Abstract
Ophiocordyceps sinensis has been utilized in China and adjacent countries for thousands of years as a rare functional food to promote health and treat diverse chronic diseases. In recent years, adulterants are usually identified in the processed products of wild O. sinensis. However, the effective adulteration examination has to be additionally performed except their routine test, and accordingly is time- and money-consuming. Recently, arsenic determination has become a necessary test for confirming whether the concentrations of inorganic arsenic are over the O. sinensis limit. In this work, the contents of total arsenic and As species in cultivated O. sinensis, Cordyceps militaris, and other edible fungi were determined by ICP-MS and HPLC-ICP-MS. The results suggest that the As speciation exhibits a species-specific behavior, and accompanies the effect of the As background. The proportions of unknown organic As and contents of total As may be considered as sensitive markers for discriminating wild O. sinensis. This result provides a novel clue for discriminating wild and artificially cultivated mushrooms/their products, with emphasis on arsenic markers for authenticating wild O. sinensis.
1. Introduction
Ophiocordyceps sinensis [1] (Figure 1A, syn. Cordyceps sinensis), a mysterious entomogenous fungus distributed on the Qinghai-Tibetan Plateau, is popularly referred as winter-worm-summer-grass (Dong Chong Xia Cao in Chinese) [2,3,4,5]. O. sinensis has been utilized in China and adjacent countries for thousands of years as a rare functional food to promote health and treat diverse chronic diseases [2]. In 1993, O. sinensis attracted worldwide attention because Chinese women athletes broke several world records in running events at the National Games, and the meritorious performances were later attributed (at least in part) to the consumption of this fungus [6]. Subsequently, modern pharmaceutical studies have increasingly shown that it has multiple remarkable functions such as anti-tumor, anti-inflammatory, nephroprotective, antioxidant, antihyperglycemic, anti-apoptosis, immunoregulatory, and hepatoprotective [7,8].

Figure 1.
Morphological characters of the samples for this study. (A) wild Ophiocordyceps sinensis, (B) cultivated Ophiocordyceps sinensis, (C) Cordyceps militaris, (D) Agaricus blazei, (E) Lentinus edodes, and (F) Auricularia auricula.
Correspondingly, the significant medicinal functions have resulted in a large demand for wild O. sinensis and sharp increase of the retail price (15,000 USD/kg for medium quality) in recent decades. To satisfy its market need, cultivated O. sinensis (Figure 1B) [9,10] (and multifarious processed products of wild O. sinensis have rapidly emerged and widely merchandised [11], with the presence of adulterated products made from its cheap substitutes. To standardize the Cordyceps market, the most urgent problem is to establish the effective indicators for authenticating O. sinensis-related products. Traditionally, the morphology, color and odor have been applied to effectively authenticate wild O. sinensis [12], due to the direct consumption of the whole fungus-larva complex (Figure 1A) such as preparing medicinal soups or liquors. However, these routine methods are insufficient to discriminate wild and cultivated O. sinensis (Figure 1B) [9,10] because they are exactly identical in these aspects. Furthermore, the health foods related to wild O. sinensis have become increasingly popular in the convenient forms such as tablet, capsule and oral liquid [13], and the above-mentioned empirical methods have been unfeasible for adulteration test. Recently, HPLC, bio-molecular fingerprint and isotopic tracing seem to be appropriate methods for this test [14,15,16,17]. As a case study, our group has proved that the stable carbon isotope ratio may be an effective indicator for authenticating the products of wild O. sinensis [17].
In 2016, the China Food and Drug Administration (CFDA) revealed that the high contents of total As (4.4–9.0 mg/kg) [18], nearly five times the limit value of 1 mg/kg for functional foods (GB16740-2014) [19], were detected in O. sinensis. Soon afterwards, CFDA ordered that all of the pilot works of O. sinensis as a functional food be discontinued [20]. This event caused anxiety in the functional food market, and prompted to consider the concentrations of total As and iAs as two obligatory test items for wild O. sinensis and its processed products. In our latest study [21], we found that the contents of total As were massive (4.00–5.25 mg/kg), but iAs was minor (AsIII, 4.1–6.0% and AsV, 1.3–3.2%) and oAs (MMA, DMA, and AsB) was absent or trace in O. sinensis. Further, we surprisingly found that As species were consistent with each other among different bulk samples even if the samples came from different producing areas [21]. Thus, As species may be used as indicators to discriminate both wild O. sinensis and its substitutes or adulterants.
To establish novel arsenic markers for discriminating wild O. sinensis, we comparatively studied the total As and As species in wild O. sinensis (WOS, Figure 1A) and its common substitutes, including cultivated O. sinensis (COS, Figure 1B), cultivated Cordyceps militaris (CM, Figure 1C) [22], and other mushrooms [Agaricus blazei (AB, Figure 1D), Lentinus edodes (LE, Figure 1E), and Auricularia auricula (AA, Figure 1F)]. These data were scrutinized by principal component analysis, and several sensitive arsenic markers were developed for discriminating wild O. sinensis. Thus, this study provides a novel clue for distinguishing wild and artificially cultivated mushrooms/their processed products in the routine inspection of hazardous materials.
2. Results
2.1. Total Arsenic
The quality control for both total As and As species analyses in this study was validated by determining the linearity (R = 0.9997–1.000) (Table S1), limits of detection (LODs < 2 μg/kg) (Table S1), limits of quantification (LOQs < 6 μg/kg) (Table S1), relative standard deviations (RSDs < 7%) (Table S2), the recoveries (92.0–106.0%) (Table S3), CRM materials (Table S4), and extract efficiency (87.0–104.5%) (Table S5). The total ion currents of As species in standards (A), COS (B), CM (C), AB (D), LE (E), and AA (F) are presented in Figure 2, and the standard adding tests are illustrated in Figures S1–S5.
