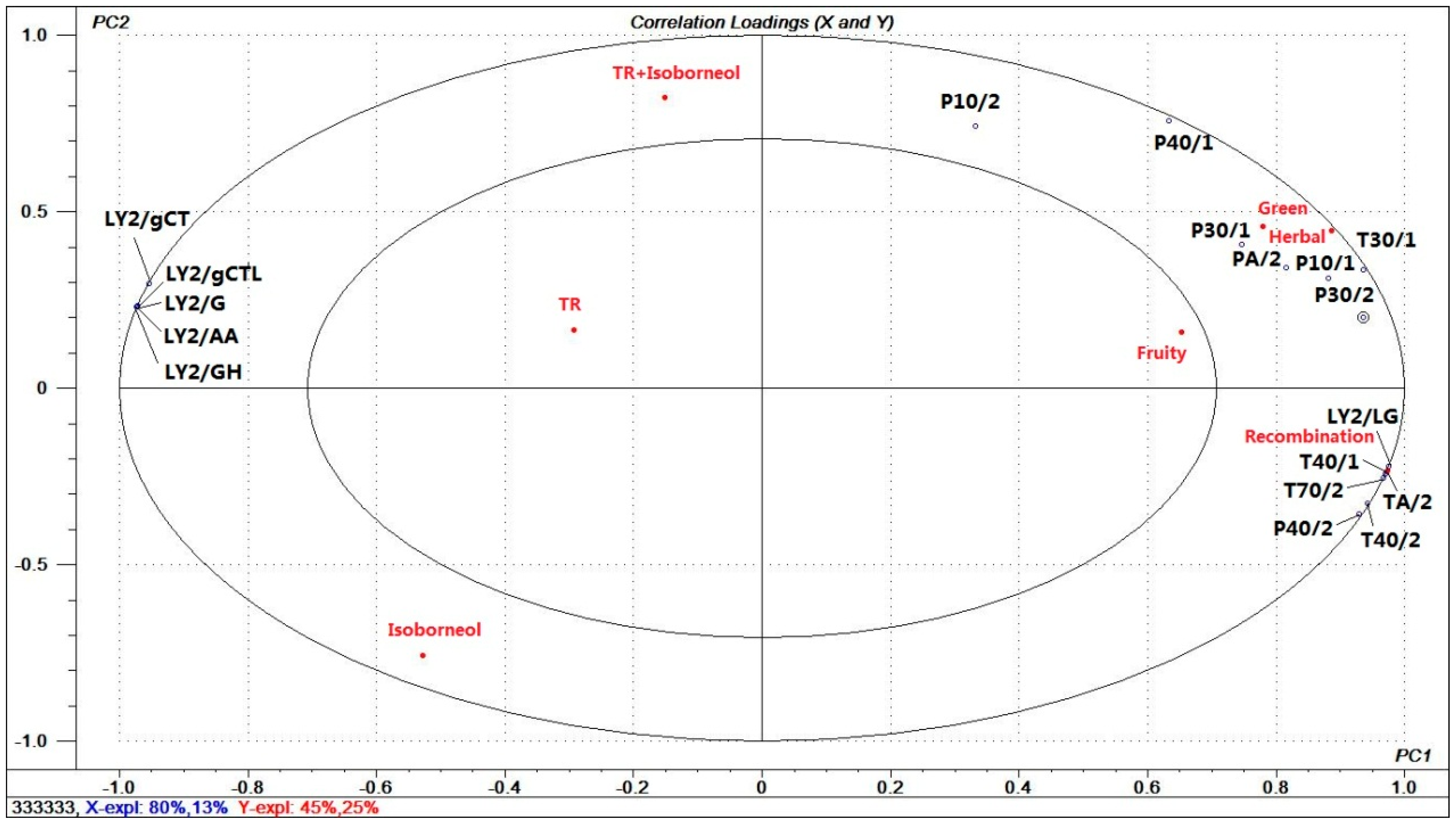
3.4.2. Electronic Nose Analysis
3.4.3. Relationship between Aromatic Recombinations, E-Nose Data, and Olfactory Descriptors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, F.; Ong, E.S.; Li, S.F.Y. A green and effective approach for characterisation and quality control of chrysanthemum by pressurized hot water extraction in combination with HPLC with UV absorbance detection. Food Chem. 2013, 141, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, C.; Fan, Z.; Wang, Z. Extraction, component analysis and antioxidant activity of plant essential oils. Sci. Technol. Food Ind. 2017, 38, 348–352. [Google Scholar]
- Shen, M.; Shao, L.; Hou, F.; Fan, Y.; Guan, Y. Response surface methodology as an approach to optimization of supercritical CO2 extraction and chemical composition analysis of volatile oil from flowers of Chrysanthemum morifolium. Food Sci. 2010, 31, 101–105. [Google Scholar]
- Li, N.; Cheng, Y. Chemical constituents and antioxidant activities of essential oils from white chrysanthemum flower. Mod. Food 2017, 11, 98–102. [Google Scholar]
- Kim, Y.; Han, J.; Sung, J.; Sung, M.; Choi, Y.; Jeong, H.-S.; Lee, J. Anti-inflammatory activity of Chrysanthemum zawadskii var. Latilobum leaf extract through haem oxygenase-1 induction. J. Funct. Foods 2012, 4, 474–479. [Google Scholar] [CrossRef]
- Sassi, A.B.; Skhiri, F.H.; Chraief, I.; Bourgougnon, N.; Hammami, M.; Aouni, M. Essential oils and crude extracts from Chrysanthemum trifurcatum leaves, stems and roots: Chemical composition and antibacterial activity. J. Oleo Sci. 2014, 63, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-P.; Liu, Y.; Zhao, S.; Zeng, Y.; Liu, S.; Hu, Z. Optimization of essential oil from Chrysanthemum morifolium ramat by enzymatic extraction and application as cigarette flavor. J. Boil. Act. Prod. Nat. 2015, 5, 255–263. [Google Scholar]
- Shafaghat, A.; Larijani, K.; Salimi, F. Composition and antibacterial activity of the essential oil of chrysanthemum Parthenium flower from Iran. Nat. Prod. Commun. 2013, 12, 708–713. [Google Scholar]
- Jung, E.-K. Chemical composition and antimicrobial activity of the essential oil of chrysanthemum indicum against oral bacteria. J. Bacteriol. Virol. 2009, 39, 61–69. [Google Scholar] [CrossRef]
- Hosni, K.; Casabianca, H. Essential oil composition and allelopathic effect of chrysanthemum coronarium (garland) extracts on the germination and root growth of four plant species. In Proceedings of the 43rd International Symposium on Essential Oils (ISEO2012), Lisboa, Portugal, 5–8 September 2012. [Google Scholar]
