The Constituents of the Stems of Cissus assamica and Their Bioactivities
Abstract
1. Introduction
2. Results and Discussion
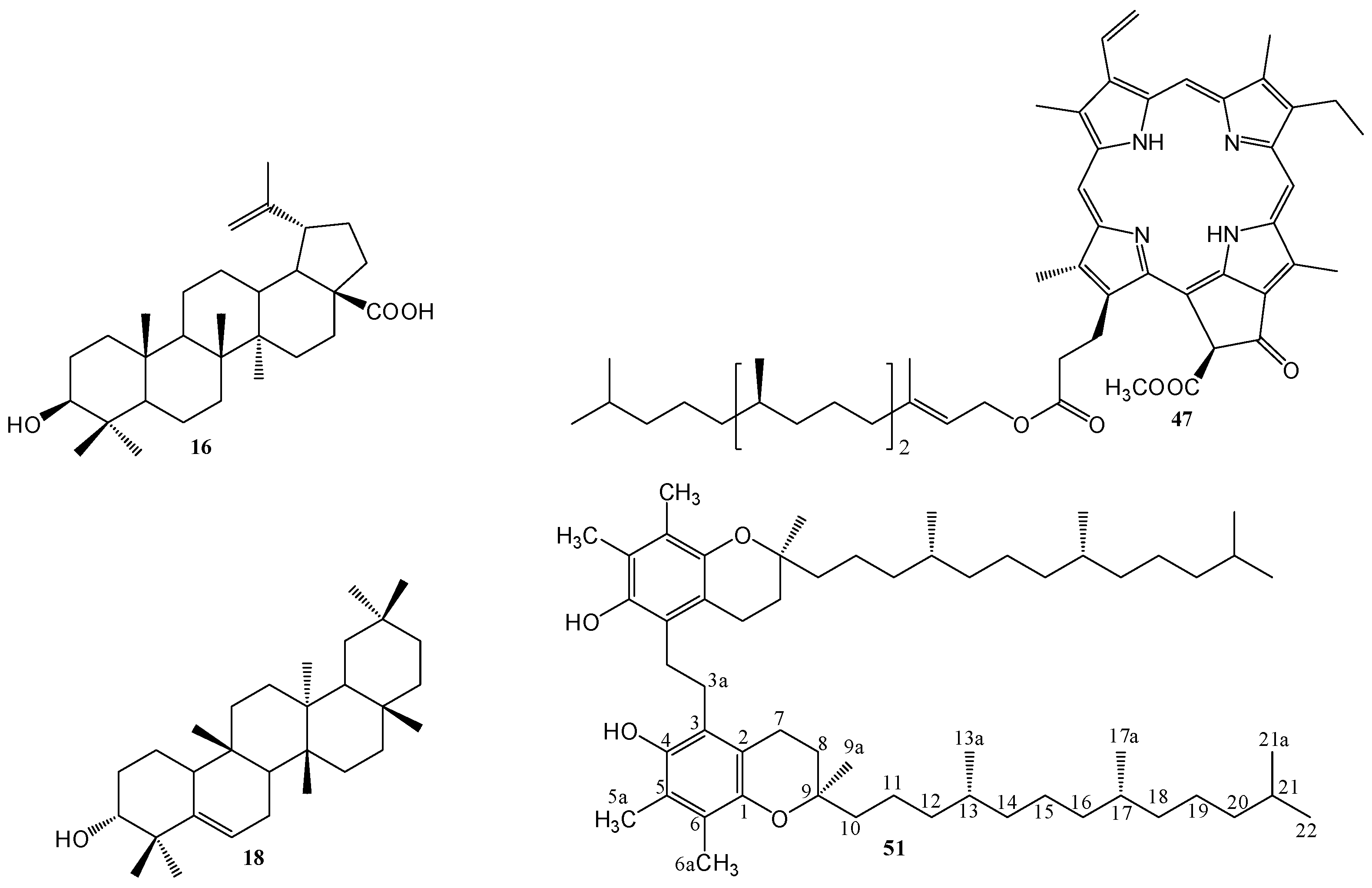
2.1. Isolation and Characterization of Compounds
2.2. Structural Elucidation of Compound 51
2.3. Anti-Inflammatory Activity
2.4. Cytotoxicity
3. Materials and Methods
3.1. General Information
3.2. Materials
3.3. Extraction and Isolation
3.4. Anti-Inflammatory Bioactivity Examination
3.4.1. Preparation of Human Neutrophils
3.4.2. Measurement of Superoxide Anion Generation and Elastase Release
3.4.3. Detection of Cytotoxicity
3.5. Determination of Anticancer Bioactivity
3.5.1. Cell Lines
3.5.2. Growth Inhibition Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, T.Y. Flora of Taiwan, 2nd ed.; Editorial Committee of the Flora of Taiwan: Taipei, Taiwan, 1998; pp. 701–703.