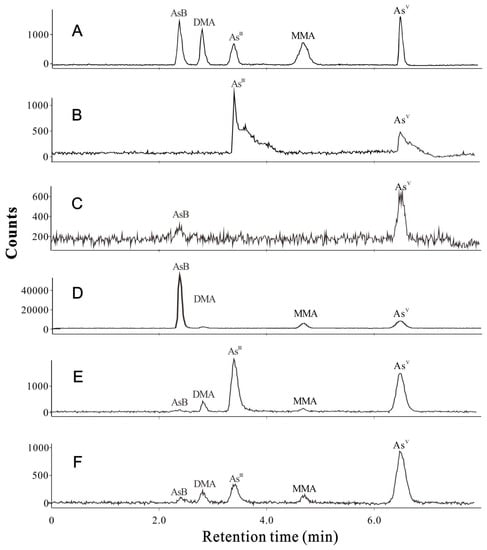
Figure 2.
Total ion currents of As species in the standards (A), COS (B), CM (C), AB (D), LE (E), and AA (F).
The contents of total As in samples are presented in Table 1 and Figure 3. It can be seen from Table 1 that the levels of total As in COS, CM, AB, LE, and AA samples are at the intervals of 0.223–0.289 mg/kg, 0.036–0.047 mg/kg, 1.680–2.677 mg/kg, 0.188–1.203 mg/kg, and 0.028–0.087 mg/kg, respectively. The concentrations of total As exhibit significant differences (p < 0.05) among different sample groups. In terms of the means of total As (Figure 3), WOS [21] > AB > 1 mg/kg (the limit for functional foods highlighted in the blue dashed line in Figure 3, GB16740-2014 [19]) > LE (except two LE samples slightly beyond the limit) > COS > AA > CM.

Table 1.
Concentrations and percentages of arsenic species in all the detected samples.
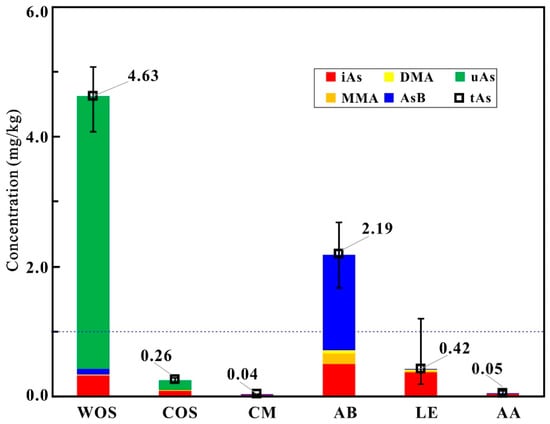
Figure 3.
Average concentrations (mg/kg dry weight) of total As and As species in the samples. The columns in red, orange, yellow, blue, and green denote iAs, MMA, DMA, AsB, and uAs, respectively. The blue dashed line denotes the arsenic limit for functional foods.
2.2. Arsenic Species
The abundances of As species in samples are presented in Table 1 and Figure 3. It can be seen that the contents of iAs in the COS samples are at the interval of 0.078–0.140 mg/kg (32–49%); while AsB, DMA and MMA are trace in most of the samples. Similar with WOS [21], the COS samples additionally have a portion of uAs with the range of 0.145–0.180 mg/kg (51–67%). The As species in CM, LE, AA, and AB are mostly confined to five As species for the routine detection; and the average relative abundances of AsB, DMA, MMA, and iAs change at the intervals of 2–67%, 0–9%, 0–8%, and 23–90%, respectively. The abundances of As species evidently vary in different sample groups (Figure 3), i.e., oAs predominant in WOS, COS, and AB; uAs rich in WOS and COS; the contents of AsB high in AB; iAs dominant in CM, LE, and AA. Figure 4 intuitively exhibits the similar profiles of As species variations in the same group but the different ones in different groups.
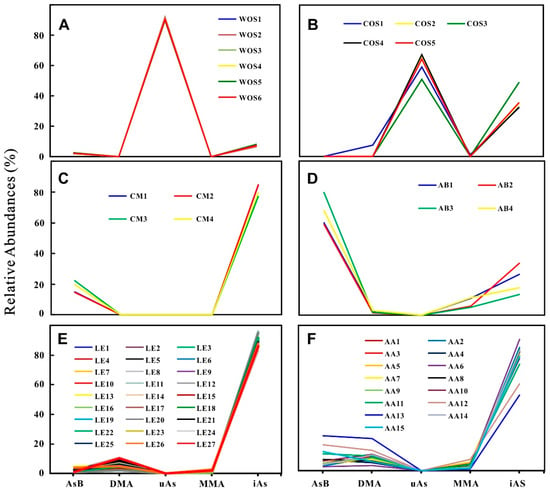
Figure 4.
Variation of the relative abundances (%) of As species in wild Ophiocordyceps sinensis (A), cultivated Ophiocordyceps sinensis (B), Cordyceps militaris (C), Agaricus blazei (D), Lentinus edodes (E), and Auricularia auricula (F).