- HekmatSorush, I.; Kalkhorani, N.M.; Rezaee, M.B.; HeroAbadi, F.; Hamisi, M. Phytochemical analysis of essential oil of Tanacetum parthenium L. With hydro-distillation and steam distillation. J. Med. Plants By-Prod. 2014, 1, 53–57. [Google Scholar]
- Rateb, E.M.M.; El-Gendy, A.-N.A.M.; El-Hawary, S.S. Phytochemical and biological investigation of Tanacetum parthenium (L.) cultivated in Egypt. J. Med. Plants Res. 2017, 1, 18–26. [Google Scholar]
- Shaaban, H.A.E.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essent. Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Basta, A.; Pavlović, M.; Couladis, M.; Tzakou, O. Essential oil composition of the flower heads of Chrysanthemum coronarium L. from Greece. Flavour Fragr. J. 2007, 22, 197–200. [Google Scholar] [CrossRef]
- Wang, W.; Dai, H.; Chen, F.; Guo, W. Correlation between floral traits of chrysanthemun (Dendranthema morifolium) and insect visitors. J. Plant Ecol. 2008, 32, 776–785. [Google Scholar]
- Adhikari, K.; Hein, K.A.; Elmore, J.R.; Heymann, H.; Willott, A.M. Flavor threshold as affected by interaction among three dairy-related flavor compounds. J. Sens. Stud. 2006, 21, 626–643. [Google Scholar] [CrossRef]
- Cain, W.S.; Drexler, M. Scope and evaluation of odor counteraction and masking. Ann. N. Y. Acad. Sci. 1974, 237, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V. Revisiting psychophysical work on the quantitative and qualitative odour properties of simple odour mixtures: A flavour chemistry view. Part 1: Intensity and detectability. A review. Flavour Fragr. J. 2012, 27, 124–140. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Xu, J.; Liu, G. Gc-ms analysis of volatile oil components in the chrysanthemum. J. Shaoguan Univ. 2013, 34, 42–46. [Google Scholar]
- Luo, J.; Wang, B. Supercritical CO2 extraction of essential oil from the flower of Chrysanthemum indicum lamb. Biomass Chem. Eng. 2007, 41, 11–14. [Google Scholar]
- Xiao, Z.; Fan, B.; Niu, Y.; Wu, M.; Liu, J.; Ma, S. Characterization of odor-active compounds of various chrysanthemum essential oils by gas chromatography-olfactometry, gas chromatography-mass spectrometry and their correlation with sensory attributes. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1009–1010, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; De Revel, G. Sensory evaluation: Scientific bases and oenological applications. J. Int. Des Sci. De La Vigne Et Du Vin 1999, 33, 81–93. [Google Scholar]
- Sensory Analysis—General Guidance for the Design of Test Rooms—ISO 8589; Analysis Sensorielle; International Organization for Standardization: Geneva, Switzerland, 2010.