- Zhang, Y.Q.; Xie, Y.H.; Huang, L.P. Studies on the chemical constituents and biological activities from Cissus L. Lishizhen Medicine and Materia Medica Research 2006, 17, 107–114. [Google Scholar]
- Otshudi, A.L.; Foriers, A.; Vercruysse, A.; Van, Z.A.; Lauwers, S. In vitro antimicrobial activity of six medicinal plants traditionally used for the treatment of dysentery and diarrhoea in Democratic Republic of Congo. Phytomedicine 2000, 7, 167–172. [Google Scholar] [CrossRef]
- Beltrame, F.L.; Sartoretto, J.L.; Bazotte, R.B.; Cuman, R.N.; Cortez, D.A.G. Phytochemical study and evaluation of the antidiabetic potential of Cissus sicyoides L. Quim. Nova 2001, 24, 783–785. [Google Scholar] [CrossRef]
- Beltrame, F.L.; Ferreira, A.G.; Cortez, D.A. Coumarin glycoside from Cissus sicyoides. Nat. Prod. Lett. 2002, 16, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Adesanya, S.A.; Nia, R.; Martin, M.T.; Boukamcha, N.; Montagnac, A.; Pais, M. Stilbene derivatives from Cissus quadrangularis. J. Nat. Prod. 1999, 62, 1694–1695. [Google Scholar] [CrossRef]
- Bhutani, K.K.; Kapoor, R.; Atal, C.K. Two unsymmetric tetracyclic triterpenoids from Cissus quadrangulari. Phytochemistry 1984, 23, 407–410. [Google Scholar] [CrossRef]
- Gupta, M.M.; Verma, R.K. Unsymmetric tetracyclic triterpenoid from Cissus quadrangularis. Phytochemistry 1990, 29, 336–337. [Google Scholar] [CrossRef]
- Gupta, M.M.; Verma, R.K. Lipid constituents of Cissus quadrangularis. Phytochemistry 1991, 30, 875–878. [Google Scholar] [CrossRef]
- Khan, M.A.; Nabi, S.G.; Prakash, S.; Zaman, A. Pallidol, a resveratrol dimer from Cissus pallida. Phytochemistry 1986, 25, 1945–1948. [Google Scholar] [CrossRef]
- Al-Said, M.S.; Khalifa, A.S.; Al-Azizi, M.M. Flavonoids from Cissus digitat. Int. J. Pharm. 1991, 29, 281–283. [Google Scholar]
- Saenz, M.T.; Garcia, M.D.; Quilez, A.; Ahumada, M.C. Cytotoxic activity of Agave intermixta L. (Agavaceae) and Cissus sicyoides L. (Vitaceae). Phytother. Res. 2000, 14, 552–554. [Google Scholar] [CrossRef]
- Garcia, M.D.; Quilez, A.M.; Saenz, M.T.; Martinez, D.M.E.; De, P.R. Anti-inflammatory activity of agave intermixta trel and Cissus sicyoides L., species used in the caribbean traditional medicine. J. Ethnopharmacol. 2000, 71, 395–400. [Google Scholar] [CrossRef]
- Atawodi, S.E.; Arneh, D.A.; Ibrahim, S.; Andrew, J.N.; Nzelibe, H.C.; Onyike, E.O.; Anigo, K.M.; Abu, E.A.; James, D.B.; Njoku, G.C.; Sallau, A.B. Indigenous knowledge system for treatment of trypanosomiasis in Kaduna state of Nigeria. J. Ethnopharmacol. 2002, 79, 279–282. [Google Scholar] [CrossRef]
- Jainu, M.; Devi, C.S.S. Effect of Cissus quadrangularis on gastric mucosal defensive factors in experimentally induced gastric ulcer–A comparative study with sucralfate. J. Med. Food 2004, 7, 372–376. [Google Scholar] [PubMed]