According to their profiles of As species, all of the studied samples may be divided into three types. Type 1 (Figure 4A,B): WOS and COS have the highest peak at uAs (Based on the chromatography behaviors under anion exchange HPLC-ICP-MS and the stability under the H2O2 treatment, it may be an arsenosugar, which is frequently reported to be co-eluted with DMA, AsB, MMA, and AsIII under anion exchange HPLC-ICP-MS [23]. More detailed analyses were reported in our previous work) [21] and a lower peak at iAs. Unlike common mushrooms cultivated on plant-based substrates [24], WOS and COS belong to a kind of the fungus-larva complex, which may contain a large part of oAs in the animal-based sclerotium, and accordingly possess the completely different profiles. Type 2 (Figure 4C,D): CM and AB have two peaks at AsB and iAs. AsB is the major As species in AB, which is consistent with the previous report for the store bought A. bisporus [24]. This result indicates that AB in the food market may be safely consumed although its total As is significantly higher than the permitted limit. AsB, an osmolyte commonly distributed in marine organisms in response to salinity changes, is also considered as an osmolyte in certain mushrooms to maintain the fruiting body structures and specific morphologies [24]. Although there is no report on AsB in mushrooms with rod-like stromata, we still infer that AsB in CM may be related with its rod-like stroma on the basis of the above theory. Type 3 (Figure 4E,F): LE and AA possess a slightly peak at DMA and a sharp peak at iAs. A previous study [24] suggested that the relative abundance of iAs in LE was approximately 40% of the total As, and the remaining over 30% As was not extracted for As species analysis. In this study, the extraction efficiencies were very high (87.0–104.2%), and the proportions of iAs reached 90% on average. The comparative analysis indicates that the As for species analysis was extracted by 2% HNO3 at 70 °C for 2 h in reference 24; while the As was extracted at 90 °C for 12 h and shaken frequently using the same solvents in this study. Thus, we consider that our procedures for extracting As species are more proper, and the unextracted As shown in reference 24 may be iAs in comparison with the iAs proportions.
3. Discussion
3.1. Arsenic Fingerprint for Adulteration Test
The species and abundances of metabolic products of hazardous materials in organisms might be species-specific. To ensure the survival and reproduction of organisms, each of them can develop a detoxifying strategy to withstand hazardous materials, which sneak into their body from environments [25,26]. Arsenic as a common element in the ecosphere can be transferred in organisms via their trophic chain. Accordingly, As is naturally accumulated in sea foods [27,28], land animals [29], terrestrial crops [30], algae [31], and mushrooms [32]. Therefore, As is one of the most concerned hazardous materials over the world due to its toxicity. The toxicity of As depends on its chemical forms. The iAs species, such as arsenite (AsIII) and arsenate (AsV) are the most toxic, and most of them exist in soils and water. When entering organisms, iAs begin to be transformed to low toxic oAs species in vivo. The initial metabolic products are MMA and DMA, which are much less toxic than iAs to human beings; and their subsequent metabolic products are other non-toxic oAs complexes such as AsC, AsB, arsenosugars, and arsenolipids [33]. Thus, the As conversion in organisms is generally considered as the detoxification of iAs. Both the species and abundances of As metabolic enzymes vary among different organisms [33], and subsequently result in the variability of oAs products among them. Correspondingly, the species and abundances of oAs exhibit a species-specific trend.
Previous studies revealed that the contents of As species varied significantly in different mushrooms [34]. The studies of As species for store-bought mushrooms (usually grown in the low As background) suggested that several mushrooms contained high oAs, e.g., Leccinum scabrum, Paxillus involutus, and Agaricus bisporus; and some mushrooms, e.g., Agaricus balieimurrill, Leucopaxillus giganteus, Pleurotas eryngii, and Lepista nuda evidently enriched toxic iAs, such as AsIII and AsV [35]. The As species in mushrooms from the high As background indicated that some of them mainly contained oAs, e.g., DMA (approximately 70% of total 1420 mg/kg As) in Laccaria amethystina from a heavily polluted site was the highest proportion [36]; the As in Collybia butyracea collected near an As smelter consisted of 8.8 mg/kg AsB and 1.9 mg/kg DMA, with the total As content of 10.9 mg/kg in the fruiting body [37]; and some mushrooms contained toxic iAs, such as AsIII and AsV [38]. Although the mechanisms of uptake, retention and biotransformation of arsenic by mushrooms are still not clear, every mushroom often contains similar arsenicals regardless of the harvest location, suggesting that the mushrooms have an ability to metabolize arsenic compounds [24,39,40]. In addition, it has been theorized that only the more highly evolved fungi (e.g., Geastrumand agaricus) can produce more complex arsenic compounds [40]. In this study, Figure 4 presents the variation in the relative abundances of MMA, DMA, AsB, uAs, and iAs among different groups; and all of the curves coincide within the same group. This coincidence may be caused by the same As metabolic ability within the same biological variety.
However, there is the evident difference in the relative abundances of As species between WOS (Figure 4A) and COS (Figure 4B). Clearly, the profiles of As species are not coincident with each other even if they belong to the same species. Furthermore, the total As in WOS is tenfold than that in COS (Figure 3). This discrepancy implies that As species are also influenced by other factors. For wild O. sinensis, as a special organism occurred in the Qinghai-Tibetan soil ecosystem, the As species and their concentrations were derived from the complicated synergy among soils (living environment and alternative foods of the host larvae [3]), plants (favorite foods of the host larvae), host larvae and O. sinensis fungus [4,5]. The average arsenic concentration in the Tibetan soils was 18.7 mg/kg (n = 205) [41], and evidently higher than the average value of the upper continental crust, 1.5 mg/kg [42]. The As in soils could be firstly accumulated into plants via their roots, and then was gradually amplified in the host Thitarodes larvae, which utilized the tender plant roots as their favorite foods for approximately 3 years [3,4], and accumulated a tremendous amount of As from the high As background in the duration of their life cycle. Thus, the total As in wild O. sinensis were plentiful, not only from the high-As host larvae as its substrate but also from the residual As in its larva-derived sclerotium. As for COS, it was cultivated in the artificially-controlled circumstance instead of the high As background in the Qinghai-Tibetan Plateau. The host larvae were fed with the artificial diet on the optimal temperature and soil conditions for promoting their growth [43] and lowering the cost of breeding time (less than a year to develop to the susceptible instars, which is greatly shorter than that in the wild [9]). Therefore, the low As background and shorter duration of the life cycle result in low As accumulation in COS. In addition, the exposure time of arsenic in the environment may also play a role in arsenic transformation [44]. Different duration of the life cycle may also induce different As metabolic processes and efficiencies in the host larvae, and ultimately result in the differentiation of As composition in COS.