- Roessler, E.B.; Pangborn, R.M.; Sidel, J.L.; Stone, H. Expanded statistical tables for estimating significance in paired-preference, paired-difference, duo-trio and triangle tests. J. Food Sci. 1978, 43, 940–947. [Google Scholar] [CrossRef]
- Sensory Analysis—Methodology—General Guidance for Measuring Odour, Flavour and Test Detection Thresholds by a Threealternative Forced-Choice (3-afc) Procedure—ISO 13301; Analysis Sensorielle; International Organization for Standardization: Geneva, Switzerland, 2002.
- Cometto-Muniz, J.E.; Cain, W.S.; Abraham, M.H.; Gil-Lostes, J. Concentration-detection functions for the odor of homologous n-acetate esters. Physiol. Behav. 2008, 95, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Feller, W. An Introduction to Probability Theory and Its Applications II; Wiley: Hoboken, NJ, USA, 1971; Volume 1. [Google Scholar]
- Miyazawa, T.; Gallagher, M.; Preti, G.; Wise, P.M. Synergistic mixture interactions in detection of perithreshold odors by humans. Chem. Senses 2008, 33, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Patte, F.; Laffort, P. An alternative model of olfactory quantitative interaction in binary mixtures. Chem. Senses 1979, 4, 267–274. [Google Scholar] [CrossRef]
- Lytra, G.; Tempere, S.; Zhang, S.; Marchand, S.; de Revel, G.; Barbe, J.C. Olfactory impact of demethyl sulfide on red wine fruity esters aroma expression in model solution. J. Int. Des Sci. De La Vigne Et Du Vin 2014, 48, 75–85. [Google Scholar]
- Lytra, G.; Tempere, S.; Le Floch, A.; de Revel, G.; Barbe, J.C. Study of sensory interactions among red wine fruity esters in a model solution. J. Agric. Food Chem. 2013, 61, 8504–8513. [Google Scholar] [CrossRef] [PubMed]
- Lorrain, B.; Tempere, S.; Iturmendi, N.; Moine, V.; de Revel, G.; Teissedre, P.-L. Influence of phenolic compounds on the sensorial perception and volatility of red wine esters in model solution: An insight at the molecular level. Food Chem. 2013, 140, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Pineau, B.; Barbe, J.-C.; Van Leeuwen, C.; Dubourdieu, D. Which impact for β-damascenone on red wines aroma? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A.; Mesa, J. Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flavour Fragr. J. 2006, 21, 207–213. [Google Scholar] [CrossRef]
- Cliff, M.; Bansal, M.; Stanich, K.; Usher, K. Comparison of new and existing threshold methods for evaluating sulfur compounds in different base wines. J. Sens. Stud. 2011, 26, 184–196. [Google Scholar] [CrossRef]