- Quilez, A.M.; Saenz, M.T.; Garcia, M.D.; de la Puerta, R. Phytochemical analysis and anti-allergic study of agave intermixta trel and Cissus sicyoides L. J. Pharm. Pharmacol. 2004, 56, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Viana, G.S.; Medeiros, A.; Lacerda, A.M.; Leal, L.K.; Vale, T.G.; Matos, F.J. Hypoglycemic and anti-lipemic effects of the aqueous extract from Cissus sicyoides. BMC Pharmacol. 2004, 4, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Chidambara, M.K.N.; Vanitha, A.; Mahadeva, S.M.; Ravishankar, G.A. Antioxidant and antimicrobial activity of Cissus quadrangularis L. J. Med. Food 2003, 6, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Pepato, M.T.; Baviera, A.M.; Vendramini, R.C.; Perez, M.P.M.S.; Kettelhut, I.C.; Brunetti, I.L. Cissus sicyoides in the long-term treatment of streptozotocin-diabetic rats. Biotechnol. Appl. Biochem. 2003, 37, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Alzoreky, N.S.; Nakahara, K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int. J. Food. Microbiol. 2003, 80, 223–230. [Google Scholar] [CrossRef]
- Garcia, X.; Cartas, H.L.; Lorenzana, J.M.; Gijon, E. Vasoconstrictor effect of Cissus sicyoides on guinea Pig aortic rings. Gen. Pharmacol. 1997, 29, 457–462. [Google Scholar] [CrossRef]
- Mori, T.; Nishikawa, Y.; Takata, Y.; Kashuichi, N.; Ishihara, N. Effect of insulina leaf extract on development of diabetes comparison between normal, streptozotocin-induced diabetic rats and hereditary diabetic mice. Nippon Eiyo Shokuryo Gakkaishi 2001, 54, 197–203. [Google Scholar] [CrossRef]
- Yang, L.; Wang, F.; Liu, M.; Lu, M.; Jia, H.; Fang, X.; Li, Z. Isolation of resveratrol and its antagonistic effects on endothelin-1. Huaxi Yaoxue Zazhi 2000, 15, 81–84. [Google Scholar]
- Wang, F.; Yang, L.; Cheng, Y.; Lu, M.; Liu, M.; Ji, X.; Jia, H. Antagonism effects of extracts of Chinese herb Cissus assamica and monomer CA-1201 on endothelin-1 responses. Zhongguo Yaoxue Zazhi 1998, 33, 337–340. [Google Scholar]
- Yang, L.C.; Wang, F.; Liu, M. A study of an endothelin antagonist from a Chinese anti-snake venom medicinal herb. J. Cardiovasc. Pharmacol. 1998, 31, S249–S250. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, L.; Liu, M.; Lu, M.; Cheng, Y.; Jia, H. A primary study on antagonizing effects of anti-snake venom Chinese herb on endothelin-1 and sarafotoxin 6b. Zhongguo Zhong Yao Za Zhi 1997, 22, 620–622. [Google Scholar] [PubMed]
- Shen, D.Y.; Juang, S.H.; Kuo, P.C.; Huang, G.J.; Chan, Y.Y.; Damu, A.G.; Wu, T.S. Chemical constituents from Andrographis echioides and their anti-inflammatory activity. Int. J. Mol. Sci. 2013, 14, 496–514. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.S.; Chan, Y.Y. Constituents of leaves of Uncaria hirsuta Haviland. J. Chin. Chem. Soc. 1994, 41, 209–212. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Chu, P.H. Studies on the constituents from the bark of Bauhinia purpurea. J. Chin. Chem. Soc. 2002, 49, 269–274. [Google Scholar] [CrossRef]