Cordyceps militaris as a fungus-pupa complex is evidently different from WOS and COS occurred as a fungus-larva complex; and its stromata are usually consumed as edible parts [22]. The stromata of C. militaris were massively cultivated on the artificial substrates in food industries, and also brisked up in food markets [45], just like C. militaris (CM) in this study. The analysis of total As in CM is usually neglected owing to the low As abundances in substrates. Similarly, the contents of total As in the two cultivated edible mushrooms, LE and AA are also low due to the low As abundances in their artificial substrates.
When A. bisporus was cultivated in the control substrate (low As background, 3.8 ± 0.2 mg As/kg), the concentrations of total As were 0.50 0.03 mg/kg, and the most abundant speciation was AsB (0.31 ± 0.08 mg/kg, about 68.4%) [38]. In this study, A. bisporus was wild and grown on the natural substrate. Although the contents of total As (1.680–2.677 mg/kg) are evidently higher than those in the controlled A. bisporus, the most abundant speciation was identical (AsB, 59 80%). The above consistency can be explained by the species-specific speculation [24]. However, when cultivated in an iAs seriously contaminated substrate (1000 mg AsV/kg), A. bisporus enriched iAs (22.8 ± 1 mg/kg; 98% of the total As) [38]. In this situation, most of the As in A. bisporus occurred in the highly toxic iAs form, and was not transformed into AsB. The same phenomenon was discovered in some marine organisms, and considered to be attributed to that the biological pathways became saturated at the higher As concentrations [46]. Therefore, higher concentrations may have inhibited the microbial transformation of As [44]. This is to say, if the background is high in iAs, the excessive iAs will be passively transferred into mushrooms from the substrate, but cannot be converted into oAs via the fungal transformation of As [38].
3.2. Novel Arsenic Markers for Discriminating Wild and Cultivated Cordyceps
Since the As-accumulated mushrooms and O. sinensis are frequently reported by CFDA, the detection of total As and five As species has become the routine test items for edible mushrooms and O. sinensis. In view of As composition dependent on biological species and As backgrounds, we may adopt As species and their abundances as markers for authenticating wild O. sinensis. In other words, the routine food safety inspection can be further utilized as an adulteration test. The discrepancy of total As (Figure 3) and As species in abundance (Figure 4) between wild O. sinensis and the other groups confirms the above speculation. To validate the possibility, the PCA scores from the data (Table 1 and Table S6) of total As and As species were examined, and the first three principal components displayed in a three-dimensional plot (Figure 5), accounting for 99.01%, 0.63%, and 0.34% (99.98% in total) of the total variation, respectively. The detailed ranges for every group were presented in Table 2. Samples in each group were classified into discrete clusters, indicating that PCA could be used to distinguish one group from another. From the PC1 axis (−2.80 to 6.55), WOS in the range of 1.40–6.55 was well separated from the other groups with the positive scores. From the PC2 axis (−0.60 to 0.80), COS at the interval of 0.45–0.80 was well discriminated from the remaining five groups with the gap of −0.40 to 0.15 on this axis. Thus, the As composition can be applied to discriminate between wild/cultivated O. sinensis and its substitutes. In addition, from the PC3 axis (−0.20 to 0.95), AB samples (0.55 to 0.95) can also be distinguished from the other samples (−0.20 to 0.35) closely distributed on the PC3 axis.
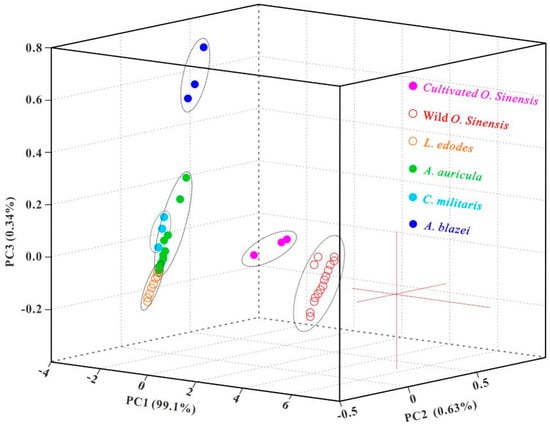
Figure 5.
Three-dimensional PCA score plot for discriminating six sample groups. The first three principal components are displayed, accounting for 99.01%, 0.63%, and 0.34% (99.98% in total) of the total variation, respectively.

Table 2.
The ranges of sample values in the three-dimensional scatter plot (PC1 vs. PC2 vs. PC3).
In this study, to discriminate wild O. sinensis, the abundances of total As and uAs can be applied as sensitive markers. Although the uAs species are still not clear, we consider that they may belong to asenosugars on the basis of the H2O2 test and their chromatographic behaviors in our previous study [21]. They were mostly formed by long-term metabolic processes in the host larva. Thus, the larva-originated sclerotium in WOS and COS may contribute to most of the uAs, which can be subsequently used to distinguishing WOS and its substitutes. In this study, we may construct the uAs (%) vs. total As (mg/kg) plot (Figure 6) from the data (Table 1 and Table S6) for discriminating adulteration. It can be seen from Figure 6 that the plot clearly exhibits different fields (A, B, C, and D) for different sample groups, i.e., O. sinensis, whatever WOS (total As, 4.1–9.2 mg/kg; uAs, 89.6–100%) or COS (total As, 0–0.29 mg/kg; uAs, 59 67%), dropped in field A or B (above the red dashed line, uAs > 59%); the other mushrooms (CM, AB, LE, and AA) except O sinensis in the field below the red dashed line; wild mushrooms (including WOS and AB) in fields B and D in the right side of the blue dashed line (1.5 mg/kg [47]); the other cultivated mushrooms (CM, LE, and AA) in the left side of the blue dashed line. Thus, Figure 6 can be evidently used to discriminate wild/cultivated O. sinensis from its substitutes. If the As composition of one Cordyceps product drops in the blue field, it may be derived from wild O. sinensis.