- Zou, Z.; Buck, L.B. Combinatorial effects of odorant mixes in olfactory cortex. Science 2006, 311, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Duchamp-Viret, P.; Duchamp, A.; Chaput, M.A. Single olfactory sensory neurons simultaneously integrate the components of an odour mixture. Eur. J. Neurosci. 2003, 18, 2690–2696. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Omura, M.; Kataoka, H.; Touhara, K. Olfactory receptor antagonism between odorants. Eur. Mol. Boil. Organ. 2003, 23, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Prazeller, P.; Antille, N.; Ali, S.; Pollien, P.; Mioche, L. Influence of in-mouth aroma release on individual perception. Dev. Food Sci. 2006, 43, 433–436. [Google Scholar]
- Guadagni, D.G.; Buttery, R.G.; Okano, S.; Burr, H.K. Additive effect of sub-threshold concentrations of some organic compounds associated with food aromas. Nature 1963, 200, 1288–1289. [Google Scholar] [CrossRef] [PubMed]
- De-la-Fuente-Blanco, A.; Sáenz-Navajas, M.P.; Ferreira, V. Levels of higher alcohols inducing aroma changes and modulating experts’ preferences in wine model solutions. Aust. J. Grape Wine Res. 2017, 23, 162–169. [Google Scholar] [CrossRef]
- Feng, Y.; Su, G.; Zhao, H.; Cai, Y.; Cui, C.; Sun-Waterhouse, D.; Zhao, M. Characterisation of aroma profiles of commercial soy sauce by odour activity value and omission test. Food Chem. 2015, 167, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Frijters, J.E. Psychophysical models for mixtures of tastants and mixtures of odorants. Ann. N. Y. Acad. Sci. 1987, 510, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Lytra, G.; Tempere, S.; de Revel, G.; Barbe, J.C. Impact of perceptive interactions on red wine fruity aroma. J. Agric. Food Chem. 2012, 60, 12260–12269. [Google Scholar] [CrossRef] [PubMed]
- Baietto, M.; Wilson, A.D. Electronic-nose applications for fruit identification, ripeness and quality grading. Sensors 2015, 15, 899–931. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Oberle, C.S.; Oberle, D.F. Detection of off-flavor in catfish using a conducting polymer electronic-nose technology. Sensors 2013, 13, 15968–15984. [Google Scholar] [CrossRef] [PubMed]

| Terpenes | Concentration (mg/L) |
|---|---|
| α-pinene | 3083 |
| camphene | 2780 |
| β-pinene | 355 |
| β-myrcene | 787 |
| α-phellandrene | 523 |
| dl-limonene | 8192 |
| cis-ocimene | 53 |
| α-terpinolen | 7566 |
| caryophyllene | 775 |
| β-farnesene | 243 |
| Germacrene B | 985 |
| alcohols | |
| linalool | 2974 |
| d-fenchyl alcohol | 1178 |
| eudesmol | 1177 |
| borneol | 183 |
| isoborneol | 3065 |
| 4-terpineol | 866 |
| L | F | E | B | I | |
|---|---|---|---|---|---|
| high concentration (mg/L) | 2974 | 1178 | 1177 | 183 | 3065 |
| Medium concentration (mg/L) | 1442 | 822 | 407 | 62 | 680 |
| low concentration (mg/L) | 81 | 123 | 13 | 13 | 66 |