- Wu, S.J.; Chan, Y.Y. Five New Iridoids from Roots of Salvia digitaloides. Molecules 2014, 19, 15521–15534. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.C.; Yang, M.L.; Hwang, T.L.; Lai, Y.Y.; Li, Y.C.; Thang, T.D.; Wu, T.S. Anti-inflammatory Diterpenoids from Croton tonkinensis. J. Nat. Prod. 2013, 76, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Chang, F.R.; Wu, Y.C. The Constituents of Lindera Glauca. J. Chin. Chem. Soc. 2000, 47, 373–380. [Google Scholar] [CrossRef]
- Thang, T.D.; Kuo, P.C.; Hwang, T.L.; Yang, M.L.; Ngoc, T.B.; Han, T.N.; Lin, C.W.; Wu, T.S. Triterpenoids and steroids from Ganoderma mastoporum and their inhibitory effects on superoxide anion generation and elastase release. Molecules 2013, 18, 14285–14292. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Gou, Y.L.; Cao, S.G.; Chen, S.X.; Sim, K.Y. Eicosenones and methylated flavonols from Amomum koenigii. Phytochemistry 1999, 50, 899–902. [Google Scholar] [CrossRef]
- Machida, K.; Osawa, K. On the flavonoid constituents from the peels of Citrus hassaku Hort.ex Tanaka. Chem. Pharm. Bull. 1989, 37, 1092–1094. [Google Scholar] [CrossRef]
- Weber, B.; Hartmann, B.; Stockigt, D.; Schreiber, K.; Roloff, M.; Bertram, H.J.; Schmidt, C.O. Liquid chromatography/mass spectrometry and liquid chromatography nuclear magnetic resonance as complementary analytical techniques for unambiguous identification of polymethoxylated flavones in residues from molecular distillation of orange peel oils (Citrus sinensis). J. Agric. Food Chem. 2006, 54, 274–278. [Google Scholar] [PubMed]
- Chatterjee, P.; Kouzi, S.A.; Pezzuto, J.P.; Hamann, M.T. Biotransformation of the antimelanoma agent betulinic acid by Bacillus megaterium ATCC 13368. Appl. Environ. Microbiol. 2000, 66, 3850–3855. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.S.; Chan, Y.Y.; Liou, M.J.; Lin, F.W.; Shi, L.S.; Chen, K.T. Platelet aggregation inhibitor from Murraya euchrestifolia. Phytother. Res. 1998, 12, S80–S82. [Google Scholar] [CrossRef]
- Tanabe, Y.; Sinoda, R.; Horikoshi, Y.; Takahashi, K. Studies on constituents of medicinal plants. XVI. The constituents of Spiracea species. Yakugaku Zasshi 1976, 96, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Li, K.H.; Chang, C.R.; Chang, Y.S. Chemical components from Triumfetta bartramia. J. Chin. Chem. Soc. 1995, 42, 93–95. [Google Scholar]
- Zhang, J.; Yin, Z.Q.; Cao, P.; Li, Y.B.; Duan, J.A. A new flavonol derivative from Fagopyrum dibotrys. Chem. Nat. Compd. 2008, 44, 701–703. [Google Scholar] [CrossRef]
- Hata, K.; Hori, K.; Takahashi, S. Differntion and apoptosis-inducing activities by pentacyclic triterpenes on a mouse melanoma cell line. J. Nat. Prod. 2009, 65, 645–648. [Google Scholar] [CrossRef]