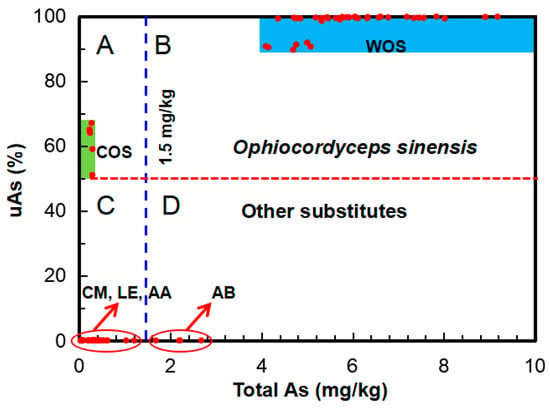
Figure 6.
uAs (%) vs. tAs (mg/kg) plot illustrating the fields of wild O. sinensis [blue in field (B)], cultivated O. sinensis [green in field (A)], and the other mushrooms. Red dashed line divides the samples into the groups of O. sinensis (WOS and COS) and the groups of the other mushrooms (CM, AB, LE, and AA). Blue dashed line divides the samples into the groups of wild (WOS and AB) and cultivated (COS, CM, LE, and AA) mushrooms.
It should be pointed out that the above-mentioned As markers can be used to authenticate not only wild O. sinensis from its substitutes/processed products, but also wild edible fungi from their cultivated ones (Figure 6), because the As contents in cultivated fungi are generally low (A. bisporus, 0.19–1.50 mg/kg), especially wood-cultivated fungi (Pleurotus ostreatus, 0.09–0.50 mg/kg; L. edodes, 0.04–0.07 mg/kg) [47]. To reduce the chances of false positives or false negatives, more accurate As indicators and ranges need to be explored and ascertained on the basis of the more data in future.
4. Materials and Methods
4.1. Reagents and Standards
All the solutions were prepared by deionized water (Millipore, Bedford, MA, USA). Anhydrous sodium acetate, potassium nitrate, sodium dihydrogen phosphate, and disodium ethylenediaminetetraacetate (HPLC grade, J & K Chemical, Beijing, China) were used for chromatographic mobile phases. Concentrated HNO3 (69%) was used to digest samples for determining the contents of total As; and dilute HNO3 (2%) was adopted to extract As species from samples. Standards for measuring both total As (10 mg As/L) and As species (AsIII, 0.233 μmol/g; AsV, 1.011 μmol/g; MMAV, 0.355 μmol/g; DMAV, 0.706 μmol/g; and AsB, 0.518 μmol/g) were obtained from diluting and compounding the standard stock solutions (The National Institute of Metrology, Beijing, China). All stock solutions were stored at 4 °C; while dilutions used for analysis were prepared daily. The following National Standard Reference Materials were used in this study: GBW10049 (Green Chinese Onion, Institute of Geophysical and Geochemical Exploration, Langfang, China), GBW10051 (Pork liver, Institute of Geophysical and Geochemical Exploration, Langfang, China), GBW08573 (Yellow fin tuna, the Second Institute of Oceanography, Hangzhou, China), and GBW(E)100358 (Rice, the National Analysis Center for Ion and Steel, Beijing, China).
4.2. Samples
Twenty-five samples of cultivated O. sinensis (COS, Figure 1B) were freshly purchased from the Horizon East Company (Shenzhen, China), and then freeze-dried in laboratory. To ensure the enough quantity for subsequent As analysis, five pieces of COS were combined into each sample, which was named as COS 1, 2, 3, 4 or 5, respectively. These samples were individually pulverized with the size of less than 40 meshes, sealed in the plastic bags, and stored at 4 °C. Cultivated Cordyceps militaris (CM, Figure 1C), wild Agaricus blazei (AB, Figure 1D), cultivated Lentinus edodes (LE, Figure 1E), and cultivated Auricularia auricula (AA, Figure 1F) were obtained from a food market (Shenzhen, China). Referring to the procedures of treating O. sinensis, we prepared these samples for As analysis.
The methods for sample digestion and As determination followed the previous reference [21]. Briefly, for total As analysis, approximately 500 mg of each powdered sample was mixed with 20 mL concentrated HNO3; and digested in a microwave-assisted system with the following procedures: (1) heated to 120 °C in 5 min and held at 120 °C for 5 min; (2) 150 °C in 5 min and held at 150 °C for 5 min; (3) 170 °C in 5 min and held at 170 °C for 5 min; (4) 190 °C in 5 min and held at 190 °C for 20 min; and (5) cooled to room temperature. For As species analysis, 1 g of each powdered sample was used to extract As species with 20 mL dilute HNO3 in a 90 °C water bath for 12 h, and shaken on a vortex for 1 min every 2 h. All of the digested products were centrifuged and filtered; and the final extracts were kept at 4 °C before analysis.
4.3. Total Arsenic Analysis
Total As analysis was conducted according to our previously-reported method [21]. The filtered supernatant was replenished with water up to 25 mL, and subjected to an Agilent 7800 ICP-MS (Agilent Technologies, Palo Alto, CA, USA). The analytical conditions were described as below: RF power, 1550 W; carrier gas, 1.03 L/min; dilution gas, 0.10 L/min; collision mode; He flow rate, 4.3 mL/min; plasma gas flow rate, 15 L/min; auxiliary gas flow rate, 0.9 L/min; and selected isotope, m/z 75. The concentrations of total As in samples were quantified through a AsV standards calibration curve (Calibration points: 5, 10, 50, 100, and 200 ppm). The corresponding digestion blanks and total As in CRMs were measured on the basis of the current method.