| 13 Terpenes | L | F | E | B | I | T | Difference Observed | |
|---|---|---|---|---|---|---|---|---|
| TR | e | - | - | - | - | - | - | |
| test 1 | e | e | - | - | - | - | - | = |
| test 2 | e | - | e | - | - | - | - | *** |
| test 3 | e | - | - | e | - | - | - | = |
| test 4 | e | - | - | - | e | - | - | = |
| test 5 | e | - | - | - | - | e | - | *** |
| test 6 | e | - | - | - | - | - | e | = |
| test 7 | e | e | e | - | - | - | - | = |
| test 8 | e | e | - | e | - | - | - | *** |
| test 9 | e | e | - | - | e | - | - | *** |
| test 10 | e | e | - | - | - | e | - | = |
| test 11 | e | e | - | - | - | - | e | = |
| test 12 | e | - | e | e | - | - | - | = |
| test 13 | e | - | e | - | e | - | - | = |
| test 14 | e | - | e | - | - | e | - | = |
| test 15 | e | - | e | - | - | - | e | = |
| test 16 | e | - | - | e | e | - | - | *** |
| test 17 | e | - | - | e | - | e | - | = |
| test 18 | e | - | - | e | - | - | e | = |
| test 19 | e | - | - | - | e | e | - | = |
| test 20 | e | - | - | - | e | - | e | = |
| test 21 | e | - | - | - | - | e | e | = |
| test 22 | e | e | e | e | - | e | - | ** |
| test 23 | e | e | e | e | e | e | - | * |
| test 24 | e | e | e | e | e | - | e | ** |
| test 25 | e | e | e | e | - | e | e | * |
| test 26 | e | e | - | e | e | e | e | ** |
| 13 Terpenes | L | F | E | B | I | T | Difference Observed | |
|---|---|---|---|---|---|---|---|---|
| CR | e | e | e | e | e | e | e | |
| test 27 | e | - | e | e | e | e | e | = |
| test 28 | e | e | - | e | e | e | e | * |
| test 29 | e | e | e | - | e | e | e | = |
| test 30 | e | e | e | e | - | e | e | = |
| test 31 | e | e | e | e | e | - | e | *** |
| test 32 | e | e | e | e | e | e | - | = |
| test 33 | e | - | - | e | e | e | e | = |
| test 34 | e | - | e | - | e | e | e | * |
| test 35 | e | - | e | e | - | e | e | * |
| test 36 | e | - | e | e | e | - | e | *** |
| test 37 | e | - | e | e | e | e | - | = |
| test 38 | e | e | - | - | e | e | e | = |
| test 39 | e | e | - | e | - | e | e | = |
| test 40 | e | e | - | e | e | - | e | *** |
| test 41 | e | e | - | e | e | e | - | = |
| test 42 | e | e | e | - | - | e | e | ** |
| test 43 | e | e | e | - | e | - | e | *** |
| test 44 | e | e | e | - | e | e | - | = |
| test 45 | e | e | e | e | - | - | e | *** |
| test 46 | e | e | e | e | - | e | - | = |
| test 47 | e | e | e | e | e | - | - | *** |
| test 48 | e | - | - | e | e | - | e | *** |
| test 49 | e | - | e | - | e | - | e | *** |
| test 50 | e | - | e | - | e | - | e | *** |
| test 51 | e | - | e | e | - | - | e | *** |
| test 52 | e | - | e | e | e | - | - | *** |
| test 53 | e | e | - | - | e | - | e | *** |