- Bolleddula, J.; Mulabagal, V.; Yanjun, Z.; David, L.D.; Muraleedharan, G.N. Impact of alkyl esters of caffeic and ferulic acids on tumor cell proliferation cyclooxygenase enzyme and lipid peroxidation. J. Agric. Food Chem. 2006, 54, 5375–5381. [Google Scholar]
- Xie, Y.H.; Zhang, Y.Q.; Deng, P.; Yu, W.S. Determination of bergenin in Cissus assamica by HPLC. Shizhen Guoyi Guoyao 2009, 20, 1086–1087. [Google Scholar]
- Chiang, C.Y.; Leu, Y.L.; Chan, Y.Y.; Wu, T.S. Sodium aristolochates from the flowers and fruits of Aristolochia zollingeriana. J. Chin. Chem. Soc. 1998, 45, 93–97. [Google Scholar] [CrossRef]
- Choi, S.E.; Yoon, J.H.; Choi, H.K.; Lee, M.W. Phenolic compounds from the root of Phragmites communis. Chem. Nat. Compd. 2009, 45, 893–895. [Google Scholar] [CrossRef]
- Jun, X.L.; Duo, L.D.; Yan, P.S. Diversity of chemical constituents from Saxifraga montana H. J. Chin. Chem. Soc. 2008, 55, 863–870. [Google Scholar]
- Cui, L.Q.; Liu, K.; Zhang, C. Effective oxidation of benzylic and alkane C–H bonds catalyzed by sodium O-iodobenzenesulfonate with oxone as a terminal oxidant under phase-transfer conditions. Org. Biomol. Chem. 2011, 9, 2258–2265. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.S.; Tsanga, Z.J.; Wu, P.L.; Lin, F.W.; Li, C.Y.; Temg, C.M.; Lee, K.H. New constituents and antiplatelet aggregation and anti-HIV principles of Artemisia capillaris. Bioorg. Med. Chem. 2001, 9, 77–83. [Google Scholar] [CrossRef]
- Wei, H.; Wen, D.X.; Liu, X.S.; Tang, R.J. Constituents in petroleum ether and ethyl acetate extract fractions of Dracaena cochinensis (Lour.). Zhong Guo Zhong Yao Za Zhi 1998, 23, 616–618. [Google Scholar]
- Loupy, A.; Chatti, S.; Delamare, S.; Lee, D.-Y.; Chung, J.-H.; Jun, C.-H. Solvent-free chelation-assisted hydroacylation of olefins rhodium(Ι) catalyst under microwave irradiation. J. Chem. Soc. 2002, 10, 1280–1285. [Google Scholar]
- Ding, H.Y.; Lin, H.C.; Teng, C.M.; Wu, Y.C. Phytochemical and pharmacological studies on Chinese Paeonia species. J. Chin. Chem. Soc. 2000, 47, 381–388. [Google Scholar] [CrossRef]
- Magdziak, D.; Rodriguez, A.A.; Van, D.W.; Ryan, W.; Pettus, T.R.R. Regioselective oxidation of phenols to O-quinones with O-iodoxybenzoic Acid (IBX). Org. Lett. 2002, 4, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wu, J.; Zou, W.; Dai, Y. Two ellagic acids isolated from roots of Sanguisorba officinalis L. promote hematopoietic progenitor cell proliferation and megakaryocyte differentiation. Molecules 2014, 19, 5448–5458. [Google Scholar] [CrossRef] [PubMed]
- Reitze, J.D.; Przewloka, S.R.; Shearer, B.J. The further chemistry of ellagic acid: I. synthesis of tetramethylellagic acid and associated polymer precursors. Holzforschung 2005, 55, 171–175. [Google Scholar] [CrossRef]