4.4. Arsenic Speciation Analysis
Analyses of As species (AsIII, AsV, MMAV, DMAV, and AsB) were conducted according to our previously-established method [21]. Briefly, As species were separated by an Agilent 1260 HPLC (Agilent Technologies, Palo Alto, CA, USA), which was equipped with an autosampler, a guard column (IonPac AG19, 4 mm × 50 mm), and a separation column (IonPacAS19, 4 mm × 250 mm). The chromatographic conditions were described as below: mobile phase, a mixed solution of 10 mmol/L anhydrous sodium acetate, 3 mmol/L potassium nitrate, 10 mmol/L sodium dihydrogen phosphate, and 0.2 mmol/L disodium ethylenediaminetetraacetate buffer; flow rate, 1.0 mL/min; column temperature, 25 °C; injection volume, 50 μL. The separated As species were determined by ICP-MS, and identified and quantified by a calibration curve of the standards (Calibration points: 0, 2.5, 5, 10, 50, 100, and 200 ppb). To correct the matrix effects which might result in the shift of retention time, considerable amounts of internal standards (2.5 ppb for COS, 5 ppb for CM, and 5 ppb for AA; while for AB, which contains a large amount of AsB, 100 ppb of the standards were firstly added in AB, and then diluted with 2% HNO3 to the ratio of 1:10) were added in each sample. Due to the complexity of the sample matrix, some of the oAs species, other than the above three oAs species (MMAV, DMAV, and AsB) might exist in O. sinensis, and they were measured using the method reported in our previous study [21]. To check the extraction efficiency for As species analysis, the concentrations of total As in the extracts of As species digested by dilute HNO3 were directly measured by ICP-MS; and then compared with the extracts of total As treated by concentrated HNO3. Extraction blanks were also determined in each work session, and the iAs species in CRMs were determined using this method.
4.5. Statistical Analysis and Data Processing
Statistical analysis was conducted using the IBM SPSS Statistics ver. 20 (Microsoft Corp., Redmond, WA, USA). The total As contents and As species were expressed as their average values of three determinations with the relative standard deviation (RSD) of less than 7%. Principle component analysis (PCA) was used to discriminate different sample groups. Based on the Fuzzy C-means (FCM) method, the three-dimensional PCA score plot was drawn by MATLAB ver. 7.0.4 (MathWorks Inc., South Natick, MA, USA).
5. Conclusions
ICP-MS and HPLC-ICP-MS have been employed to measure both total As and As species in wild and cultivated O. sinensis as well as multiple edible fungi. The results indicate that both total As and As species exhibit the species-specific behavior and the effect of the As background. The proportions of uAs and the contents of total As are considered as effective markers for discriminating wild O. sinensis from its substitutes/processed products.
6. Patents
A new method for discrimination of wild and cultivated Ophiocordyceps sinensis. Patent No. 201810564330.1
Supplementary Materials
The following are available online. Figures S1–S5: Total ion currents of the standards adding tests (Figure S1: COS; Figure S2: CM; Figure S3: AB; Figure S4: LE; and Figure S5: AA), Table S1: Analytical performances for total As and As species analysis, Table S2: Precision of the methods, Table S3: Recovery of the methods, Table S4: Values of the National Standard Reference Materials (mg/kg, mean ± standard deviation) and determined values for total and inorganic As (n = 5), Table S5: Extraction efficiency of the method for As species analysis, Table S6: Concentrations of total As and percentages of As species in the samples of wild O. sinensis.
Author Contributions
L.-X.G. and J.-H.W. conceived and designed the experiments; G.-W.Z. performed the experiments; Q.-Q.L. and X.-M.X. analyzed the data and contributed analysis tools; L.-X.G. wrote the paper.
Funding
This work was jointly supported by the Excellent College Teachers Training Program of Guangdong Province, China (No. YQ2015084), the Natural Science Foundation of Guangdong Province, China (No. 2018A030313094), the Social Science and Technology Development Project of Dongguan, China (No. 20185071521641), and the National Natural Science Foundation of China (No. 81303155).