| test 54 | e | e | - | e | - | - | e | *** |
| test 55 | e | e | - | e | e | - | - | *** |
| test 56 | e | - | - | e | - | - | e | *** |
| test 57 | e | - | - | e | e | - | - | *** |
| test 58 | e | - | e | - | - | - | - | *** |
| Sample | Diluted Concentration (mg/L) | C | D | R | OT (mg/L) |
|---|---|---|---|---|---|
| in alkanes Solution | |||||
| I | 22/11/5.50/2.75/1.88/0.94/0.47/0.24/0.12/0.06 | −0.216 | 0.267 | 0.964 | 0.61 |
| F | 380/190/95/47.50/23.75/11.88/5.94/2.97/1.48/0.74 | 1.096 | 0.367 | 0.985 | 12.47 |
| B | 84/42/21/10.50/5.25/2.63/1.82/0.91/0.46/0.23 | 0.117 | 0.433 | 0.991 | 2.03 |
| E | 3120/1560/780/390/195/97.50/48.75/24.38/12.19/6.10 | 1.991 | 0.218 | 0.992 | 97.95 |
| L | 460/230/115/57.50/28.75/14.38/7.19/3.60/1.80/0.90 | 0.585 | 0.367 | 0.980 | 3.85 |
| BE | 145/72.50/36.25/18.13/9.07/4.53/2.27/1.13/0.57/0.28 | 0.190 | 0.424 | 0.979 | 1.55 |
| BL | 2.89/1.45/0.72/0.36/0.18/0.09/0.05/0.02/0.01/0.006 | −1.284 | 0.445 | 0.970 | 0.05 |
| EL | 6.25/3.13/1.57/0.79/0.40/0.20/0.10/0.05/0.03/0.01 | −1.088 | 0.604 | 0.957 | 0.08 |
| BEL | 88/44/22/11/5.50/2.75/1.88/0.94/0.47/0.24 | −0.060 | 0.536 | 0.954 | 0.87 |
| TR | 800/400/200/100/50/25/12.5/6.25/3.13/1.57 | 1.487 | 0.388 | 0.978 | 30.69 |
| in TR Solution | |||||
| I | 100/50/25/12.5/6.25/3.13/1.57/0.79/0.39/0.20 | 0.507 | 0.488 | 0.986 | 3.22 |
| F | 500/250/125/62.50/31.25/15.63/7.81/3.91/1.95/0.98 | 1.105 | 0.469 | 0.979 | 12.74 |
| BE | 3400/1700/850/425/212.50/106.25/53.13/26.56/13.28/6.64 | 1.841 | 0.482 | 0.986 | 69.34 |
| BL | 280/140/70/35/17.50/8.75/4.38/2.19/1.09/0.55 | 0.944 | 0.440 | 0.976 | 8.79 |
| EL | 928/464/232/116/58/29/14.50/7.25/3.63/1.81 | 1.335 | 0.294 | 0.989 | 21.63 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Y.; Sun, X.; Xiao, Z.; Wang, P.; Wang, R. Olfactory Impact of Terpene Alcohol on Terpenes Aroma Expression in Chrysanthemum Essential Oils. Molecules 2018, 23, 2803. https://doi.org/10.3390/molecules23112803
Niu Y, Sun X, Xiao Z, Wang P, Wang R. Olfactory Impact of Terpene Alcohol on Terpenes Aroma Expression in Chrysanthemum Essential Oils. Molecules. 2018; 23(11):2803. https://doi.org/10.3390/molecules23112803
Chicago/Turabian StyleNiu, Yunwei, Xiaoxin Sun, Zuobing Xiao, Pinpin Wang, and Ruolin Wang. 2018. "Olfactory Impact of Terpene Alcohol on Terpenes Aroma Expression in Chrysanthemum Essential Oils" Molecules 23, no. 11: 2803. https://doi.org/10.3390/molecules23112803
APA StyleNiu, Y., Sun, X., Xiao, Z., Wang, P., & Wang, R. (2018). Olfactory Impact of Terpene Alcohol on Terpenes Aroma Expression in Chrysanthemum Essential Oils. Molecules, 23(11), 2803. https://doi.org/10.3390/molecules23112803