- Almeida, E.R.D.; Rafael, K.R.D.O.; Couto, G.B.L.; Ishigmi, A.B. Anxiolytic and anticonvulsant effects on mice of flavonoids, linalool, and α-tocopherol presents in the extract of leaves of Cissus sicyoides L. J. Biomed. Biotechnol. 2009, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lie, C.R.; Jiau, C.H.; Chiu, M.C. Cerebrosides and tocopherol trimers from the seeds of Euryale ferox. J. Nat. Prod. 2007, 70, 1214–1217. [Google Scholar]
- Rosenau, T.; Kloser, E.; Gille, L.; Mazzini, F.; Netscher, T. Vitamin E chemistry. studies into initial oxidation intermediates of α-tocopherol: disproving the involvement of 5a-C-centered chromanol methide radicals. J. Org. Chem. 2007, 72, 3268–3281. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.M.; Kuo, Y.H. Two novel α-tocopheroids from the aerial roots of Ficus microcarpa. Tetrahedron Lett. 2003, 44, 5125–5128. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Butler, T.; Lower, A. Controlling regiochemistry in negishi carboaluminations fine tuning the ligand on zirconium. J. Am. Chem. Soc. 2006, 128, 15396–15398. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.X.; Ma, W.H.; Li, Y.L.; Han, T.; Zheng, C.J.; Qin, L.P. Two new diterpenes from Solidago canadensis. Helv. Chim. Acta. 2012, 95, 1121–1125. [Google Scholar] [CrossRef]
- Zeng, Z.X.; Li, Y.L.; Dong, L.L.; Fan, G.X.; Fei, D.Q. Chemical constituents of the roots of Ligularia lapathifolia. Chem. Nat. Compd. 2015, 51, 375–377. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Investig. 2000, 80, 617–653. [Google Scholar] [CrossRef] [PubMed]
- Borregaard, N. The human neutrophil. Function and dysfunction. Eur. J. Haematol. 1998, 41, 401–413. [Google Scholar] [CrossRef]
- Roos, D.; van Bruggen, R.; Meischl, C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003, 5, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Faurschou, M.; Borregaard, N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003, 5, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.J.; Wang, Y.L.; Kuo, L.M.; Lin, C.F.; Chen, C.Y.; Tsai, Y.F.; Shen, J.J.; Hwang, T.L. Honokiol suppresses TNF-α-induced neutrophil adhesion on cerebral endothelial cells by disrupting polyubiquitination and degradation of IκBα. Sci. Rep. 2016, 6, 26554. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Chung, P.J.; Ho, C.M.; Kuo, C.Y.; Hung, M.F.; Huang, Y.T.; Chang, W.Y.; Chang, Y.W.; Chan, K.H.; Hwang, T.L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973, 52, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.L.; Li, G.L.; Lan, Y.H.; Chia, Y.C.; Shieh, P.W.; Wu, Y.H.; Wu, Y.C. Potent inhibition of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii. Free Radic. Biol. Med. 2009, 46, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]
Sample Availability: Samples of all the isolated compounds are available from the authors. |
| Position | 48 | 51 | ||
|---|---|---|---|---|