Acknowledgments
We thank for the financial supports from the National Natural Science Foundation of China, the Foundation from Guangdong Province, and the Science and Technology Bureau of Dongguan.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
| CFDA | China Food and Drug Administration |
| As | arsenic |
| iAs | inorganic arsenic |
| oAs | organic arsenic |
| AsIII | arsenite |
| AsV | arsenate |
| uAs | unknown organic As |
| AsB | arsenobetaine |
| AsC | arsenocholine |
| MMA | monomethylarsonic acid |
| DMA | dimethylarsinic acid |
| ICP-MS | inductively coupled plasma mass spectroscopy |
| HPLC | high-performance liquid chromatography |
| WOS | wild Ophiocordyceps sinensis |
| COS | cultivated Ophiocordyceps sinensis |
| CM | Cordyceps militaris |
| AB | Agaricus blazei |
| LE | Lentinus edodes |
| AA | Auricularia auricular |
| PCA | principle component analysis |
| CRMs | certified reference materials |
References
- Dong, C.H.; Li, W.J.; Li, Z.Z.; Yan, W.J.; Li, T.H.; Liu, X.Z. Cordyceps industry in China: Current status, challenges and perspectives: Jinhu declaration for Cordyceps industry development. Mycosystema 2016, 35, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, E.; Wang, C.; Li, Y.; Liu, X. Ophiocordyceps sinensis, the flagship fungus of China: Terminology, life strategy and ecology. Mycology 2012, 3, 2–10. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, J.P.; Xu, S.P.; Zhou, X.G.; Zhang, Y.; Xu, X.M.; Zou, Z.W.; Zhang, G.R.; Wang, J.H. Stable carbon isotope evidence for tracing the diet of the host Hepialus larva of Cordyceps sinensis in the Tibetan Plateau. Sci. China Ser. D-Earth Sci. 2009, 52, 655–659. [Google Scholar] [CrossRef]
- Zou, Z.W.; Xin, L.; Zhang, G.R. Revision of taxonomic system of the genus Hepialus (Lepidoptera, Hepialidae) currently adopted in China. Hunan Univ. Sci. Technol. 2010, 25, 114–120. [Google Scholar]
- Guo, L.X.; Hong, Y.H.; Zhou, Q.Z.; Zhu, Q.; Xu, X.M.; Wang, J.H. Fungus-larva relation in the formation of Cordyceps sinensis as revealed by stable carbon isotope analysis. Sci. Rep. 2017, 7, 789. [Google Scholar] [CrossRef] [PubMed]
- Stone, R. Mycology: Last stand for the body snatcher of the Himalayas? Science 2008, 322, 1182. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Lei, W.; Yan, Y.; Zhang, Y.; Liu, S.; Cao, P.; Fan, Y. Pharmacological study progress of the Cordyceps sinensis. Glob. Tradit. Chinese Med. 2014, 7, 227–232. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.H.; Wang, W.; Zhang, H.Y.; Zhang, X.L.; Han, C.C. The chemical constituents and pharmacological actions of Cordyceps sinensis. Evid.-Based Complem. Altern. Med. 2015, 2015, 575063. [Google Scholar] [CrossRef]
- Li, W.J.; Dong, C.H.; Liu, X.Z.; Li, Q.P.; Xia, J.M.; Liang, L. Research advances in artificial cultivation of Chinese Cordyceps. Mycosystema 2016, 35, 375–387. [Google Scholar] [CrossRef]
- Wei, J.C.; Wei, X.L.; Zheng, W.F.; Guo, W.; Liu, R.D. Species identification and component detection of Ophiocordyceps sinensis cultivated by modern industry. Mycosystema 2016, 35, 404–410. [Google Scholar] [CrossRef]
- Qiu, X.H.; Cao, L.; Han, R.C. The progress, issues and perspectives in the research of Ophiocordyceps sinensis. Environ. Entomol. 2016, 38, 1–23. [Google Scholar] [CrossRef]
- Pan, X.Y. Identification of Cordyceps sinensis by experience. China Pharm. 2006, 9, 380–381. [Google Scholar]
- Lo, H.C.; Hsieh, C.; Lin, F.Y.; Hsu, T.H. A systematic review of the mysterious caterpillar fungus Ophiocordyceps sinensis in DongChongXiaCao (dōng chóng xià căo) and related bioactive ingredients. J. Tradit. Complement. Med. 2013, 3, 16–32. [Google Scholar] [CrossRef]
- Qian, Z.M.; Liao, N.; Li, W.Q.; Ai, Z.; Tian, Y.; Li, W.J. Application of modern instrumental analytical techniques in quality evaluation of Cordyceps sinensis. Mod. Chin. Med. 2016, 18, 682–688. [Google Scholar] [CrossRef]
- Zhang, P.; Li, S.; Li, J.; Wei, F.; Cheng, X.; Zhang, G.; Ma, S.; Liu, C. Identification of Ophiocordyceps sinensis and its artificially cultured Ophiocordyceps mycelia by ultra-performance liquid chromatography/Orbitrap fusion mass spectrometry and chemometrics. Molecules 2018, 23, 1013. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Su, Y.L.; Yang, H.L.; Chen, T.Y. Identification of Chinese medicinal fungus Cordyceps sinensis by PCR-single-stranded conformation polymorphism and phylogenetic relationship. J. Agric. Food. Chem. 2005, 53, 3963–3968. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.X.; Xu, X.M.; Hong, Y.H.; Li, Y.; Wang, J.H. Stable carbon isotope composition of the lipids in natural Ophiocordyceps sinensis from major habitats in China and its substitutes. Molecules 2017, 22, 1567. [Google Scholar] [CrossRef] [PubMed]
- China Food and Drug Administration (CFDA). Consumption tips on Ophiocordyceps sinensis products. Available online: http://samr.cfda.gov.cn/WS01/CL1986/144020.html (accessed on 4 February 2016).
- The Common Standards of Functional Food; GB16740-2014; The National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2014.
- China Food and Drug Administration (CFDA). Notice on the Suspension of All the Pilot Work on Ophiocordyceps sinensis for Health Food. Available online: http://samr.cfda.gov.cn/WS01/CL0847/146100.html (accessed on 4 March 2016).
- Guo, L.X.; Zhang, G.W.; Wang, J.T.; Zhong, Y.P.; Huang, Z.G. Determination of arsenic species in Ophiocordyceps sinensis from major habitats in China by HPLC-ICP-MS and the edible hazard assessment. Molecules 2018, 23, 1012. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.P.; Liu, S.C.; Tang, C.H.; Chan, Y.; Elshazly, M.; Lee, C.L.; Du, Y.C.; Wu, T.Y.; Chang, F.R.; Wu, Y.C. Anti-inflammatory cerebrosides from cultivated Cordyceps militaris. J. Agric. Food Chem. 2016, 64, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Niegel, C.; Matysik, F.M. Analytical methods for the determination of arsenosugars—A review of recent trends and developments. Anal. Chim. Acta 2010, 657, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Nearing, M.M.; Koch, I.; Reimer, K.J. Arsenic speciation in edible mushrooms. Environ. Sci. Technol. 2014, 48, 14203–14210. [Google Scholar] [CrossRef] [PubMed]
- Yendrek, C.R.; Koester, R.P.; Ainsworth, E.A. A comparative analysis of transcriptomic, biochemical, and physiological responses to elevated ozone identifies species-specific mechanisms of resilience in legume crops. J. Exp. Bot. 2015, 66, 7101–7112. [Google Scholar] [CrossRef] [PubMed]
- Strauss, A.S.; Sven, P.; Wilhelm, B.; Antje, B. ABC transporter functions as a pacemaker for sequestration of plant glucosides in leaf beetles. eLife 2013, 2, e01096. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.; Goodale, B.; Raab, A.; Schwerdtle, T.; Reimer, K.; Conklin, S.; Karagas, M.R.; Francesconi, K.A. Human exposure to organic arsenic species from seafood. Sci. Total Environ. 2017, 580, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.J.; Jiang, S.J. Application of HPLC-ICP-MS and HPLC-ESI-MS procedures for arsenic speciation in seaweeds. J. Agric. Food Chem. 2012, 60, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Eisler, R. Arsenic hazards to fish, wildlife, and invertebrates: a synoptic review. U.S. Fish Wildlife Serv. Biol. Rep. 1988, 85, 1–65. [Google Scholar]
- Hettick, B.E.; Cañas-Carrell, J.E.; French, A.D.; Klein, D.M. Arsenic: A review of the element’s toxicity, plant interactions, and potential methods of remediation. J. Agric. Food Chem. 2015, 63, 7097–7107. [Google Scholar] [CrossRef] [PubMed]
- Andreae, M.O. Distribution and speciation of arsenic in natural waters and some marine algae. Deep-Sea Res. 1978, 25, 391–402. [Google Scholar] [CrossRef]
- Falandysz, J.; Rizal, L.M. Arsenic and its compounds in mushrooms: A review. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Yan, X.J.; Chen, Z. Arsenic in Tissues, Organs, and Cells. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 135–138. [Google Scholar]
- Chen, S.; Guo, Q.; Liu, L. Determination of arsenic species in edible mushrooms by high-performance liquid chromatography coupled to inductively coupled plasma mass spectrometry. Food Anal. Method. 2017, 10, 740–748. [Google Scholar] [CrossRef]
- Gonzálvez, A.; Llorens, A.; Cervera, M.; Armenta, S.; De la Guardia, M. Non-chromatographic speciation of inorganic arsenic in mushrooms by hydride generation atomic fluorescence spectrometry. Food Chem. 2009, 115, 360–364. [Google Scholar] [CrossRef]
- Larsen, E.H.; Hansen, M.; Gössler, W. Speciation and health risk considerations of arsenic in the edible mushroom Laccaria amethystina collected from contaminated and uncontaminated locations. Appl. Organomet. Chem. 1998, 12, 285–291. [Google Scholar] [CrossRef]
- Kuehnelt, D.; Goessler, W.; Irgolic, K. Arsenic compounds in terrestrial organisms I: Collybia maculata, Collybia butyracea and Amanita muscaria from arsenic smelter sites in Austria. Appl. Organomet. Chem. 2010, 11, 289–296. [Google Scholar] [CrossRef]
- Soeroes, C.; Kienzl, N.; Ipolyi, I.; Dernovics, M.; Fodor, P.; Kuehnelt, D. Arsenic uptake and arsenic compounds in cultivated Agaricus bisporus. Food Control. 2005, 16, 459–464. [Google Scholar] [CrossRef]
- Braeuer, S.; Goessler, W.; Kameník, J.; Konvalinková, T.; Žigová, A.; Borovička, J. Arsenic hyperaccumulation and speciation in the edible ink stain bolete (Cyanoboletus pulverulentus). Food Chem. 2018, 242, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Sÿlejkovec, Z.; Byrne, A.R.; Stijve, T.; Goessler, W.; Irgolic, K.J. Arsenic compounds in higher fungi. Appl. Organomet. Chem. 1997, 11, 673–682. [Google Scholar] [CrossRef]
- Zhang, X.P.; Deng, W.; Yang, X.M. The background concentrations of 13 soil trace elements and their relationships to parent materials and vegetation in Xizang (Tibet), China. J. Asian Earth Sci. 2002, 21, 167–174. [Google Scholar] [CrossRef]
- Zhao, Z.H. The distribution and abundances of elements. In Advanced Geochemistry, 1st ed.; The Institute of Geochemistry, Chinese Academy of Sciences, Eds.; Science Press: Beijing, China, 1988; p. 38. [Google Scholar]
- Li, W.J.; Li, Q.P.; Wei, Z.H.; Jiang, C.J.; Zhang, Z.Y.; Zhang, Y.F. Effects of soil and host plants on the artificial feeding of Hepialus sp. larvae, the host of Ophiocordyceps sinensis. Mycosystema 2016, 35, 467–475. [Google Scholar] [CrossRef]
- Nearing, M.M.; Koch, I.; Reimer, K.J. Uptake and transformation of arsenic during the reproductive life stage of Agaricus bisporus and Agaricus campestris. J. Environ. Sci. 2016, 49, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Won, S.Y.; Park, E.H. Anti-inflammatory and related pharmacological activities of cultured mycelia and fruiting bodies of Cordyceps militaris. J. Ethnopharm. 2005, 96, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Whaley-Martin, K.J.; Koch, I.; Moriarty, M.; Reimer, K.J. As speciation in blue mussels (Mytilus edulis) along a highly contaminated As gradient. Environ. Sci. Technol. 2012, 46, 3110–3118. [Google Scholar] [CrossRef] [PubMed]
- Haldimann, M.; Bajo, C.; Haller, T.; Venner, T.; Zimmerli, B. Occurrence of arsenic, lead, cadmium, mercury and selenium in cultivated mushrooms. Mitt. Geb. Lebensmittelunters Hyg. 1995, 86, 463–484. [Google Scholar]
Sample Availability: Samples of the compounds AsB, DMA, MMA, AsIII, AsV and uAs are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).