| δH (mult., J in Hz) | δC | δH (mult., J in Hz) | δC | |
| 1 | 145.5 | 146.3 | ||
| 2 | 117.2 | 117.0 | ||
| 3 | 118.5 | 123.4 | ||
| 4 | 144.5 | 145.5 | ||
| 5 | 121.1 | 122.1 | ||
| 6 | 122.6 | 124.1 | ||
| 7 | 2.64 (t, 4.5) | 21.0 | 2.74 (m) | 21.7 |
| 8 | 1.79 (m) | 31.5 | 1.83 (m) | 32.3 |
| 9 | 74.5 | 75.3 | ||
| 10 | 1.56 (m) | 39.8 | 1.56 (m) | 40.7 |
| 11 | 1.54 (m) | 22.6 | 1.54 (m) | 21.3 |
| 12 | 1.26~1.25 (m) | 37.4 | 1.28~1.25 (m) | 38.2 |
| 13 | 1.31 (d, 7.4) | 32.7 | 1.32 (d, 7.2) | 33.4 |
| 14 | 1.26~1.25 (m) | 37.2 | 1.28~1.25 (m) | 37.9 |
| 15 | 1.26~1.25 (m) | 24.8 | 1.28~1.25 (m) | 25,5 |
| 16 | 1.26~1.25 (m) | 37.5 | 1.28~1.25 (m) | 38.2 |
| 17 | 1.31 (d, 7.4) | 32.8 | 1.32 (d, 7.2) | 33.5 |
| 18 | 1.16~1.13 (m) | 37.4 | 1.16~1.13 (m) | 38.2 |
| 19 | 1.16~1.13 (m) | 25.1 | 1.16~1.13 (m) | 25,2 |
| 20 | 1.16~1.13 (m) | 39.4 | 1.16~1.13 (m) | 40.1 |
| 21 | 1.52 (m) | 27.9 | 1.52 (m) | 28.7 |
| 22 | 0.91 (d, 6.4) | 23.7 | 0.86 (d, 7.2) | 23.4 |
| 3a | 2.17 (s) | 11.2 | 2.73 (s) | 26.7 |
| 5a | 2.22 (s) | 12.8 | 2.18 (s) | 12.8 |
| 6a | 2.19 (s) | 11.8 | 2.13 (s) | 12.6 |
| 9a | 1.28 (s) | 24.4 | 1.24 (s) | 24.5 |
| 13a | 0.90 (d, 7.4) | 20.3 | 0.85 (d, 7.2) | 20.3 |
| 17a | 0.89 (d, 7.4) | 20.7 | 0.83 (d, 7.2) | 20.5 |
| 21a | 0.93 (d, 6.5) | 22.7 | 0.87 (d, 7.2) | 23.3 |
| OH | 4.25 (s) | 5.41 (s) | ||
| Compound | Superoxide Anion Generation | Elastase Release |
|---|---|---|
| IC50 (μM) a | IC50 (μM) | |
| 16 | 0.2 ± 0.1 *** | 2.7 ± 0.3 *** |
| 47 | >10 | 5.3 ± 1.0 *** |
| LY294002b | 0.4 ± 0.1 *** | 1.5 ± 0.3 *** |
| Compounds | Cell Lines | |
|---|---|---|
| NCI-H226 | HCT-116 | |
| IC50 (μM) | IC50 (μM) | |
| 16 | 2.0 | 1.6 |
| 18 | 9.1 | 6.0 |
| 20 | 15.8 | 16.7 |
| 21 | 38.0 | 24.0 |
| 41 | 31.6 | 30.3 |
| 52 | >50 | 39.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, Y.-Y.; Wang, C.-Y.; Hwang, T.-L.; Juang, S.-H.; Hung, H.-Y.; Kuo, P.-C.; Chen, P.-J.; Wu, T.-S. The Constituents of the Stems of Cissus assamica and Their Bioactivities. Molecules 2018, 23, 2799. https://doi.org/10.3390/molecules23112799
Chan Y-Y, Wang C-Y, Hwang T-L, Juang S-H, Hung H-Y, Kuo P-C, Chen P-J, Wu T-S. The Constituents of the Stems of Cissus assamica and Their Bioactivities. Molecules. 2018; 23(11):2799. https://doi.org/10.3390/molecules23112799
Chicago/Turabian StyleChan, Yu-Yi, Chiu-Yuan Wang, Tsong-Long Hwang, Shin-Hun Juang, Hsin-Yi Hung, Ping-Chung Kuo, Po-Jen Chen, and Tian-Shung Wu. 2018. "The Constituents of the Stems of Cissus assamica and Their Bioactivities" Molecules 23, no. 11: 2799. https://doi.org/10.3390/molecules23112799
APA StyleChan, Y.-Y., Wang, C.-Y., Hwang, T.-L., Juang, S.-H., Hung, H.-Y., Kuo, P.-C., Chen, P.-J., & Wu, T.-S. (2018). The Constituents of the Stems of Cissus assamica and Their Bioactivities. Molecules, 23(11), 2799. https://doi.org/10.3390/molecules23112799






